Abstract
Recent advances in multiparametric magnetic resonance imaging (mp-MRI) have led to a paradigm shift in the diagnosis and management of prostate cancer (PCa). Its sensitivity in detecting clinically significant cancer and the ability to localize the tumor within the prostate gland has opened up discussion on targeted diagnosis and therapy in PCa. Use of mp-MRI in conjunction with prostate-specific antigen followed by targeted biopsy allows for a better diagnostic pathway than transrectal ultrasound (TRUS) biopsy and improves the diagnosis of PCa. Improved detection of PCa by mp-MRI has also opened up opportunities for focal therapy within the organ while reducing the incidence of side-effects associated with the radical treatment methods for PCa. This review discusses the evidence and techniques for in-bore MRI-guided prostate biopsy and provides an update on the status of MRI-guided targeted focal therapy in PCa.
Keywords: Focal therapy in prostate cancer, multiparametric magnetic resonance imaging, MRI-guided prostate biopsy, prostate cancer
INTRODUCTION
Widespread use of prostate-specific antigen (PSA) screening and increased number of transrectal prostate biopsy encounters have led to an increased diagnosis of prostate cancer (PCa) along with stage migration toward early-stage, organ-confined disease.[1] It is estimated that 233,000 new PCas will be diagnosed in the US in 2014.[2] Recently, the US Preventive Services Task Force attributed grade D to PSA screening for detecting PCa, implying that PSA screening may cause harm to the patients.[3] But, the “random” nature of the verification test following the PSA test, the TRUS biopsy, may be partly to blame for the poor screening yield of PSA. Only about one in four men subjected to TRUS biopsy reveal cancer, and many of them are indolent and low grade. On the other hand, many significant tumors in anterior gland (prostate evasive anterior tumor), midline and at the apex go undetected because of the random nature of TRUS biopsy.[4]
Multiparametric magnetic resonance imaging (mp-MRI) of the prostate combining T2-weighted imaging with diffusion-weighted imaging (DWI) and perfusion imaging has been extensively studied in recent years.[5,6,7,8] It has been shown to have a high sensitivity, particularly in detecting clinically significant cancers.[9,10] The localizing strength of mp-MRI of the prostate has opened up opportunities for targeted diagnosis and treatment strategies.[11,12,13,14,15,16] Transrectal ultrasound-guided biopsy has historically shown low concordance with radical prostatectomy histology. Several studies have shown that there is undergrading of the Gleason score on TRUS biopsy when compared with radical prostatectomy specimens.[17,18] The effectiveness of mp-MRI used along with PSA, followed by targeted biopsy of the MRI visible lesion, is now accepted as a better alternative to systematic TRUS biopsy.[19,20,21] Schoots et al. recently published a meta-analysis of 16 studies, where the authors concluded that MRI-guided targeted biopsy had a higher detection rate for significant cancer and lower rate of detection of insignificant cancer and therefore benefits the diagnosis of PCa.[22] Targeted biopsy of the MRI visible lesion can be performed by two different techniques, either in-bore MRI-guided biopsy of the visible target[23,24] or registration of MR images with TRUS images using fusion software for biopsy under TRUS guidance (out of bore).[25] Both approaches use the advantage of tumor delineation on mp-MRI for accurate localization and sampling.
Apart from the role in risk stratification of PCa,[26,27,28,29] the localizing ability of mp-MRI has also opened up opportunities for its focal treatment. At present, men diagnosed with PCa are offered one of two options – either active surveillance or a radical form of treatment (surgery/radiation therapy). Significant deterioration of urinary and sexual function remain prevalent side-effects of radical therapies.[30] Men with low or low-intermediate risk disease may be candidates for active surveillance. However, there is usually low adherence to active surveillance with the perceived possibility of disease progression and missing the opportunity for cure.[31] Focal therapy is an intuitive new organ-sparing technique that aims to selectively ablate locally confined, clinically significant index lesions while sparing the majority of the prostate gland and the surrounding delicate neurovascular bundles and urinary sphincter.[32,33] Although PCa is often multifocal, evidence suggests that the natural history of the disease is predominantly determined by the largest lesion, which is most often also the highest grade,[34] the so-called index lesion.[35] Evidence supporting the theory that the largest volume tumor is the index tumor includes the observations that recurrence after radiation therapy nearly always occurs at this site and extracapsular extension arises from the largest tumor in 90% of cases.[36] The index lesion if clinically significant will often be visualized on mp-MRI, can be confirmed on biopsy and targeted for ablation.
Various experts have proposed different ablative templates for focal therapy [Figure 1], but in our opinion “focal therapy” is somewhat of a misnomer for hemiablation and subtotal zonal ablation templates. The goal of focal therapy should be to perform targeted ablation of the MRI visible index tumor with the aim to eradicate the index lesion and minimizing side-effects of treatment, thereby providing the best balance between oncologic control and maintenance of quality of life (QoL).[15] Visualization of tumor allows in-bore MR-guided focal treatment to be more suitable for targeted ablation and accurate targeting without any misregistration concerns.[37] In-bore treatment also offers the advantage of MR thermometry, which provides real-time monitoring of the thermal map during the treatment to ensure selective and adequate tumor ablation and preservation of sensitive surrounding structures. Contrast-enhanced scan at the end of the procedure shows the non-perfused volume and helps confirm adequate treatment coverage before the patient is taken off the table.
Figure 1.
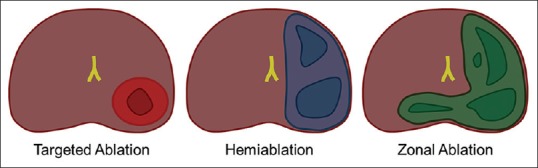
Focal therapy templates. Magnetic resonance-guided in-bore ablation is ideally suited for targeted ablation
This article reviews the current status of MRI-guided prostate biopsies as well as minimally invasive in-bore targeted treatment of organ-confined focal PCa. Ongoing clinical trials for in-bore focal PCa treatment via the transperineal, transrectal and transurethral routes using different energy modalities for the ablation are discussed. Focal therapy and prostate biopsy performed under TRUS guidance, with or without use of the newer MRI-TRUS fusion technologies, are beyond the scope of this review and are therefore not included.
