Abstract
Advances in imaging technology, especially in the last two decades, have led to a paradigm shift in the field of image-guided interventions in urology. While the traditional biopsy and drainage techniques are firmly established, image-based stone management and endovascular management of hematuria have evolved further. Ablative techniques for renal and prostate cancer and prostate artery embolization for benign prostatic hypertrophy have evolved into viable alternative treatments. Many urologic diseases that were earlier treated surgically are now effectively managed using minimally invasive image-guided techniques, often on a day care basis using only local anesthesia or conscious sedation. This article presents an overview of the technique and status of various image-guided urological procedures, including recent emerging techniques.
Keywords: Angiography, fluoroscopy, imaging, ultrasonography, urology
INTRODUCTION
Percutaneous image-guided interventions have changed the management of various urologic diseases. Their applications are growing because of the minimally invasive nature, lower procedure-related morbidity and reduced hospital stay. These have evolved from a few traditional diagnostic procedures to current state-of-art therapeutic techniques and include a broad range of non-vascular and vascular applications. Today, they continue to play an important role in drainage procedures, the management of urolithiasis, tumor ablation and renovascular diseases. Multiple imaging modalities are used for this purpose, with fluoroscopy, ultrasound and digital subtraction angiography (DSA) being the mainstay. A close multidisciplinary collaboration between urologists, nephrologists and interventional radiologists is important. This article reviews an outline of various aspects of interventional uroradiology.
General principles
Uroradiological interventions may be broadly classified as non-vascular and vascular. Non-vascular procedures mainly include tissue sampling (fine needle aspiration or biopsy), percutaneous nephrostomy (PCN) and stone extraction. Vascular procedures mainly include embolization of pseudoaneurysms, arterio-venous malformations and fistulas most commonly presenting with hematuria. Others include prostate artery embolization (PAE), recanalization of renal artery stenosis (RAS), gonadal vein embolization (varicocele, pelvic congestion syndrome), etc. A few common principles apply to all interventional uroradiology procedures.
Image guidance
Anatomical location, use of iodinated contrast and real-time guidance are the most important factors in choosing a preferred imaging modality. Fluoroscopy helps to access the urinary collecting system with the use of iodinated contrast material. Urinary drainage procedures and uereteric stenting are routinely performed under fluoroscopic guidance. Digital subtraction angiography (DSA) also involves the administration of intra-arterial iodinated contrast medium and use of radiation for obtaining images for the treatment of vascular disorders. Intravascular carbon dioxide and gadolinium-based magnetic resonance imaging (MRI) contrast media are other less commonly used alternatives to conventional iodinated contrast media. Both fluoroscopy and DSA provide real-time guidance with full view of catheters, wires and stents. Evaluation of vascular anatomy is now commonly performed by non-invasive techniques like computed tomography (CT) or magnetic resonance (MR) angiography. Ultrasonography (USG) provides excellent radiation-free and real-time guidance and is therefore routinely used in urinary drainage procedures, cryotherapy or other ablative procedures and robotic surgery. CT guidance has a distinct advantage of providing 3D anatomical details without hindrance from gas or bony structures. It is commonly used for the drainage of perirenal collections, adrenal biopsies due to their strategic location and in radiofrequency ablation (RFA) of renal tumors. Cone beam CT uses the newest technology to acquire volumetric imaging data with one rotation of the gantry. It is a compact, low-cost and low-radiation equipment that can be mounted on C-arm in interventional urology suites. The use of MRI in guiding interventional urologic procedures is evolving. The excellent image contrast leading to improved lesion detection coupled with lack of ionizing radiation make MRI an attractive modality for targeting pathologies for biopsy and treatment. However, the dependence on expensive MRI-compatible equipment and long procedure time are its main limitations. Currently, the chief role of interventional MRI is for performing accurate biopsy and minimally invasive therapy of early prostate cancer and cryoablation of renal tumors.
Routes of access
While the urethra is the main portal of entry for endourological procedures, the vascular route is employed by interventional radiologists. The percutaneous route is common to both. For percutaneous procedures, the target is reached by a needle following a straight and the shortest route, avoiding large bowel and vascular structures. The pancreas and spleen are other organs that must not be transgressed. For vascular procedures, the transfemoral approach is most preferred.
Tools of trade
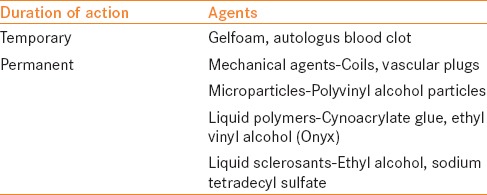
These include puncture needles, guide wires, dilators, various drainage catheters, stents, balloon catheters and embolizing materials etc., Balloon expandable stents are used for RAS. Embolic materials are used to treat leaking pseudoaneurysms, arterio-venous shunts and devascularization of tumors. Intravascular embolic agents are generally classified based on their duration of occlusion or physical state [Table 1].[1] The choice of an agent depends on target vessel size, duration of occlusion required, tissue viability and clinical setting. Coils are used to occlude a bleeding artery identified on DSA. Metallic embolization coils act by occluding a feeding artery similar to a surgical ligature. Commercially available vascular plugs are more suitable for occlusion of a larger vessel, like the main renal artery.
