Abstract
Background:
A promising approach to treat diabetes is the development of fully automated artificial/bionic pancreas systems that use both insulin and glucagon to maintain euglycemia. A physically and chemically stable liquid formulation of glucagon does not currently exist. Our goal is to develop a glucagon formulation that is stable as a clear and gel-free solution, free of fibrils and that has the requisite long-term shelf life for storage in the supply chain, short-term stability for at least 7 days at 37°C, and pump compatibility for use in a bihormonal pump.
Methods:
We report the development of two distinct families of stable liquid glucagon formulations which utilize surfactant or surfactant-like excipients (LMPC and DDM) to “immobilize” the glucagon in solution potentially through the formation of micelles and prevention of interaction between glucagon molecules.
Results:
Data are presented that demonstrate long-term physical and chemical stability (~2 years) at 5°C, short-term stability (up to 1 month) under accelerated 37°C testing conditions, pump compatibility for up to 9 days, and adequate glucose responses in dogs and diabetic swine.
Conclusions:
These stable glucagon formulations show utility and promise for further development in artificial pancreas systems.
Keywords: liquid glucagon, bihormonal pump, artificial pancreas, stabilized glucagon
Because glucagon in solution is chemically and physically unstable for extended periods, the only available pharmaceutical preparations of glucagon require reconstitution of a dry powder immediately prior to each use. Although this is the only FDA approved procedure for using glucagon, clinical research in the areas of “mini-dosing”1 and artificial/bionic pancreas systems2,3 has shown that current glucagon preparations can retain bioactivity for at least 1 day after reconstitution. However, these studies have not reported in-use chemical stability over this time period. As the promise of fully automated artificial/bionic pancreas systems which use both insulin and glucagon to maintain euglycemia comes closer to reality, there is intense interest in developing liquid formulations of glucagon which can meet regulatory standards for stability in pump delivery devices. A stable liquid formulation of glucagon would also be of use for the currently approved clinical indication, that is, rescue of patients experiencing severe hypoglycemia, and potentially for “mini-dosing” of patients for the prevention or treatment of mild to moderate hypoglycemia. Success in achieving stability in nonaqueous solvents such as DMSO has been reported, however, one such formulation appears to have diminished bioavailability relative to an approved comparator in a human trial.4 This article summarizes a process for achieving stability of glucagon in aqueous solution using surfactant or surfactant-like excipients and provides an update on the most promising formulations developed to date.
Specifications, Target Product Profile, and Overview of Methods
USP Specifications
The USP Monograph for Glucagon for Injection5 states that the content of glucagon be (as measured by HPLC methods) “not less than 65% and not more than 110% of the labeled amount of glucagon.” The dose used for rescue from severe hypoglycemia is 1 mg glucagon for an adult, which is a supraphysiologic dose exceeding what is needed to induce a maximal pharmacodynamic effect in most adults with available glycogen stores; therefore, up to 45% degradation may be acceptable for this indication. Glucagon for mini-dosing or artificial pancreas systems would involve much smaller doses (in the low microgram range) and therefore it is likely that a narrower glucagon content range for these indications may be required by regulators. Other USP specifications for the rescue medication include not more than (NMT) 14% of all 4 desamido glucagons and NMT 31% of total impurities and related compounds. In addition to the measurement of the “chemical purity” of glucagon and degradation products, there are USP requirements for testing the biological potency of the preparation using a rat hepatocyte assay USP<123>.6 The USP specifications for biological potency state that “the potency is not less than 80.0% rGlucagon U/mg.” Newer assays utilizing stable transfectants of the glucagon receptor are being developed and are being used to determine the biological potency of glucagon formulations.7
Target Product Profile
Table 1 details potential minimal and optimal target product profiles (TPPs) for a pumpable glucagon formulation. These TPPs are based, in part, on TPPs for insulin formulations on the market. A certain amount of stability at ≥5°C is required for distribution in the supply chain following manufacturing. The optimal “in-use” temperature for “mini-dosing” is unknown; however, it would likely require room temperature stability for >7 days. With regard to use in pumps, labeling for 3 to 4 days of pump usage will likely require data supporting 6 to 8 days of stability at 37°C within specifications in pumps. We believe that pharmacokinetic (PK) and pharmacodynamic (PD) bioequivalence to the Lilly or Novo marketed glucagons may not be required or relevant for utility in the pump. However, it is important to determine the PK/PD profiles with respect to onset of action and dose-response that would be required to counteract the effects of insulin and to develop the algorithms necessary for development of a bihormonal pump.