MRI-guided prostate biopsy
MRI-guided prostate biopsy was initially reported in 2000 when investigators reported its success via a transperineal route using an open-configuration 0.5T strength MRI.[38,39] Although the open-bore MRI configuration provides easier patient access, it is limited by its low signal to noise ratio, which leads to non- or poor visualization of the target lesion at the time of biopsy on the low-strength (typically 0.5T) open-bore magnets. The pre-biopsy diagnostic images must therefore be registered to the real-time images at time of biopsy in low-strength MRI scanners. In 2005, Beyersdorff et al.[40] reported transrectal MR-guided biopsy in a cohort of 12 patients using a robotic device in a closed-bore 1.5T magnet. Since then, several studies have reported successful MR-guided prostate biopsy within closed-bore higher strength magnets (1.5T and 3T), most using the transrectal approach in prone position.[21,23,41,42] The reported cancer detection rates in these studies ranged from 8% to 70%.[23,24] In the largest published series on in-bore prostate biopsy, 265 patients with PSA over 4.0 ng/mL and at least one previous negative TRUS biopsy were sampled following mp-MRI in a 3T magnet. Cancer was detected in 33% of the sites called on MRI, and 87% of these were clinically significant.[43] DWI has been shown to be a biomarker for PCa aggressiveness.[10,44] Using DWI for MR-guided in-bore biopsy, Hambrock et al. reported correct representation of the Gleason grade with prostatectomy specimens in 34 patients, and therefore representing the pre-treatment risk stratification.[45] Utilization of T2-weighted turbo spin echo (TSE), T1– weighted spolied gradient echo, ultrafast gradient echo and T2-weighted true fast imaging with steady-state precession sequences (bSSFP) enabling good visualization of the needle during the procedure have been reported in most studies.
Several MR-compatible biopsy devices, registered to the MR images using a coordinate system, have been used along with complex software to guide the needle.[41] Although most of the reported transrectal prostate needle guidance devices have been in the prone position, Schwab et al. recently reported the results of MR-guided biopsy preformed in wide-bore 1.5T and 3T magnets in the more comfortable supine lithotomy position in 50 patients.[46] They used a modified version of a previously used biopsy device[47] with a custom bed allowing elevation of the pelvis and legs and leaving a gap for the biopsy device. More recently, Cepek et al.[48,49,50] described a MR-compatible mechatronic (robotic) system for in-bore needle guidance via a transperineal route, which could be used for prostate biopsies and focal ablation. They demonstrated a reliable method for accurate needle placement in a short needle delivery time. The transperineal approach for prostate intervention is associated with decreased risk of urosepsis, which may occur in about 4% of patients following transrectal prostate biopsies.[51]
The reported procedure time for transrectal MR-guided biopsy varies from 30 to 68 min,[23] which is substantially longer than those reported for TRUS biopsy. Repeatedly moving the table to bring the patient out of the scanner to provide access to the needle for advancing or adjusting the biopsy needle, followed by repeat imaging, is one of the main reasons that prolongs the procedure time of in-bore procedures, including biopsy. Some investigators have therefore looked at MR-compatible robotic or mechatronic devices using rectal,[52] gluteal[53] and perineal approaches.[48,49,50] All investigators concluded that robot-assisted prostate procedures were feasible and can be performed safely and accurately, although in at least one of these studies the procedure time did not decrease with use of the robotic device when compared with the manual technique.[52] Cepek et al.[48] reported needle delivery time to the target of 9 min using their transperineal mechatronic device, although this assessment was performed at the time of focal laser ablation (FLA) and not for biopsy and did not take into account the time needed to set up the device at the start of the procedure. This brings into question the cost-effectiveness of MR-guided in-bore prostate biopsy. de Rooij et al.[54] compared the QoL and health care costs for the “blind” TRUS-guided biopsy strategy to the imaging-based strategy where MRI and directed MR-guided biopsies were performed, modeled to include the cascading effects for a period of 10 years following initial referral for biopsy. Their results suggested comparable healthcare costs in the two strategies but an improved QoL in the imaging arm. The benefit in QoL is derived from decrease in overdiagnosis and overtreatment in the imaging arm. The benefits of mp-MRI in the diagnostic pathway of PCa include the potential of performing less number of biopsies, based on the negative predictive value of mp-MRI, and therefore decreasing the incidence of complications from biopsies (multiresistant sepsis), increased sampling efficiency, decreased histopathology costs, better characterization of Gleason grade and fewer missed clinically significant cancers.[55] MRI-TRUS fusion biopsy exploits the high accuracy of mp-MRI with the real-time capability and ease of TRUS biopsy. It is less expensive than MR-guided biopsy and can also be performed in a shorter time, although there have not been any published studies directly comparing the two techniques for targeted prostate biopsy. Recent results of MRI-TRUS fusion biopsy with different available software platforms have shown promising results[25,56,57,58,59,60] and therefore this technique is likely to provide an alternative targeted biopsy method to overcome the costs of in-bore prostate.
MR-guided focal laser ablation
Laser is one of the energy modalities currently being evaluated for MR-guided focal therapy of PCa. Laser-induced thermal therapy utilizes laser light to deposit high-energy photons to generate coagulation through rapid heating. The thermal effect depends on the amount of heat energy delivered and on the depth of light distribution, which is regulated by the wavelength of the laser fiber. Improvements in the design of diode laser sources currently used for interstitial therapy have made them smaller, portable and less expensive. The diameter of the optical fibers for light delivery vary from 300 to 600 um (typically 400 um for the 15W fiber and 600 um for the 30W fiber), with the length of the cylindrical diffuser tip varying from 10 to 40 mm,[61] but most commonly 10 mm and 15 mm for prostate application. Laser ablation is MRI compatible, predictable and precise with sharply defined margins, as has been shown in some pre-clinical and phase I clinical studies, and therefore is ideally suited for targeted ablation as opposed to hemi-ablation or zonal ablation.[62] The intensity of the energy can also be controlled by the operator in real time based on the thermal feedback at the time of treatment. This integrated system is less expensive when compared with other sources of energy delivery.