Table 1.
Embolic agents used in interventional urology[1]
Patient preparation and anesthetic requirements
Most image-guided interventions are performed under local anesthesia with conscious sedation and analgesia. As a general rule, the INR should be ≤1.5 and platelets greater than 50,000/cu mm for most procedures.
Non-vascular procedures
Tissue sampling
Image-guided urologic biopsies include non-focal and focal renal biopsies, renal cyst aspiration, adrenal biopsy and transrectal prostate biopsies.
Non-focal renal biopsy: Indicated for histological characterization of renal parenchymal diseases, renal grafts suspicious for rejection or unexplained renal failure. After placing the patient in the appropriate position (supine for transplant and prone or prone oblique with target side dependent for native kidneys), the biopsy needle (15–18 G) is advanced obliquely into the renal cortex near the lower pole [Figure 1]. The cortical sample ensures that the specimen contains a few glomeruli necessary to make a diagnosis. Puncture of the renal medulla or renal sinus must be avoided.
Figure 1.
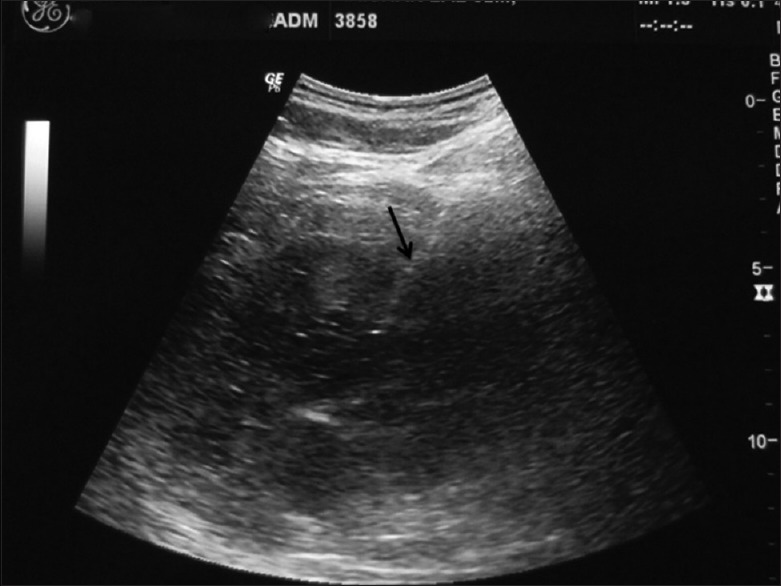
Ultrasound-guided biopsy from the renal parenchyma. The renal cortex is being targeted with a coaxial needle (arrow) from the posterior paravertebral approach with the patient in prone oblique position
Biopsy of renal masses: The purpose is to save these patients from unnecessary nephrectomies. Large enhancing renal masses are considered to be renal cell carcinoma (RCC) and are therefore operated without a biopsy. However, smaller, indeterminate masses are now often biopsied as many of them are thought to be benign. Such indications are rising due to the increased detection of small renal masses due to widespread use of cross-sectional imaging. Renal mass biopsy is also considered prior to a percutaneous thermal (radiofrequency/cryo) ablation, when it is suspected to be infective, in borderline surgical candidates or sometimes to differentiate a concurrent renal cancer from a metastasis in known primary extra-renal malignancy. Renal mass biopsy may be performed under CT or USG guidance. If the mass is not visible on a non-contrast CT scan, it may be demonstrated following administration of intravenous iodinated contrast [Figure 2]. The mass is first approached using a 17 G cannula into which an 18 G coaxial biopsy needle or gun is introduced to obtain several cores. The use of a co-axial system allows multiple samples to be taken by only one puncture of the renal capsule.
Figure 2.
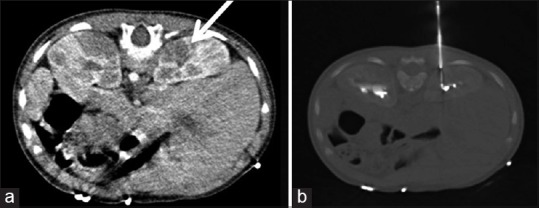
Right renal mass seen as a hypodense lesion that was visualized better with the use of intravenous contrast (a) (arrow). It was targeted using CT-guided biopsy (b). It turned out to be a non-Hodgkins lymphoma
Renal cyst aspiration: Aspiration is performed to differentiate benign cysts from malignant cystic RCC. The technique is identical to focal renal biopsies. Aspiration can be coupled with biopsy if the cyst wall has solid nodule(s). Various techniques like injection of water-soluble contrast or air may be used to make such nodules apparent on non-contract CT.