Table 1.
| Minimal | Optimal | |
|---|---|---|
| Presentation | Liquid | Liquid |
| Distribution conditions | 18 months, 5°C | 24 months, 5°C |
| In-use conditions | Continued storage at 5°C | 30°C for 30 days |
| Pump duration | 3 to 4 days | 7 days |
| PK/PD profile | TBD—sufficient based on algorithm to counter insulin | PK/PD equivalence to Lilly/Novo rescue products |
Methods
Experimental formulations of liquid glucagon are routinely evaluated for chemical and physical stability at various temperatures (5°C, 25°C, 30°C, and/or 37°C) and under static or agitated conditions. Chemical degradation is routinely quantified by reverse phase UPLC. We have used a variety of methods to assess physical stability of the various formulations including visual observations for particle/gel formation and the Thioflavin T fluorescence assay for the detection and measurement of fibril formation. In preparation for pump use, select formulations have been evaluated in normal beagle dogs and in diabetic miniature swine following subcutaneous injection. The diabetic swine model is no longer preferred because of increased variability related to lack of compensatory insulin secretion. Normal beagle dogs are preferred for initial screening and determination of the PK profiles of glucagon formulations because PD responses are limited by compensatory endogenous insulin secretion thereby minimizing variability. We routinely use a crossover experimental design when comparing several different formulations to minimize the variability between animals within a study.
Development of Stable Liquid Glucagon Formulations
Overview of LMPC and DDM
Our general approach to stabilization has been to “immobilize” the glucagon in solution to prevent it from interacting with itself. A second objective is to enhance the solubility of glucagon which is limited at neutral pH. This has been accomplished in one case by using a combination of a lysolecithin (LMPC; 1-Myristoyl-2-hydroxy-sn-glycero-3-phosphocholine or lyso myristoyl-phosphocholine; see Figure 1), a simple sugar and an alcohol to form micelles, minimizing glucagon-glucagon interactions. Lysolecithins are a minor component (~3%) of all cell membranes and in plasma (8-12%). Lyso-phosphocholines are surface active agents with varied alkyl chain length, typically used in the food industry as emulsifiers and also presented in liposome drug delivery systems. Lysolecithins in aqueous environments act like a nonionic surfactant. When added to an acidic glucagon solution at concentrations above the critical micelle concentration (CMC), lipid micelles are formed that interact with glucagon. This association allows glucagon solubility in water at neutral pH.
Figure 1.
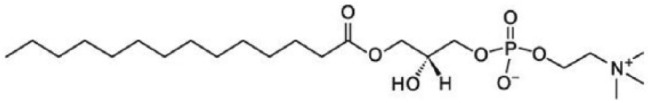
1-Myristoyl-2-hydroxy-sn-glycero-3-phosphocholine.
A second excipient utilized to stabilize glucagon in solution and enhance solubility is N-dodecyl-β-D-Maltoside (DDM; see Figure 2). DDM-related stabilizing agents utilize a disaccharide coupled to a long chain alcohol. They are safe, odorless, tasteless, nontoxic, nonmutagenic, and nonirritating and are metabolized to H2O and CO2. They are listed as “generally regarded as safe (GRAS)” for food purposes and have been studied in over 12 animal toxicology studies and 4 human trials.8-10 The mechanism of protein stabilization involves the disaccharide terminal which acts as a sugar, reducing aggregation and protecting the peptide/protein from oxidative degradation. In addition, the hydrophobic tail binds to exposed hydrophobic areas in the protein further reducing denaturation risk due to elevated temperature and pH changes.
Figure 2.