The procedure is performed under deep sedation in the MRI suite in supine position with the legs elevated on support to provide access to the perinuem. T2-weighted turbo spin echo (TSE), T1-weighted spoiled gradient echo, ultrafast gradient echo and T2-weighted true fast imaging with bSSFP sequences have been used for visualization of the needle during the procedure [Figure 2]. Our group has previously reported on concentration of the contrast agent to be used for filling up the catheter for better visualization on spoiled gradient echo sequences.[63] Real-time thermal maps are obtained using the proton resonance frequency (PRF) shift [Figure 2]. 3D thermal mapping can also be obtained, which helps to show the ablation zone in relation to important surrounding structures. Pre-clinical trials using 980 nm diode laser (Visualase, Houston, TX, USA) as a thermal energy source for MRgFLA have shown precise and accurate ablation zones.[64,65,66] Linder et al.[65] compared whole mount histology with MRI in four patients who underwent FLA followed by radical prostatectomy and found that FLA created confluent ablation and that post-ablation MRI was accurate in determining the ablated area. Two separate phase 1 trials using the transperineal approach on a cohort of 38[67] and nine patients,[68] respectively, were reported in 2013, demonstrating its feasibility with minimal or no side-effects of the treatment. Both studies showed near-identical results with no residual disease at the treated site in approximately 75% of the patients, and most of the patients with residual disease had low-volume low-grade disease not visible on mp-MRI at 4–6 months post-treatment. Ongoing phase II clinical trial results at our institute and the University of Chicago are expected in 2015. Our investigators are also presently using a MR-compatible mechatronic device for needle guidance and ablation.[47,48,49,69] In their initial evaluation of 37 needle insertions using the mechatronic device in the course of 10 FLA treatments, the median needle guidance error was 3.5 mm and the needle delivery time was 9 min.[48] Lee et al. used a transrectal approach for MRgFLA with the patient in the prone position and reported their experience in 23 patients.[70] Although these initial results are encouraging, larger series with longer follow-up will be needed for validation.
Figure 2.
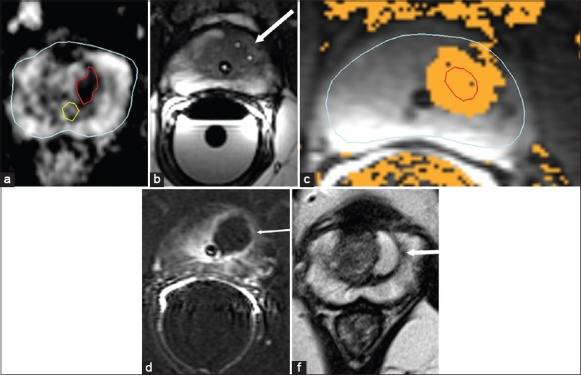
Magnetic resonance-guided focal laser ablation treatment. A 54-year-old male with biopsy-confirmed Gleason 7 (3+4) prostate carcinoma. (a) Pre-treatment axial apparent diffusion coefficient map (ADC) shows a well-demarcated magnetic resonance imaging (MRI) visible lesion (outlined in red) in the left transition zone at the level of mid gland. The prostatic urethra is outlined in yellow. (b) Intra-operative axial balanced steady-state precession sequence MRI scan confirming final position of two transperineally advanced cannulas with gadolinium markers (arrow) prior to initiating power. (c) Thermal map image during treatment showing areas of heat deposition color coded (orange) overlaid on tumor outline (in red). (d) Immediate post-treatment axial post-contrast Gd-DTPA (Magnevist®, Bayer Healthcare) enhanced subtraction image highlights the devascularized ablated volume (arrow), showing no damage to the rectal mucosa or neurovascular bundle or even the adjacent urethra, which is outlined by Foleys catheter. (e) Post-treatment axial T2-weighted image at 6 months shows devascularized cystic area at the site of treatment. All six samples obtained from the site at the 6-month follow-up biopsy were negative
MR-guided focused ultrasound therapy
Focused ultrasound is a completely non-invasive treatment method of tissue ablation and has been proved effective in a wide variety of benign and malignant tumors. The high-energy ultrasound waves are converted to thermal energy, resulting in raised temperature and tissue coagulation from protein denaturation.[71] As with other energy modes for focal treatment of PCa, integration of focused ultrasound with MRI (MRgFUS) provides for accurate targeting and closed-loop real-time monitoring of temperature by MRI thermography thus also allowing for a safer ablation procedure. Focused ultrasound has been shown to ablate prostate tissue with sharply demarcated transition between the coagulated zone and the surrounding gland. Two different systems, one for transrectal and the other for transurethral ablation, have been developed[72,73] and are being presently evaluated in phase 1 clinical trials.
Transrectal MRgFUS
The ExAblate 2100 Prostate (Insightec Inc., Haifa, Israel) is a transrectal MRgFUS system. The system consists of an endorectal transducer made of 990 elements and filled with degassed water at 140°C to eliminate air in the beam path and to cool the rectum during treatment. The ultrasound beam can be steered to the desired location in the prostate [Figure 3]. A proof-of-principle study published in 2013 demonstrated extensive coagulative necrosis without any residual tumor in the ablated area in five patients who underwent radical prostatectomy within 2 weeks of the MRgFUS treatment.[72] The average procedure time in the study was 84 min. Our group initially reported the feasibility of transrectal MRgFUS treatment for PCa with the ExAblate system,[74] and have recently reported the results with >18 months follow-up on the first four patients treated with MRgFUS system at our institution in an ongoing phase I trial.[75] Six sites of disease confirmed on biopsy were treated in the four patients. All six treatment sites were clear on MRI at 6 months, and five of the six target lesions (83%) were free of disease on follow-up biopsy at 6 months post-treatment, while low-risk MR-invisible disease was seen at one of the six treated sites at the 6-month biopsy. The median treated volume was 3.55 cc and the median procedure time was 215 min, although this was predominantly from system error during one of the treatments leading to increase in procedure time. There was no significant difference in the pre- and post-treatment QoL, IIEF-15 (International Index of Erectile Function) and IPSS (International Prostate Symptom Score) scores.