Core biopsies typically have a better diagnostic yield than fine-needle biopsies. The fine-needle aspiration (FNA) material is spread on slides and fixed in liquid preservatives, while the cores are sent to the pathologist in saline or formalin vials. In suspected lymphoma, the cores are additionally sent for flow cytometry.
Renal biopsy/aspiration with either CT or USG guidance is safe and has a high success rate, with a sensitivity of 70–100% and specificity of 100%.[2] Percutaneous core biopsy continues to provide an accurate and safe tool for pre-operative tissue diagnosis of indeterminate, incidentally detected, asymptomatic, small (≤4 cm) renal masses (SRM) and should be offered to patients before considering surgical intervention.[3] It is a safe and accurate technique for distinguishing malignant from benign tumors.[4] The biopsy of SRMs is associated with a relatively high rate of technical failures.[4] Renal biopsy may sometimes lead to complications like hemorrhage, pneumothorax, infection and adjacent visceral injury. Bleeding occurs due to injury to renal vessels or from vascular tumors. Sometimes, arterio-venous fistulas or pseudoaneurysms may develop and present with persistent bleeding. Bleeding is common, but it is usually mild and self-limiting.[5,6] Patients should be instructed to report in case of frank hematuria or symptoms suggestive of hypotension. The risk of needle tract seeding is low (0.01%).[7]
Adrenal biopsy: Biopsy of an adrenal mass is indicated when it cannot be characterized by standard imaging or laboratory tests. It can be easily performed using CT or sonographic guidance.[8,9,10] For the histologic diagnosis of right adrenal masses that are either invisible or inaccessible via the standard extrahepatic route, the transhepatic core route appears to be feasible and safe.[11] Martinez et al. reported that endoscopic ultrasound (EUS)-FNA is a safe, minimally invasive and sensitive technique with significant impact in the management of adrenal gland mass or enlargement.[12] CT-guided adrenal biopsy [Figure 3] has a high success rate (80–95%).[13,14] The overall sensitivity for diagnosis of malignancy is 94.6% and specificity is 95.3%.[15] Apart from the hemorrhage and infection, pneumothorax may be a complication due to its proximity to pleural recess. When it is impossible to avoid lung/pleura, a transrenal or transhepatic route may be taken to reach the target. Another rare but unique complication might be a hypertensive crisis.[16]
Figure 3.
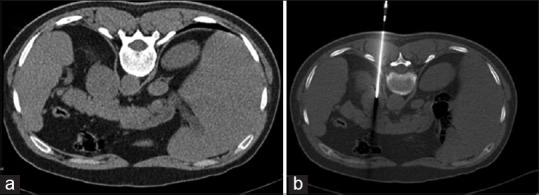
CT-guided biopsy from the left adrenal mass (a). Note the accurate placement of the biopsy needle (b). It was proven to be a metastatic adenocarcinoma
Prostate biopsy: Suspicion of prostate cancer based on abnormal digital rectal examination and/or elevated serum prostate-specific antigen (PSA) levels is currently the leading indication for prostate biopsy. It is most commonly performed using transrectal ultrasound (TRUS) guidance with the patient in the left lateral decubitus position. Hematuria and hematospermia are seen in up to 80% of patients in the first 2 weeks, and are self-limiting.[17] Conventional TRUS-guided biopsy misses up to 20% cancers and frequently underestimates the grade of malignancy, but, despite its limitations, it is still used due to its universal availability, ease of use and real-time capability.
Drainage procedures
Drainage procedures are either used to divert an obstruction or to drain collections.
Percutaneous nephrostomy: A posterolateral sub-costal approach targeting the lower (or middle) calyx prevents entering through the pleural recess and permits access through the Brodel's avascular plane of the kidney [Figures 4 and 5]. A soft tip wire is then inserted into the collecting system and is replaced with a stiff wire using a 6 F fascial dilator. The tract is serially dilated up to 8 or 10 F and a pigtail catheter or Malecot nephrostomy tube is then placed into the renal pelvis [Figure 6]. The latter is a better choice if the collecting system is either small or filled with calculi. PCN has a high success rate, approaching 100%, in dilated systems and around 80% in undilated systems.[18] Complications include bleeding, infection and injury to adjacent organs.
Figure 4.
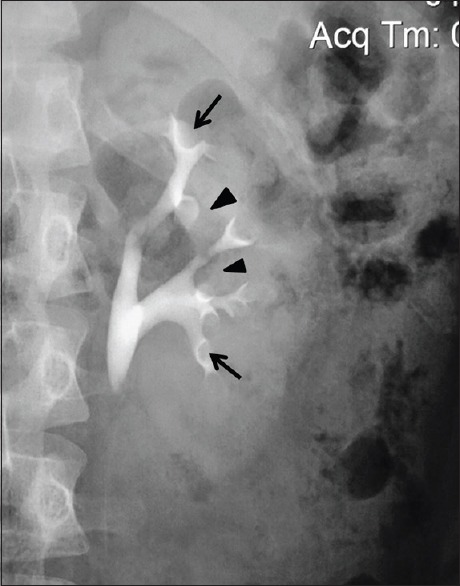
An intravenous urographic image of the left kidney depicting the ideal site of entering the pelvicalyceal system via minor calyces (arrows). This avoids the plane of interlobar arteries (arrow heads)
Figure 5.