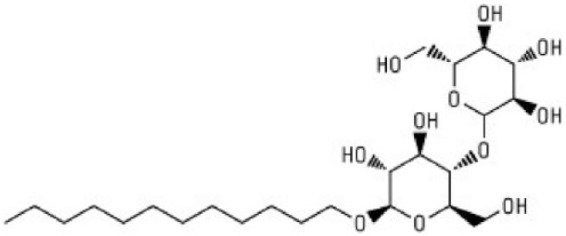
N-dodecyl-β-D-maltoside.
Stability of Prototype LMPC and DDM Glucagon Formulations
Extensive studies have been carried out to optimize liquid glucagon formulations at various conditions (e.g. temperature, pH, agitation) to achieve chemical and physical stability and sufficient absorption in animal models following subcutaneous administration. These detailed results are beyond the scope of this article and data on the most promising prototype formulations are presented.
Results
BIOD-903—Stability Profile of the Prototype LMPC-Based Glucagon Liquid Formulation
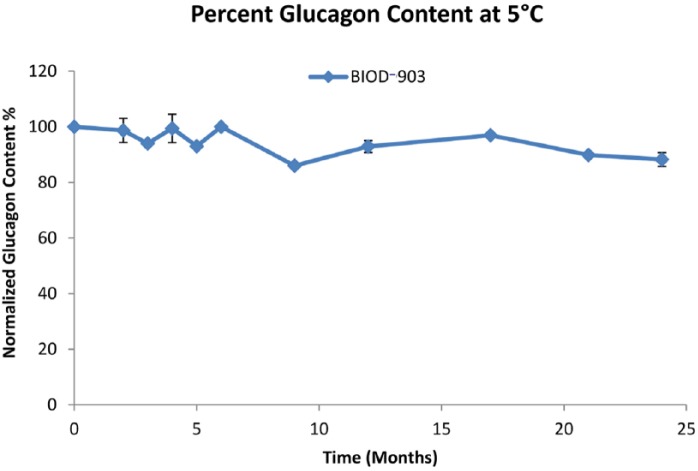
BIOD-903 and related LMPC-based glucagon liquid formulations contain glucagon (1 mg/mL), LMPC (2 mg/mL), simple sugar, alcohol and preservative in phosphate buffer at pH 7.0. Detailed data on early LMPC-based glucagon formulations have been published.11 The long-term stability of BIOD-903 at 5°C is excellent and remains above 80% of the original glucagon content for at least 24 months (Figure 3). The total impurities and related compounds at 24 months was 7.7%. Physical stability studies demonstrate that the solutions at 5°C are clear, gel and particle free, and show no detectable change of fluorescence intensity in the Thioflavin T assay indicative of having low to negligible fibril content (data not shown). This overall chemical and physical stability profile would be in keeping with the optimal TPP required for distribution in the commercial supply chain. Some limited studies have been done assessing the biological potency of BIOD-903 stored at 5°C for time points exceeding a year in a cell line that has been stably transfected with the glucagon receptor. The data suggest very little to no loss in the biological potency of the formulation over time.
Figure 3.
BIOD-903 long-term stability at 5°C, n=3.
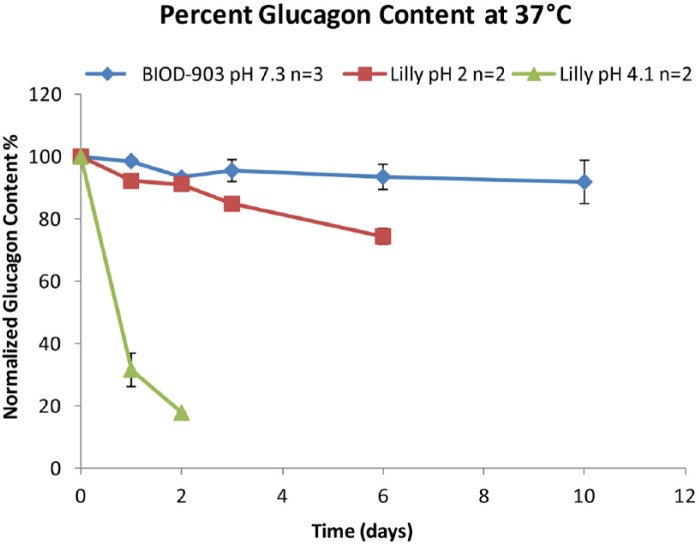
The short-term stability of BIOD-903 in comparison with Lilly reconstituted glucagon under accelerated testing conditions at 37°C is shown in Figure 4.11 The chemical degradation of glucagon was quantitated by UPLC over a 10-day period. Samples that gelled were not quantifiable, thus Lilly glucagon at pH 4 past 3 days and Lilly glucagon at pH 2 after 6 days have no data (Figure 4). BIOD-903 did not gel over the 10-day study.