Figure 3.
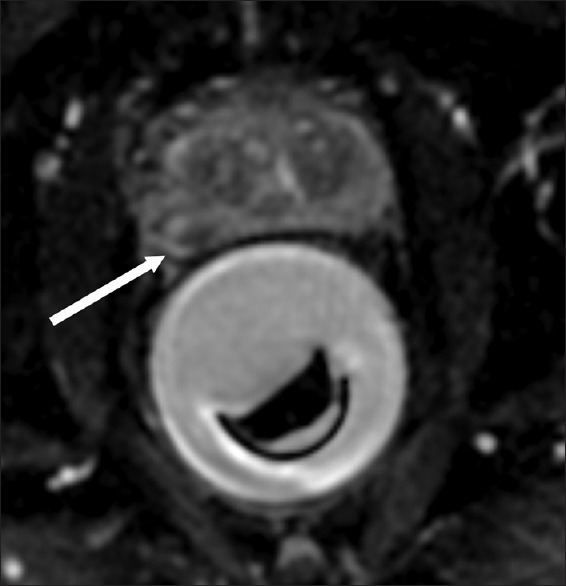
MR-guided focused ultrasound therapy treatment. A 64-year-old male with biopsy-confirmed Gleason 6 prostate carcinoma. Intra-operative axial ADC map prior to heating shows the endorectal focused ultrasound device steered to the direction of the tumor (arrow)
MR-guided transurethral focused ultrasound therapy
The transurethral device (PAD – 105, Profound Medical Inc., Toronto, Canada) includes a transurethral ultrasound device and a rotational positioning system, which allows the transducer to rotate along a defined angular sector of the gland. The device is inserted into the urethra over a guidewire and positioned in place in the prostatic urethra under MRI guidance. Pre-clinical studies have shown the device to be safe, capable of producing highly accurate volumes of ablation and treat entire prostate volumes in a short time.[76,77,78,79] A suprapubic catheter is placed prior to the procedure for continuous bladder drainage during the procedure. The procedure is performed under spinal or general anesthesia. The first proof-of-principle study demonstrating the safety and feasibility of transurethral prostate ablation in eight human subjects was reported by Chopra et al.[73] They treated a 1800 angular sector of the gland along the posterior half of the gland in this study. Ongoing phase 1 multi-center trial across three sites to evaluate MR-guided transurethral whole gland ablation has completed enrollment of 30 patients, and the 12-month results are due in 2015. Preliminary results show it to be a safe and feasible technique with low side-effect profile.[80]
MR-guided focal cryoablation
Transrectal ultrasound-guided percutaneous prostate cryoablation was first described by Onik et al. in 1993.[81] However, the ice ball is not visualized under ultrasound guidance because of the shadowing artifact from the posterior margin of the ice ball once the cooling process is initiated. This makes it somewhat of a “blind” procedure and increases the potential for complications such as urtheral or rectal fistula.
In-bore MRI-guided focal cryoablation allows real-time visualization of ice ball formation with the added benefit of the spatial resolution provided by MRI, which is paramount to identify the sensitive surrounding structures, such as rectal wall, ureters, urethra and external urinary sphincter. A hyperintense rim can be seen progressing at the margin of the ice ball, which is caused by shortening of T1 and seen in areas cooled to <20°C but as yet not frozen.[82] This allows monitoring of the ice ball growth in near real time with T1-weighted gradient echo MRI.[83] MR thermography information is less accurate from cooling tissue and hence not used for real-time monitoring of the thermal damage. Also, susceptibility artifacts are seen at the interface between the ice ball and normal tissue, which hinders thermal mapping. T2W-BLADE sequence, with its reduced sensitivity to movement, was used to monitor the ice ball in one study.[84] As in other in-bore prostate interventional procedures, T2-weighted true fast imaging with bSSFP can be used for confirming the final position of the cryoprobes.
Three studies have been recently published documenting feasibility of MR-guided cryoablation in a different cohort of patients.[83,84,85] Gangi et al.[84] assessed the feasibility of whole gland cryoablation in 11 patients, eight with newly diagnosed PCa and three for salvage therapy following radiation therapy. They placed four to seven cryoprobes transperineally under MRI guidance with an intent to ablate the entire gland. The procedure was performed in the supine position. Urethral and rectal warmers were used in most of the cases and two freeze–thaw cycles were performed in all treatments. The reported procedure time was from 2 to 4.5 h and the mean hospital stay was 5 days. One of the initial patients in the study in whom the rectal warmer was not used developed a recto–urethral fistula, which healed in 3 months. They concluded that MRI allows precise positioning of the cryoprobes and excellent 3D monitoring of the ice ball growth, thereby overcoming the two limitations of ultrasound guidance. In another study, Woodrum et al.[85] treated 18 patients with local recurrence after radical prostatectomy (six of these patients had salvage radiation therapy and subsequent recurrence) in a 1.5T wide-bore MR magnet. Two or three freeze–thaw cycles were performed in this cohort of patients. They reported better oncologic results in the group who were treated with three freeze–thaw cycles.