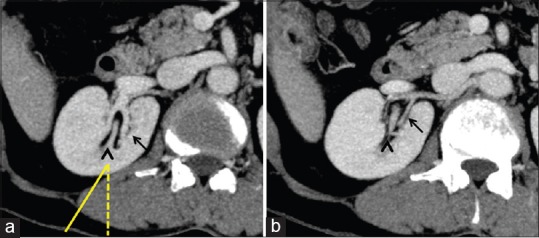
Axial contrast enhanced CT images showing the relationship of posterior calyx (arrowheads) with renal veins (a) and arteries (b). Note the relative avascular zone of Brodel (solid line), 20–30 degree lateral to the imaginary sagittal plane (interrupted line) (a)
Figure 6.
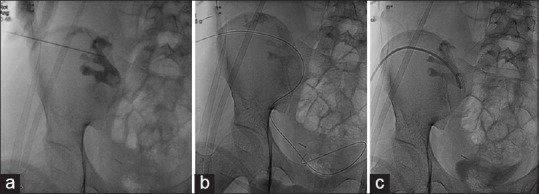
Percutaneous nephrostomy in an obstructed transplant kidney: Contrast medium administered into the pelvicalyceal system following ultrasonography-guided needle puncture (a). A guide wire left in the bladder (b) and pigtail catheter introduced after serial dilatations over the wire (c)
Suprapubic catheter: It may be inserted under ultrasound guidance or even fluoroscopy in a contrast-opacified urinary bladder. A 12 F Foley catheter loaded over a trocar is used to enter the bladder. Care must be taken to avoid injury to the sigmoid colon. It is a simple procedure with almost 100% success rate.[19]
Stone management
Ureteric stent: An antegrade, percutaneous route may be required to place a ureteric stent [Figure 7]. The choice of approach (retrograde or antegrade) is based on the accessibility and the location of ureteric pathology.[20] The procedure has near 100% success rate with patency rates of 95% at 3 months.[20]
Figure 7.

Placement of a DJ ureteric stent in a patient with urinary diversion by ileal conduit. A guide wire placed into the opacified urinary tract and ileal loop (a) followed by the deployment of a DJ stent (b)
Vascular interventions
Endovascular embolization
It is a minimally invasive procedure where the lumen of the intrarenal vessel is occluded by embolizing material. Indications include persistent hematuria as a result of pseudo-aneurysm [Figure 8] or arteriovenous fistula [Figure 9] following a biopsy, surgical or accidental trauma. Superselective embolization of the feeding artery saves more functioning nephrons than conventional surgery. It is also employed to reduce vascularity of renal tumors, e.g. angiomyolipoma [Figure 10] or renal carcinoma prior to surgery. Gel foam and vascular coils are the usual materials used for this purpose. This procedure has a success rate of around 90%. Complications include bleeding, infection, renal arterial injury, infarction and allergic reaction.[21]
Figure 8.
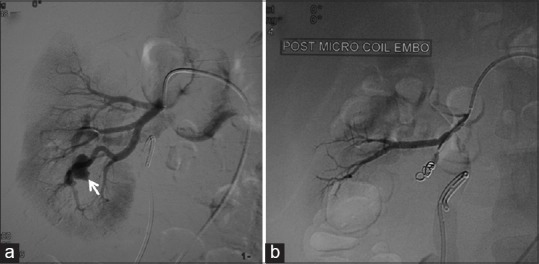
Post-biopsy renal pseudoaneurysm coil embolization. A small contrast filled outpouching seen from the lower polar branch of the right renal artery (arrow) (a). Following selective coil embolization (b), the branch supplying the pseudoaneurysm shows absent flow
Figure 9.