Figure 4.
BIOD-903 versus Lilly Glucagon: short-term stability at 37°C.
Physical degradation was assessed using light obscuration at 37°C. The Lilly glucagon at pH 4 immediately showed a decrease in transmitted light, indicating a precipitated gel. The pH 2 Lilly glucagon formulation forms a clear gel but does not have a reduction in transmitted light for ~20 days, because the transmitted light is not sensitive to clear gel formation. The BIOD-903 glucagon formulation resists opaque and clear gel formation for a considerably longer period of time, out to 50 days at 37°C. This is a marked improvement over the commercially available glucagon formulation.
Stability Profile of DDM-Based Liquid Glucagon Formulations
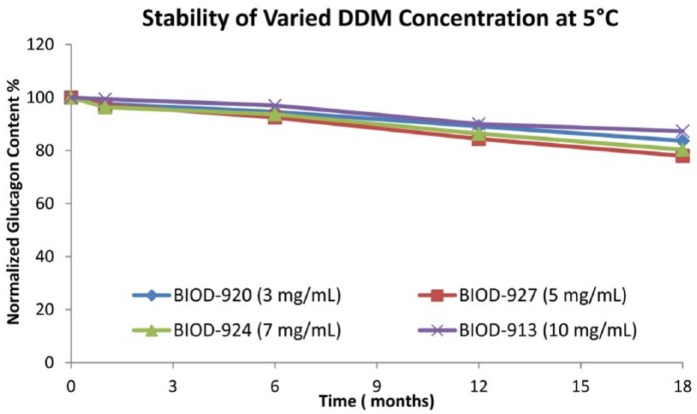
A series of DDM-based liquid formulations containing various combinations of glucagon (1 mg/mL), DDM (range of 3-10 mg/mL), glycerin (18.5 mg/mL), various buffers (5 mM phosphate or Tris), various antimicrobials (0.5 mg/mL phenol and 5 mg/mL benzyl alcohol) and a range of pH have been evaluated. The stability profiles of DDM-based formulations at 5°C are comparable at varying levels of DDM ranging from 3 to 10 mg/mL. Furthermore the long-term stability at 5°C for all the formulations is excellent and remains above 70% of the original glucagon content for at least 18 months (Figure 5). For the four compounds BIOD- 920, BIOD-927, BIOD-924 and BIOD-913, the range of total impurities and related compounds at t = 0 was between 5 and 15 percent. After 18 months at 5°C, the range of total impurities and related compounds was 10 to 20%, and after 28 days at 37°C the range was 20-24%, all within the USP specifications. Physical stability studies of select formulations evaluated demonstrate that the solutions at 5°C are clear, gel and particle free, and show no detectable change of fluorescence intensity in the Thioflavin T assay indicative of having low to negligible fibril content (data not shown). This overall chemical and physical stability profile is in keeping with the optimal TPP required for distribution in the commercial supply chain.
Figure 5.
Long-term stability of DDM-based glucagon formulations at 5°C.