Bomers et al.[83] reported the feasibility of MR-guided cryoablation in 10 patients with locally recurrent PCa after radiation therapy. Unlike the other two studies cited above, they evaluated focal treatment, ablating only the lesion with margins in order to keep complications at a minimum. The patients were treated under general anesthesia. The median focal tumor size was 20 mm and the treatment time was 210.5 min, very similar to our own initial study[75] that treated four patients with transrectal MRgFUS. The investigators reported a steep learning curve and that the last two procedures in their study took substantially less time. All patients were discharged one to two days following the treatment. Three patients had recurrent/residual disease at the margin of the treated area in the first 12 months and were retreated. Urinary stricture was noted in one patient.
Apart from the treatment modalities described above, there are other techniques such as microwave ablation[86] and photodynamic therapy,[87] which have used MRI for planning and follow-up for PCa therapy, but the procedures have been performed under ultrasound guidance in most studies. More recently, early studies on focal irreversible electrophoration[88] for PCa under ultrasound guidance have been performed using MRI imaging for planning and follow-up. Given the excellent spatial resolution and the multiplanar capability of MRI, the feasibility of irreversible electroporation techniques for PCa therapy may also be assessed in-bore, in a similar manner to cryoablation, to increase the procedure safety.
The increased costs associated with performing focal therapy in the MRI magnet remain a disadvantage. Other limitations include need for MR-compatible materials, time availability on the MRI machine and the limited amount of working space for the physician when performing these procedures in the MR system. Use of newer wider bore MRI systems and dedicated MRI interventional suites in the future would make it easier for the physicians to work in the MRI environment. Also, use of MRI-compatible robotic/mechatronic systems, as highlighted in some of the recent studies, may also help to speed up the treatment and thereby decrease the overall costs of the treatment.
CONCLUSION
Advances in prostate MRI have opened up opportunities for targeted diagnosis and treatment of PCa. Real-time MRI thermometry feedback during treatment and the spatial resolution provided by MRI are added advantages of treating in the MRI suite, making it ideally suited for targeted therapeutics. At this time, MRgFLA and MRgFUS techniques appear to be the most promising of the available in-bore techniques for the primary treatment of focal PCa. While MRgFLA has the advantage of being precise and interstitial with lower cost of the integrated system, MRgFUS is a non-invasive technique. Initial phase 1 in-bore studies for focal therapy of PCa have shown promising results, although larger phase II multi-center trial results are awaited for assessing widespread clinical application.
Footnotes
Source of Support: Nil
Conflict of Interest: Both authors are Co-Principal Investigators for the MRgFLA and MRgFUS programs for focal treatment of PCa at their institute.
REFERENCES
- 1.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: Risk assessment and treatment. J Urol. 2007;178:S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, Fu R, et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–71. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Clinical applications of multiparametric MRI within the prostate cancer diagnostic pathway. Urol Oncol. 2013;31:281–4. doi: 10.1016/j.urolonc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection-histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langer DL, van der Kwast TH, Evans AJ, Plotkin A, Trachtenberg J, Wilson BC, et al. Prostate tissue composition and MR measurements: Investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology. 2010;255:485–94. doi: 10.1148/radiol.10091343. [DOI] [PubMed] [Google Scholar]
- 7.Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 8.Tan CH, Wei W, Johnson V, Kundra V. Diffusion-weighted MRI in the detection of prostate cancer: Meta-analysis. AJR Am J Roentgenol. 2012;199:822–9. doi: 10.2214/AJR.11.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: A meta-analysis. AJR Am J Roentgenol. 2014;202:343–51. doi: 10.2214/AJR.13.11046. [DOI] [PubMed] [Google Scholar]
- 10.Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–61. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 11.Cornud F, Khoury G, Bouazza N, Beuvon F, Peyromaure M, Flam T, et al. Tumor target volume for focal therapy of prostate cancer-does multiparametric magnetic resonance imaging allow for a reliable estimation? J Urol. 2014;191:1272–9. doi: 10.1016/j.juro.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Sankineni S, Wood BJ, Rais-Bahrami S, Walton Diaz A, Hoang AN, Pinto PA, et al. Image-guided focal therapy for prostate cancer. Diagn Interv Radiol. 2014;20:492–7. doi: 10.5152/dir.2014.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penzkofer T, Tempany-Afdhal CM. Prostate cancer detection and diagnosis: The role of MR and its comparison with other diagnostic modalities-a radiologist's perspective. NMR Biomed. 2014;27:3–15. doi: 10.1002/nbm.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller BG, Fütterer JJ, Gupta RT, Katz A, Kirkham A, Kurhanewicz J, et al. The role of magnetic resonance imaging (MRI) in focal therapy for prostate cancer: Recommendations from a consensus panel. BJU Int. 2014;113:218–27. doi: 10.1111/bju.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghai S, Trachtenberg J. Prostate cancer: A consensus on trial design for focal therapy. Nat Rev Urol. 2014;11:190–2. doi: 10.1038/nrurol.2014.64. [DOI] [PubMed] [Google Scholar]