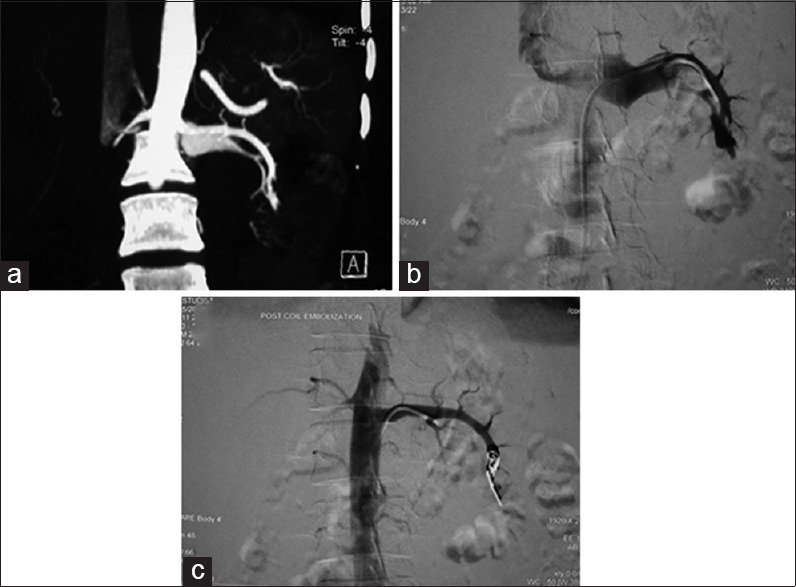
Post-biopsy intrarenal AV fistula embolization. Computed tomography (CT) angiography coronal maximum intensity projection (MIP) images showing early filling of a vein draining the lower pole of the left kidney (a). Digital subtraction angiography (b) confirms the CT finding. Post-coil embolization (c) showing non-filling of the draining vein
Figure 10.
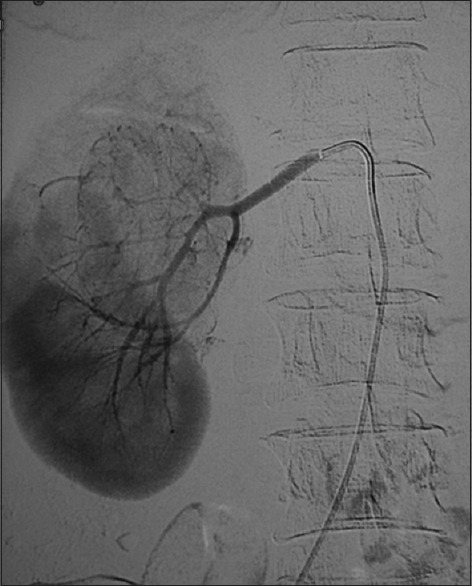
Upper polar angiomyolipoma from the right kidney. Digital subtraction angiography showing marked increase in vascularity. The vascularity of such tumors can be reduced pre-operatively by injecting embolic agents into the feeding arteries
Transjugular renal biopsy
The procedure is carried out in patients where percutaneous biopsy is contraindicated, usually due to a deranged coagulation profile. A special biopsy needle is wedged into the peripheral renal vein branch via the transjugular route. It provides diagnostic yield and safety similar to percutaneous renal biopsy. It has an added advantage that it allows multiorgan biopsy during the same procedure, e.g. simultaneous liver and renal biopsy.[22]
Gonadal vein embolization
It is performed in males for scrotal varicocele causing pain and infertility and in women for pelvic congestion syndrome as a result of retrograde flow in incompetent ovarian veins, causing chronic pelvic pain. The veins are approached via a jugular or femoral route. After diagnostic angiography, the veins are embolized using steel coils.[23] The clinical outcomes of technically successful percutaneous internal spermatic vein embolization are similar to surgical treatment.[24,25]
Recanalization of RAS
RAS is found in 2% and 40% of general and high cardiovascular risk populations, respectively.[26] Recanalization involves balloon angioplasty and/or stenting of hemodynamically significant RAS defined by a transstenotic pressure gradient of ≥ 20 mmHg. The renal artery is approached via the transfemoral route. Preliminary diagnostic angiography defines the site of stenosis. The stenosis is crossed using a guide wire and balloon angioplasty ± stenting is performed [Figure 11]. The technical success of renal angioplasty depends on the site of stenosis and the underlying etiology. It ranges from 40% in ostial arthersclerosis to 90% in fibromuscular dysplasia. Stenting, coupled with angioplasty, has a higher success rate.[27] The procedure is associated with a significant risk of complications. Lately, there has been a marked decline in the number of these recanalization procedures conducted worldwide. This is a result of few recently published trials that have not shown any benefit of renal revascularization over medical therapy for blood pressure control, preserving renal function or reducing cardiovascular events. However, it is agreed that selected high-risk patients, viz severe or refractory hypertension, heart failure or rapid loss of renal function may benefit from the revascularization procedure. Medical therapy remains the optimal choice for the majority of patients.[28,29]
Figure 11.