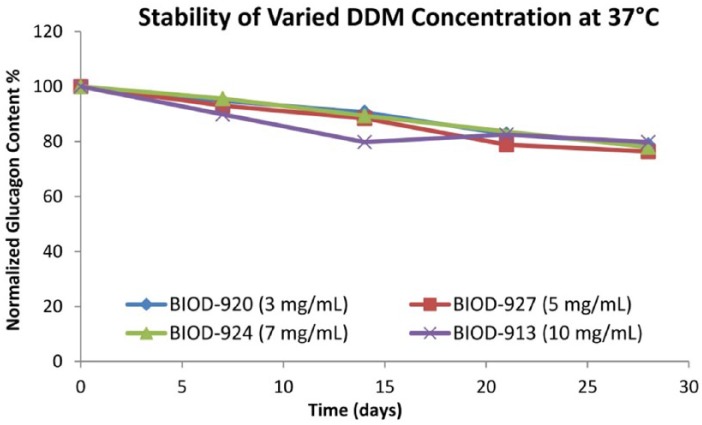
The short-term stability of the DDM-based formulations was evaluated at 37°C under static conditions (Figure 6). The chemical degradation profiles under static conditions at 37°C were comparable at the various DDM concentrations (3-10 mg/mL) and within the range that might be acceptable for a pump indication. Physical stability was evaluated with visual inspection under both static and agitated conditions. In general, the physical stability as indicated by particle formation or gel formation under agitation conditions was significantly improved at higher concentrations of DDM (7 and 10 mg/mL of DDM). Furthermore, physical stability appeared to be improved at basic pH ranges while chemical stability was improved at neutral or acidic pH. The mechanism of physical stabilization has not been fully characterized. Preliminary data show that glucagon alpha-helix content can be increased in the presence of a certain amount of DDM or LMPC at the expense of beta-sheets. The beta conformation is thought to be the predominant form when glucagon gels or forms fibrils.12,13
Figure 6.
Short-term stability of DDM-based formulations at 37°C.
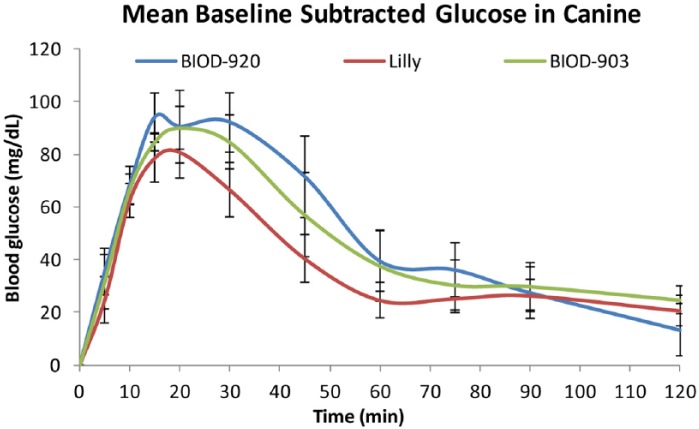
Preliminary Assessment in the Canine Animal Model
Select LMPC (BIOD-903) and DDM (BIOD-920 containing 3 mg/mL of DDM) formulations were assessed in normal male and female beagle dogs for their ability to increase blood glucose in comparison with the marketed Lilly glucagon product. On the day before the study, the dogs were fed double their typical daily food amounts to build up adequate glycogen stores. On the day of the study, animals were fasted and blood samples taken at −10 minutes and time zero. Glucagon (0.5 mg) was administered subcutaneously via syringe in the scruff of the neck and blood samples were taken over the next 2 hours. Glucose concentration was determined via strip method (Alpha Track, Abbott) immediately post blood draw. As shown in Figure 7, all three formulations produced comparable and rapid increases in glucose concentrations, which peaked between 10 and 20 minutes after the injection and returned toward baseline concentrations after 1 hour. The rapid return toward baseline is most likely a consequence of endogenous insulin secretion in the normal dogs, which limits the rise in glucose concentration.
Figure 7.
Comparison of the glucose response to LMPC- (BIOD-903), DDM- (BIOD-920) based formulations and Lilly reconstituted glucagon in normal beagle dogs following subcutaneous injection. ±SEM, n = 8.