- 16.Sommer G, Bouley D, Gill H, Daniel B, Pauly KB, Diederich C. Focal ablation of prostate cancer: Four roles for magnetic resonance imaging guidance. Can J Urol. 2013;20:6672–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Pinthus JH, Witkos M, Fleshner NE, Sweet J, Evans A, Jewett MA. Prostate cancers scored as Gleason 6 on prostate biopsy are frequently Gleason 7 tumors at radical prostatectomy: Implication on outcome. J Urol. 2006;176:979–84. doi: 10.1016/j.juro.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 18.Rajinikanth A, Manoharan M, Soloway CT, Civantos FJ, Soloway MS. Trends in Gleason score: Concordance between biopsy and prostatectomy over 15 years. Urology. 2008;72:177–82. doi: 10.1016/j.urology.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Panebianco V, Barchetti F, Sciarra A, Ciardi A, Indino EL, Papalia R, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urol Oncol. 2015;33:17.e1–7. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Shakir NA, George AK, Siddiqui MM, Rothwax JT, Rais-Bahrami S, Stamatakis L, et al. Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy. J Urol. 2014;192:1642–9. doi: 10.1016/j.juro.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann S, Kruck S, Kramer U, Gatidis S, Stenzl A, Roethke M, et al. Direct Comparison of Targeted MRI-Guided Biopsy with Systematic Transrectal Ultrasound-Guided Biopsy in Patients with Previous Negative Prostate Biopsies. Urol Int. 2014 doi: 10.1159/000365397. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: A systematic review and Meta-analysis. (01220-2).Eur Urol. 2014;pii(14):S0302–2838. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Overduin CG, Fütterer JJ, Barentsz JO. MRI-guided biopsy for prostate cancer detection: A systematic review of current clinical results. Curr Urol Rep. 2013;14:209–13. doi: 10.1007/s11934-013-0323-z. [DOI] [PubMed] [Google Scholar]
- 24.Pokorny MR, de Rooij M, Duncan E, Schröder FH, Parkinson R, Barentsz JO, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol. 2014;66:22–9. doi: 10.1016/j.eururo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Valerio M, Donaldson I, Emberton M, Ehdaie B, Hadaschik BA, Marks LS, et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: A systematic review. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Marcus DM, Rossi PJ, Nour SG, Jani AB. The impact of multiparametric pelvic magnetic resonance imaging on risk stratification in patients with localized prostate cancer. Urology. 2014;84:132–7. doi: 10.1016/j.urology.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Bjurlin MA, Meng X, Le Nobin J, Wysock JS, Lepor H, Rosenkrantz AB, et al. Optimization of prostate biopsy: The role of magnetic resonance imaging targeted biopsy in detection, localization and risk assessment. J Urol. 2014;192:648–58. doi: 10.1016/j.juro.2014.03.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turkbey B, Choyke PL. Multiparametric MRI and prostate cancer diagnosis and risk stratification. Curr Opin Urol. 2012;22:310–5. doi: 10.1097/MOU.0b013e32835481c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rastinehad AR, Baccala AA, Jr, Chung PH, Proano JM, Kruecker J, Xu S, et al. D’Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011;185:815–20. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Tol-Geerdink JJ, Leer JW, van Oort IM, van Lin EJ, Weijerman PC, Vergunst H, et al. Quality of life after prostate cancer treatments in patients comparable at baseline. Br J Cancer. 2013;108:1784–9. doi: 10.1038/bjc.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A, et al. Active surveillance for low-risk prostate cancer worldwide: The PRIAS study. Eur Urol. 2013;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: Modalities, findings and future considerations. Nat Rev Urol. 2010;7:562–71. doi: 10.1038/nrurol.2010.142. [DOI] [PubMed] [Google Scholar]
- 33.Lindner U, Trachtenberg J. Focal therapy for localized prostate cancer -choosing the middle ground. Can Urol Assoc J. 2009;3:333–5. doi: 10.5489/cuaj.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arora R, Koch MO, Eble JN, Ulbright TM, Li L, Cheng L. Heterogeneity of Gleason grade in multifocal adenocarcinoma of the prostate. Cancer. 2004;100:2362–6. doi: 10.1002/cncr.20243. [DOI] [PubMed] [Google Scholar]
- 35.Karavitakis M, Winkler M, Abel P, Livni N, Beckley I, Ahmed HU. Histological characteristics of the index lesion in whole-mount radical prostatectomy specimens: Implications for focal therapy. Prostate Cancer Prostatic Dis. 2011;14:46–52. doi: 10.1038/pcan.2010.16. [DOI] [PubMed] [Google Scholar]
- 36.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–9. doi: 10.1016/s0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 37.Ghai S, Trachtenberg J. In-bore MRI interventions: Current status and future applications. Curr Opin Urol. 2015;25:205–11. doi: 10.1097/MOU.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 38.Cormack RA, D’Amico AV, Hata N, Silverman S, Weinstein M, Tempany CM. Feasibility of transperineal prostate biopsy under interventional magnetic resonance guidance. Urology. 2000;56:663–4. doi: 10.1016/s0090-4295(00)00698-1. [DOI] [PubMed] [Google Scholar]
- 39.D’Amico AV, Tempany CM, Cormack R, Hata N, Jinzaki M, Tuncali K, et al. Transperineal magnetic resonance image guided prostate biopsy. J Urol. 2000;164:385–7. [PubMed] [Google Scholar]
- 40.Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: Initial results. Radiology. 2005;234:576–81. doi: 10.1148/radiol.2342031887. [DOI] [PubMed] [Google Scholar]
- 41.Pondman KM, Fütterer JJ, ten Haken B, Schultze Kool LJ, Witjes JA, Hambrock T, et al. MR-guided biopsy of the prostate: An overview of techniques and a systematic review. Eur Urol. 2008;54:517–27. doi: 10.1016/j.eururo.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Polanec SH, Helbich TH, Margreiter M, Klingler HC, Kubin K, Susani M, et al. Magnetic resonance imaging-guided prostate biopsy: Institutional analysis and systematic review. Rofo. 2014;186:501–7. doi: 10.1055/s-0033-1355546. [DOI] [PubMed] [Google Scholar]