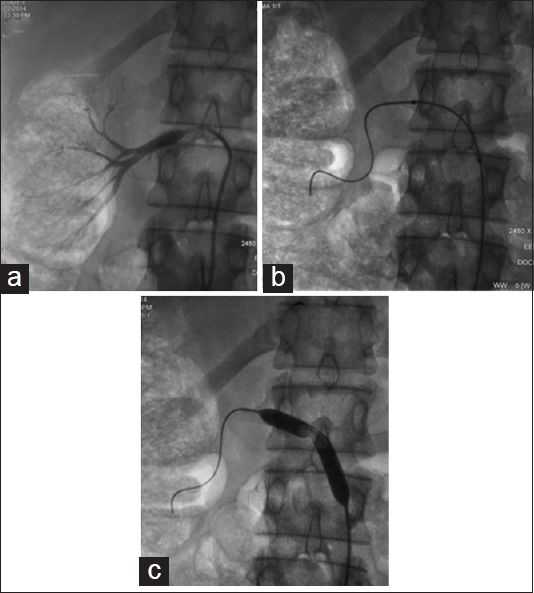
Angioplasty for right renal artery stenosis. Diagnostic angiography (a) shows ostial right renal artery stenosis. An angiographic balloon was placed across the stenosis (b) and uneventful angioplasty was performed (c)
Recent advances
Thermal ablation for renal cancer
Of all such techniques, RFA and cryo-ablation have been best studied. These provide local treatment for renal carcinomas in patients unsuitable for surgery [Figure 12]. Patients having renal insufficiency at presentation and tumors requiring a more nephron-conserving approach (solitary kidney, multiple synchronous RCC, von Hippel Lindau/familial RCC) are also candidates for these minimally ablative therapies. In the short term, 90–95% local control rates and 6% significant complication rates are seen for tumors <3.0 cm for both techniques.[30,31] The major limitations for both these techniques are lack of prospective randomized clinical trials or long-term follow-up data. A meta-analysis of 46 series (28 percutaneous, 18 surgical) by Hui et al.[32] compared results of surgical and percutaneous treatment of renal tumors. The analysis showed a significantly lower primary effectiveness rate for the percutaneous group (87% vs. 94%; P < 0.05) compared with the surgical group. There was no significant difference in the retreatment rates (92% vs. 95%; P > 0.05). The major complication rate was significantly lower in the percutaneous treatment group (3% vs. 7%; P < 0.05) than the surgical treatment group. Microwave ablation has potential advantages of being able to treat cystic renal tumors, but has even lesser data available.[33]
Figure 12.
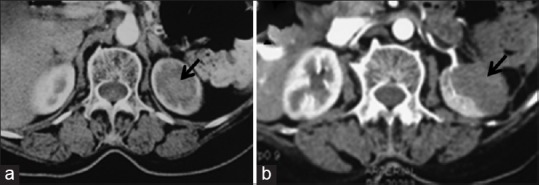
Pre-radiofrequency ablation contrast enhanced CT image (a) showing a mass at the upper pole of the left kidney (arrow). The mass shows complete ablation (arrow) in post-ablation CECT axial image (b)
MR-guided ablation of prostate cancer
Focal ablative therapy of prostate cancer is an emerging therapy that is being investigated for the treatment of localized tumor. It is a minimally invasive therapeutic option with favorable outcome while preserving continence and sexual function. The technique is described in detail elsewhere in this issue.
Prostate artery embolization
This is a new, minimally invasive endovascular technique to treat lower urinary tract symptoms due to benign prostatic hypertrophy. It is undertaken sporadically in few centers in patients, especially with prostates over 80 g, who are refractory to medication or at high risk for surgery. It is performed under local anesthesia as an outdoor procedure with low morbidity and rapid recovery. The prostate arteries arising from the anterior division of the internal iliac artery are super selectively catheterized followed by slow injection of diluted embolic agents. Both polyvinyl alcohol (PVA) (size 100–200 μm) and gelatin microspheres have been used. The end point is identified by near stasis of flow in the injected arteries and gland opacification. Preliminary data have shown that it relieves symptoms, improves quality of life and preserves sexual function in short- and medium-term follow-up and may therefore be seen as an effective alternative to surgery.[34,35] However, the present limitation is paucity of long-term data. The published studies lack standard inclusion criteria and have heterogeneous post-procedure follow-up.
Renal sympathetic denervation
The procedure is performed like renal angiography using the transfemoral route under local anesthesia. A 6 F sheath is placed in the femoral artery through which an electrode-tipped catheter is advanced into the renal artery under real-time fluoroscopic guidance. Once the desired position is reached, the catheter is connected to a RF generator and low-level radiofrequency energy is delivered through the renal artery wall to disrupt the surrounding renal nerves. Symplicity HTN-2 trial involving patients with uncontrolled hypertension showed that this procedure leads to notable and sustained reductions in blood pressure.[36]
Contrast-enhanced ultrasound (CEUS)-aided renal biopsy
CEUS uses stabilized microspheres filled with gas as a new type of ultrasound contrast (sulfur hexafluoride) medium. It enhances tumors and other vascular structures without the use of potentially nephrotoxic iodinated contrast media. Ultrasound contrast is excreted via the lungs and has no serious side-effects. Currently, there is paucity of literature using CEUS-assisted renal biopsy. We sometimes use this technique in guiding renal biopsy by highlighting the solid portion of the necrotic renal tumor or accurately localizing small renal masses by their enhancement compared with the adjoining normal parenchyma.