Pump Compatibility Studies
A glucagon formulation that is chemically and physically stable as a clear solution, free of fibrils at a neutral pH for at least 7 days when kept at 37°C is likely to be needed for approval in pumps with an in-use time of 3 days. Six Animas OneTouch® Ping™ pumps were fitted with Animas 2 mL Cartridges and Unomedical Inset Infusion Sets (110 cm; 9 mm needle). Each pump was tested prior to this study to insure proper accuracy and reproducibility of dose delivery and six sets of reservoirs and infusion sets were used for each 12 mL of formulation. Sufficient BIOD-903 (LMPC-based formulation) was removed from refrigeration and was brought to room temperature. The pump reservoirs were filled and the infusion sets attached. The pumps were activated to insure that the drug product was in contact with all interior surfaces and was run at a basal delivery rate of 0.192 U glucagon/day/pump (1 glucagon IU = 1 mg). The unit was placed in a 37°C oven with continuous agitation (25 RPM × 24 hours with 45° tilt). The effluent was collected externally into separate vials. These vials were removed on the testing day and consolidated into a single vial. The effluent sample vial was stored at 5°C until the analyses were performed. Glass cartridges and additional reservoir syringes were filled with the same BIOD-903 formulation and were kept under the same 37°C conditions (without agitation) to serve as stability controls. The infusion sets were not replaced during the study.
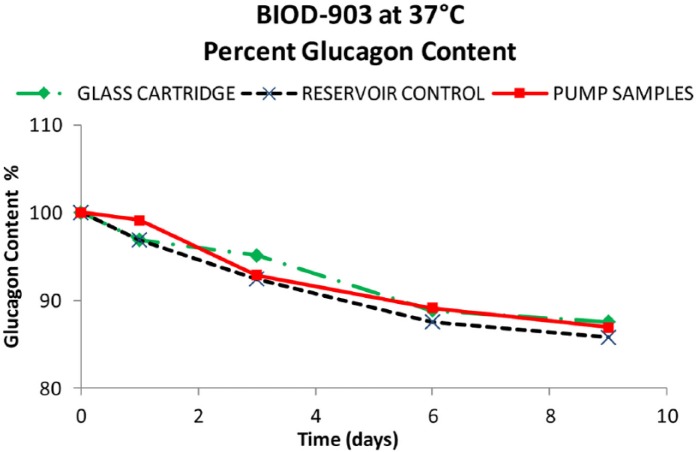
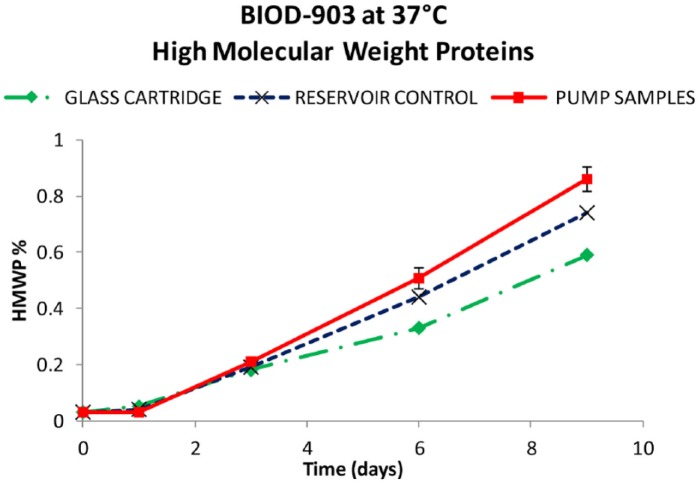
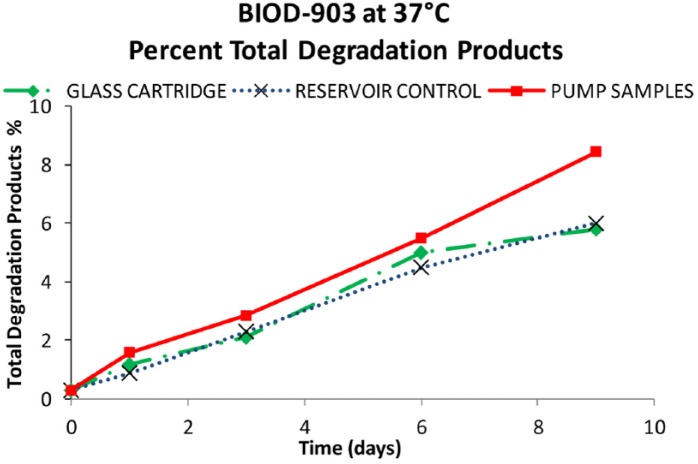
Results from the BIOD-903 pump study are shown in Figures 8 to 10. After 9 days at 37ºC, about 87% of the original glucagon content remained in the pump samples. The rest was identified as ~8% total other degradants and 0.9% high molecular weight protein (HMWP). These data were comparable with the control samples (within experimental error) in either glass cartridges or reservoir syringes at the same testing points. The short-term moderate agitation did not significantly impact glucagon stability and there were no catheter occlusions during the study.