- 43.Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: Detection of clinically significant prostate cancers. Eur Urol. 2012;62:902–9. doi: 10.1016/j.eururo.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 44.Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–95. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Fütterer J, Bouwense S, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012;61:177–84. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 46.Schwab SA, Kuefner MA, Adamietz B, Engelhard K, Keck B, Kunath F, et al. MRI-guided core biopsy of the prostate in the supine position--introduction of a simplified technique using large-bore magnet systems. Eur Radiol. 2013;23:1415–9. doi: 10.1007/s00330-012-2698-5. [DOI] [PubMed] [Google Scholar]
- 47.Engelhard K, Hollenbach HP, Kiefer B, Winkel A, Goeb K, Engehausen D. Prostate biopsy in the supine position in a standard 1.5-T scanner under real time MR-imaging control using a MR-compatible endorectal biopsy device. Eur Radiol. 2006;16:1237–43. doi: 10.1007/s00330-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 48.Cepek J, Lindner U, Ghai S, Louis AS, Davidson SR, Gertner M, et al. Mechatronic system for in-bore MRI-guided insertion of needles to the prostate: An in vivo needle guidance accuracy study. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24742. In Press. [DOI] [PubMed] [Google Scholar]
- 49.Cepek J, Chronik BA, Lindner U, Trachtenberg J, Davidson SR, Bax J, et al. A system for MRI-guided transperineal delivery of needles to the prostate for focal therapy. Med Phys. 2013;40:012304. doi: 10.1118/1.4773043. [DOI] [PubMed] [Google Scholar]
- 50.Cepek J, Chronik B, Lindner U, Trachtenberg J, Fenster A. Development of an MRI-compatible device for prostate focal therapy. Med Image Comput Comput Assist Interv. 2012;15:455–62. doi: 10.1007/978-3-642-33415-3_56. [DOI] [PubMed] [Google Scholar]
- 51.Nam RK, Saskin R, Lee Y, Liu Y, Law C, Klotz LH, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 Suppl):S12–8. doi: 10.1016/j.juro.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Schouten MG, Bomers JG, Yakar D, Huisman H, Rothgang E, Bosboom D, et al. Evaluation of a robotic technique for transrectal MRI-guided prostate biopsies. Eur Radiol. 2012;22:476–83. doi: 10.1007/s00330-011-2259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zangos S, Melzer A, Eichler K, Sadighi C, Thalhammer A, Bodelle B, et al. MR-compatible assistance system for biopsy in a high-field-strength system: Initial results in patients with suspicious prostate lesions. Radiology. 2011;259:903–10. doi: 10.1148/radiol.11101559. [DOI] [PubMed] [Google Scholar]
- 54.de Rooij M, Crienen S, Witjes JA, Barentsz JO, Rovers MM, Grutters JP. Cost-effectiveness of magnetic resonance (MR) imaging and MR-guided targeted biopsy versus systematic transrectal ultrasound-guided biopsy in diagnosing prostate cancer: A modelling study from a health care perspective. Eur Urol. 2014;66:430–6. doi: 10.1016/j.eururo.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Emberton M. Is prostate magnetic resonance imaging going to break the bank? Eur Urol. 2014;66:437–8. doi: 10.1016/j.eururo.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 56.Mozer P, Rouprêt M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115:50–7. doi: 10.1111/bju.12690. [DOI] [PubMed] [Google Scholar]
- 57.Villers A. Words of wisdom. Re: Improving detection of clinically significant prostate cancer: MRI/TRUS fusion-guided prostate biopsy. Eur Urol. 2014;65:1218–9. doi: 10.1016/j.eururo.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 58.Logan JK, Rais-Bahrami S, Turkbey B, Gomella A, Amalou H, Choyke PL, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int. 2014;114:641–52. doi: 10.1111/bju.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delongchamps NB, Lefevre A, Bouazza N, Beuvon F, Legman P, Cornud F. Detection of significant prostate cancer with MR-targeted biopsies: Should TRUS-MRI fusion guided biopsies alone be a standard of care? J Urol. 2014 doi: 10.1016/j.juro.2014.11.002. In Press. [DOI] [PubMed] [Google Scholar]
- 60.Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index Tumor: Correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.08.077. In Press. [DOI] [PubMed] [Google Scholar]
- 61.Colin P, Mordon S, Nevoux P, Marqa MF, Ouzzane A, Puech P, et al. Focal laser ablation of prostate cancer: Definition, needs, and future. Adv Urol. 2012;2012:589160. doi: 10.1155/2012/589160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van den Bos W, Muller BG, Ahmed H, Bangma CH, Barret E, Crouzet S, et al. Focal therapy in prostate cancer: International multidisciplinary consensus on trial design. Eur Urol. 2014;65:1078–83. doi: 10.1016/j.eururo.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Sussman MS, Lindner U, Haider M, Kucharczyk W, Hlasny E, Trachtenberg J. Optimizing contrast agent concentration and spoiled gradient echo pulse sequence parameters for catheter visualization in MR-guided interventional procedures: An analytic solution. Magn Reson Med. 2013;70:333–40. doi: 10.1002/mrm.24830. [DOI] [PubMed] [Google Scholar]
- 64.Colin P, Nevoux P, Marqa M, Auger F, Leroy X, Villers A, et al. Focal laser interstitial thermotherapy (LITT) at 980 nm for prostate cancer: Treatment feasibility in Dunning R3327-AT2 rat prostate tumour. BJU Int. 2012;109:452–8. doi: 10.1111/j.1464-410X.2011.10406.x. [DOI] [PubMed] [Google Scholar]
- 65.Lindner U, Lawrentschuk N, Weersink RA, Davidson SR, Raz O, Hlasny E, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: Validation of focal therapy and imaging accuracy. Eur Urol. 2010;57:1111–4. doi: 10.1016/j.eururo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Raz O, Haider MA, Davidson SR, Lindner U, Hlasny E, Weersink R, et al. Real-time magnetic resonance imaging-guided focal laser therapy in patients with low-risk prostate cancer. Eur Urol. 2010;58:173–7. doi: 10.1016/j.eururo.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 67.Lindner U, Sean RH, Neil EF, Anthonio F, Alexandre RZ, Michael AS, et al. 554 initial results of mr guided laser focal therapy for prostate cancer. J Urol. 189:e227–8. [Google Scholar]