CONCLUSION
Multimodal team-based image-guided interventional procedures now form an integral part of contemporary urologic practice. These are minimally invasive treatment strategies often with very little procedure-related morbidity. The domain is all set to expand further with the upcoming applications and novel imaging techniques.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Medsinge A, Zajko A, Orons P, Amesur N, Santos E. A case based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol. 2014;203:699–708. doi: 10.2214/AJR.14.12480. [DOI] [PubMed] [Google Scholar]
- 2.Herts BR, Baker ME. The current role of percutaneous biopsy in the evaluation of renal masses. Semin Urol Oncol. 1995;13:254–61. [PubMed] [Google Scholar]
- 3.Menogue SR, O’Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: A diagnostic tool to better stratify patients for surgical intervention. BJU Int. 2013;111:E146–51. doi: 10.1111/j.1464-410X.2012.11384.x. [DOI] [PubMed] [Google Scholar]
- 4.Shannon BA, Cohen RJ, de Bruto H, Davies RJ. The value of preoperative needle core biopsy for diagnosing benign lesions among small, incidentally detected renal masses. J Urol. 2008;180:1257–61. doi: 10.1016/j.juro.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Lechevallier E, André M, Barriol D, Daniel L, Eghazarian C, De Fromont M, et al. Fineneedle percutaneous biopsy of renal masses with helical CT guidance. Radiology. 2000;216:506–10. doi: 10.1148/radiology.216.2.r00au01506. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RP, Bush WH, Jr, Burnett LL. Needle tract seeding following aspiration of renal cell carcinoma. J Urol. 1977;118:865–7. doi: 10.1016/s0022-5347(17)58226-9. [DOI] [PubMed] [Google Scholar]
- 7.Kiser GC, Totonchy M, Barry JM. Needle tract seeding after percutaneous renal adenocarcinoma aspiration. J Urol. 1986;136:1292–3. doi: 10.1016/s0022-5347(17)45318-3. [DOI] [PubMed] [Google Scholar]
- 8.Mignon F, Mesurolle B. CT guided adrenal biopsies: Remaining indications? J Radiol. 2002;83:419–28. [PubMed] [Google Scholar]
- 9.Harisinghani MG, Maher MM, Hahn PF, Gervais DA, Jhaveri K, Varghese J, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol. 2002;57:898–901. doi: 10.1053/crad.2002.1054. [DOI] [PubMed] [Google Scholar]
- 10.König CW, Pereira PL, Trübenbach J, Fritz J, Duda SH, Schick F, et al. MR imaging-guided adrenal biopsy using an open low-field-strength scanner and MR fluoroscopy. AJR Am J Roentgenol. 2003;180:1567–70. doi: 10.2214/ajr.180.6.1801567. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Park BK, Kim CK. Sonographically guided transhepatic core biopsies of right renal and adrenal masses: Safety and short-term follow-up. J Ultrasound Med. 2013;32:2013–21. doi: 10.7863/ultra.32.11.2013. [DOI] [PubMed] [Google Scholar]
- 12.Martinez M, LeBlanc J, Al-Haddad M, Sherman S, DeWitt J. Role of endoscopic ultrasound fine-needle aspiration evaluating adrenal gland enlargement or mass. World J Nephrol. 2014;3:92–100. doi: 10.5527/wjn.v3.i3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeger W, Fassnacht M, Chita R, Prager G, Nies C, Lorenz K, et al. High diagnostic accuracy of adrenal core biopsy: Results of the German and Austrian adrenal network multicenter trial in 220 consecutive patients. Hum Pathol. 2003;34:180–6. doi: 10.1053/hupa.2003.24. [DOI] [PubMed] [Google Scholar]
- 14.Silverman SG, Mueller PR, Pinkney LP, Koenker RM, Seltzer SE. Predictive value of image-guided adrenal biopsy: Analysis of results of 101 biopsies. Radiology. 1993;187:7158. doi: 10.1148/radiology.187.3.8497619. [DOI] [PubMed] [Google Scholar]
- 15.Moore TP, Moulton JS. Coaxial percutaneous biopsy technique with automated biopsy devices: Value in improving accuracy and negative predictive value. Radiology. 1993;186:515–22. doi: 10.1148/radiology.186.2.8421758. [DOI] [PubMed] [Google Scholar]
- 16.Atwell TD, Wass CT, Charboneau JW, Callstrom MR, Farrell MA, Sengupta S. Malignant hypertension during cyroablation of an adrenal gland tumor. J Vasc Interv Radiol. 2006;17:573–5. doi: 10.1097/01.RVI.0000197370.83569.33. [DOI] [PubMed] [Google Scholar]
- 17.Ghani KR, Dundas D, Patel U. Bleeding after transrectal ultrasonography-guided prostate biopsy: A study of 7-day morbidity after a six-, eight- and 12-core biopsy protocol. BJU Int. 2004;94:1014–20. doi: 10.1111/j.1464-410X.2004.05096.x. [DOI] [PubMed] [Google Scholar]