Figure 8.
Percentage glucagon content from the glass cartridges (♦), reservoir controls (X), and pumped BIOD-903 (■).
Figure 10.
High molecular weight protein content of glass cartridges (♦), reservoir controls (X), and pumped BIOD-903 (■).
Figure 9.
Total percentage degradation from the glass cartridge (♦), reservoir controls (X), and pumped BIOD-903 (■).
A preliminary, short-term, 4-day study has also been carried out with a DDM-based formulation in Animas pumps in comparison with Lilly reconstituted glucagon. Over the 4-day period there was no decrease in glucagon content in the DDM-based formulation (101% of time 0), while there was a 40% decrease in the Lilly glucagon content over the same time period. Studies to determine the compatibility of both the LMPC- and DDM-based glucagon formulations in additional insulin pumps are planned.
Preliminary Evaluation of BIOD-903 in Diabetic Swine
BIOD-903 was studied in swine rendered diabetic with streptozocin, which eliminates endogenous insulin reserve, resulting in an animal model of type 1 diabetes physiology. We designed a study to evaluate the effects of freshly prepared BIOD-903 on glucose concentrations in 5 diabetic Yucatan Miniature swine and compared the effects of freshly prepared BIOD-903 with the same formulation that had been stored for 3 days at 37ºC to simulate the time frame and temperature for use in a pump. On the day of the glucagon study, glycogen stores were built up by an early morning meal and dose of prandial insulin was provided. Three hours after the meal, an intravenous dose of insulin was given to reduce the glucose levels to approximately 100 mg/dL. When this glucose level was achieved, 50 µg (50 µL of a 1 mg/mL solution) of glucagon was subcutaneously administered and glucose levels followed for 2 hours.
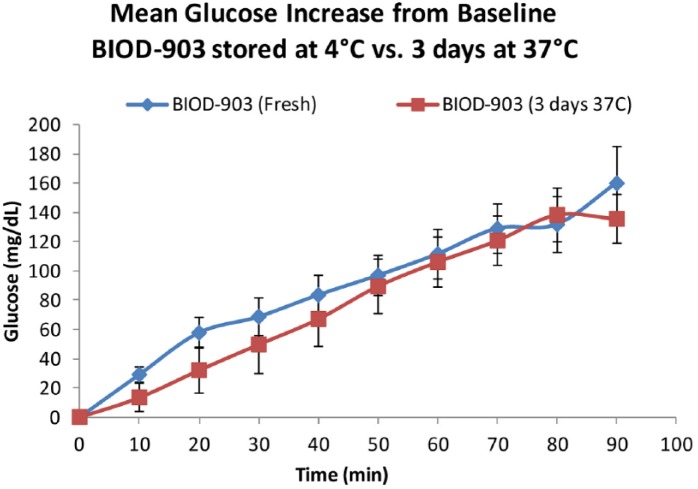
As shown in Figure 11, glucose concentrations increased following the subcutaneous injection of BIOD-903. However, in contrast to the response in normal dogs (Figure 7), the glucose levels continued to rise for the duration of the 90 minute study presumably due to the lack of insulin response. Furthermore, we observed comparable glucose responses to the freshly prepared BIOD-903 and to BIOD-903 which had been “stressed” for 3 days at 37ºC to simulate pump use in a patient. Studies are ongoing in diabetic swine to evaluate the effects of the stabilized glucagon formulations delivered through pumps in a continuous subcutaneous infusion and bolus paradigm to evaluate the PK and PD profiles and dose response relationships.