- 68.Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, Stadler WM, et al. MR imaging-guided focal laser ablation for prostate cancer: Phase I trial. Radiology. 2013;267:932–40. doi: 10.1148/radiol.13121652. [DOI] [PubMed] [Google Scholar]
- 69.Cepek J, Lindner U, Davidson SR, Haider MA, Ghai S, Trachtenberg J, et al. Treatment planning for prostate focal laser ablation in the face of needle placement uncertainty. Med Phys. 2014;41:013301. doi: 10.1118/1.4842535. [DOI] [PubMed] [Google Scholar]
- 70.Lee T, Mendhiratta N, Sperling D, Lepor H. Focal laser ablation for localized prostate cancer: Principles, clinical trials, and our initial experience. Rev Urol. 2014;16:55–66. [PMC free article] [PubMed] [Google Scholar]
- 71.Hynynen K, McDannold N. MRI guided and monitored focused ultrasound thermal ablation methods: A review of progress. Int J Hyperthermia. 2004;20:725–37. doi: 10.1080/02656730410001716597. [DOI] [PubMed] [Google Scholar]
- 72.Napoli A, Anzidei M, De Nunzio C, Cartocci G, Panebianco V, De Dominicis C, et al. Real-time magnetic resonance-guided high-intensity focused ultrasound focal therapy for localised prostate cancer: Preliminary experience. Eur Urol. 2013;63:395–8. doi: 10.1016/j.eururo.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Chopra R, Colquhoun A, Burtnyk M, N’djin WA, Kobelevskiy I, Boyes A, et al. MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: Initial feasibility in humans. Radiology. 2012;265:303–13. doi: 10.1148/radiol.12112263. [DOI] [PubMed] [Google Scholar]
- 74.Lindner U, Ghai S, Spensieri P, Hlasny E, Van Der Kwast TH, McCluskey SA, et al. Focal magnetic resonance guided focused ultrasound for prostate cancer: Initial North American experience. Can Urol Assoc J. 2012;6:E283–6. doi: 10.5489/cuaj.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghai S, Louis AS, Van Vliet M, Lindner U, Haider MA, Hlasny E, Spensieri P, et al. Real time magnetic resonance guided focused ultrasound for focal therapy of locally confined low risk prostate cancer: Feasibility and preliminary outcomes. AJR Am J Roentgenol. 2015 doi: 10.2214/AJR.14.13098. In Press. [DOI] [PubMed] [Google Scholar]
- 76.Burtnyk M, Chopra R, Bronskill MJ. Quantitative analysis of 3-D conformal MRI-guided transurethral ultrasound therapy of the prostate: Theoretical simulations. Int J Hyperthermia. 2009;25:116–31. doi: 10.1080/02656730802578802. [DOI] [PubMed] [Google Scholar]
- 77.Siddiqui K, Chopra R, Vedula S, Sugar L, Haider M, Boyes A, et al. MRI-guided transurethral ultrasound therapy of the prostate gland using real-time thermal mapping: Initial studies. Urology. 2010;76:1506–11. doi: 10.1016/j.urology.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 78.N’djin WA, Burtnyk M, Kobelevskiy I, Hadjis S, Bronskill M, Chopra R. Coagulation of human prostate volumes with MRI-controlled transurethral ultrasound therapy: Results in gel phantoms. Med Phys. 2012;39:4524–36. doi: 10.1118/1.4730288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burtnyk M, Hill T, Cadieux-Pitre H, Welch I. MRI-guided transurethral ultrasound prostate ablation; A pre-clinical safety and feasibility study with 28-day follow-up. J Urol. 2014 doi: 10.1016/j.juro.2014.11.089. In Press. [DOI] [PubMed] [Google Scholar]
- 80.Billia M, Billia M, Burtnyk M, Kuru T, Pahernik S, Roethke M, Schlemmer HP, et al. 1133 MRI-guided transurethral ultrasound ablation of prostate cancer: Preliminary outcomes of a phase I clinical trial. Eur Urol. 2014;13:e1133. [Google Scholar]
- 81.Onik GM, Cohen JK, Reyes GD, Rubinsky B, Chang Z, Baust J. Transrectal ultrasound-guided percutaneous radical cryosurgical ablation of the prostate. Cancer. 1993;72:1291–9. doi: 10.1002/1097-0142(19930815)72:4<1291::aid-cncr2820720423>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 82.Overduin CG, Bomers JG, Jenniskens SF, Hoes MF, Ten Haken B, de Lange F, et al. T1-weighted MR image contrast around a cryoablation iceball: A phantom study and initial comparison with in vivo findings. Med Phys. 2014;41:112301. doi: 10.1118/1.4896824. [DOI] [PubMed] [Google Scholar]
- 83.Bomers JG, Yakar D, Overduin CG, Sedelaar JP, Vergunst H, Barentsz JO, et al. MR imaging-guided focal cryoablation in patients with recurrent prostate cancer. Radiology. 2013;268:451–60. doi: 10.1148/radiol.13121291. [DOI] [PubMed] [Google Scholar]
- 84.Gangi A, Tsoumakidou G, Abdelli O, Buy X, de Mathelin M, Jacqmin D, et al. Percutaneous MR-guided cryoablation of prostate cancer: Initial experience. Eur Radiol. 2012;22:1829–35. doi: 10.1007/s00330-012-2411-8. [DOI] [PubMed] [Google Scholar]
- 85.Woodrum DA, Kawashima A, Karnes RJ, Davis BJ, Frank I, Engen DE, et al. Magnetic resonance imaging-guided cryoablation of recurrent prostate cancer after radical prostatectomy: Initial single institution experience. Urology. 2013;82:870–5. doi: 10.1016/j.urology.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 86.Trachtenberg J, Chen J, Kucharczyk W, Toi A, Lancaster C. Microwave thermoablation for localized prostate cancer after failed radiation therapy: Role of neoadjuvant hormonal therapy. Mol Urol. 1999;3:247–50. [PubMed] [Google Scholar]
- 87.Trachtenberg J, Weersink RA, Davidson SR, Haider MA, Bogaards A, Gertner MR, et al. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: A study of escalating light doses. BJU Int. 2008;102:556–62. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]
- 88.Valerio M, Stricker PD, Ahmed HU, Dickinson L, Ponsky L, Shnier R, et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:343–7. doi: 10.1038/pcan.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]