- 18.Maher MM, Fotheringham T, Lee MJ. Percutaneous nephrostomy. Semin Interv Radiol. 2000;17:329–39. [Google Scholar]
- 19.Lee MJ, Papanicolaou N, Nocks BN, Valdez JA, Yoder IC. Fluoroscopically guided percutaneous suprapubic cystostomy for long-term bladder drainage: An alternative to surgical cystostomy. Radiology. 1993;188:787–9. doi: 10.1148/radiology.188.3.8351348. [DOI] [PubMed] [Google Scholar]
- 20.Lu DS, Papanicolaou N, Girard M, Lee MJ, Yoder IC. Percutaneous internal ureteral stent placement: Review of technical issues and solutions in 50 consecutive cases. Clin Radiol. 1994;49:256–61. doi: 10.1016/s0009-9260(05)81852-5. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson AI, Amukele SA, Marcovich R, Shapiro O, Shetty R, Aldana JP, et al. Efficacy and morbidity of therapeutic renal embolization in the spectrum of urologic disease. J Endourol. 2003;17:385–91. doi: 10.1089/089277903767923164. [DOI] [PubMed] [Google Scholar]
- 22.Cluzel P, Martinez F, Bellin MF, Michalik Y, Beaufils H, Jouanneau C, et al. Transjugular versus percutaneous renal biopsy for the diagnosis of parenchymal disease: Comparison of sampling effectiveness and complications. Radiology. 2000;215:689–93. doi: 10.1148/radiology.215.3.r00ma07689. [DOI] [PubMed] [Google Scholar]
- 23.Bittles MA, Hoffer EK. Gonadal vein embolization: Treatment of varicocele and pelvic congestion syndrome. Semin Intervent Radiol. 2008;25:261–70. doi: 10.1055/s-0028-1085927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsch EM, Schill WB, Erlinger C, Tauber R, Pfeifer KJ. Semen parameters and conception rates after surgical treatment and sclerotherapy of varicocele. Andrologia. 1990;22:275–8. doi: 10.1111/j.1439-0272.1990.tb01979.x. [DOI] [PubMed] [Google Scholar]
- 25.Gat Y, Bachar G, Everaert K, Levinger U, Gornish M. Induction of spermatogenesis in azoospermic men after internal spermatic vein embolization for the treatment of varicocele. Hum Reprod. 2005;20:1013–7. doi: 10.1093/humrep/deh706. [DOI] [PubMed] [Google Scholar]
- 26.Piecha G, Wiecek A, Januszewicz A. Epidemiology and optimal management in patients with renal artery stenosis. J Nephrol. 2012;25:872–8. doi: 10.5301/jn.5000206. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ. Percutaneous genitourinary interventions. In: Kaufman JA, Lee MJ, editors. The requisites: Vascular and interventional radiology. 1st ed. St. Louis (MO): Mosby; 2004. [Google Scholar]
- 28.ASTRAL Investigators; Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–62. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 29.Liang P, Hurks R, Bensley RP, Hamdan A, Wyers M, Chaikof E, et al. The rise and fall of renal artery angioplasty and stenting in the United States, 1988-2009. J Vasc Surg. 2013;58:1331–8.e1. doi: 10.1016/j.jvs.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: Part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 31.Littrup PJ, Ahmed A, Aoun HD, Noujaim DL, Harb T, Nakat S, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–92. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Hui GC, Tuncali K, Tatli S, Morrison PR, Silverman SG. Comparison of percutaneous and surgical approaches to renal tumor ablation: Metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol. 2008;19:1311–20. doi: 10.1016/j.jvir.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Clark PE, Woodruff RD, Zagoria RJ, Hall MC. Microwave ablation of renal parenchymal tumors before nephrectomy: Phase I study. AJR Am J Roentgenol. 2007;188:1212–4. doi: 10.2214/AJR.05.2190. [DOI] [PubMed] [Google Scholar]
- 34.isco JM, Tinto HR, Pinheiro LC, Bilhim T, Duarte M, Fernandes L, et al. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: Results of short- and mid-term follow-up. Eur Radiol. 2013;23:2561–72. doi: 10.1007/s00330-012-2714-9. [DOI] [PubMed] [Google Scholar]
- 35.Carnevale FC, da Motta-Leal-Filho JM, Antunes AA, Baroni RH, Marcelino AS, Cerri LM, et al. Quality of life and clinical symptom improvement support prostatic artery embolization for patients with acute urinary retention caused by benign prostatic hyperplasia. J Vasc Interv Radiol. 2013;24:535–42. doi: 10.1016/j.jvir.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Cao L, Fu Q, Wang B, Li Z. Renal denervation: A new therapeutic approach for resistant hypertension. Chin Med J (Engl) 2014;127:3302–8. [PubMed] [Google Scholar]