Figure 11.
Mean increase from baseline blood glucose levels over time (±SEM), comparing BIOD-903 glucagon stored at 4°C (fresh) versus storage for 3 days at 37°C. n = 5.
Discussion and Conclusions
This article summarizes progress made in the use of surfactant or surfactant-like excipients to achieve viable stable liquid formulations of glucagon. Some of these formulations appear to meet physical and chemical stability, pump compatibility, and in vivo PD response criteria for use in a bihormonal pump. These data suggest that this approach could lead to formulations suitable for clinical development and subsequent commercialization.
Acknowledgments
We thank Drs. Solomon Steiner and Robert Hauser for their contributions to the early work on the LMPC-based formulations and Bryan Wilson and Michael Crick for their continuing contributions to the ongoing work in this program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Abbreviations: C, Celsius; CMC, critical micelle concentration; DDM, N-dodecyl-β-D-Maltoside; dL, deciliter; DMSO, dimethyl sulfoxide; GRAS, generally regarded as safe; HMWP, high molecular weight protein; HPLC, high performance liquid chromatography; IU, international unit; LMPC, 1-Myristoyl-2-hydroxy-sn-glycero-3-phosphocholine; mL, milliliter; NLT, not less than; NMT, not more than; PD, pharmacodynamic; PK, pharmacokinetic; TBD, to be determined; TPP, target product profile; UPLC, ultrahigh performance liquid chromatography.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of Biodel Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Diabetes and Digestive Kidney Diseases of the National Institutes of Health under grant award number 1R43DK096602 and a State SBIR acceleration and commercialization grant from CT Innovations.
References
- 1. Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643-645. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs PG, Youssef JE, Castle J, et al. Automated control of an adaptive bi-hormonal, dual-sensor artificial pancreas and evaluation during inpatient studies. IEEE Trans Biomed Eng. 2014;61:2569-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes [published online ahead of print June 15, 2014]. N Engl J Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cersosimo E, Cummins MJ, Kinzel JH, et al. A phase 2 comparative safety PK/PD study of stable nonaqueous glucagon (G-Pen) vs. Lilly glucagon for treatment of severe hypoglycemia. Diabetes. 2014;63(suppl 1A):LB1. [Google Scholar]
- 5. USP 37-NF 32. Official monographs. Glucagon for injection. May 1, 2014 3158-3159. [Google Scholar]
- 6. USP 37-NF 32. Biological tests. (123) Glucagon bioidentity tests. May 1, 2014 127-129. [Google Scholar]
- 7. Morris TS, Singer R, Ambrose MR, Hauck WW. Biological potency assays are key to assessing product consistency. BioPharm Int. 2009;22(6). Available from: http://www.bio-pharminternational.com. [Google Scholar]
- 8. Kocher K, Wiegand HJ. Toxicology and dermatology In: Balzer D, Luder H, eds. Nonionic Surfactants—Alkylpoly-glucosides, Surfactant Science Series. Vol. 91 New York, NY: Marcel Dekker; 2000:365-384. [Google Scholar]
- 9. Maggio ET. Absorption enhancing excipients in systemic nasal drug delivery. J Excipients Food Chem. 2014;5:100-112. [Google Scholar]
- 10. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharma-cokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105:362-367. [DOI] [PubMed] [Google Scholar]
- 11. Steiner SS, Li M, Hauser R, Pohl R. Stabilized glucagon formulation for bihormonal pump use. J Diabetes Sci Tech-nol. 2010;4(6):1332-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki K, Dockerill S, Adamiak D, Tickle I, Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975;257:751-756. [DOI] [PubMed] [Google Scholar]
- 13. Chou P, Fasman G. The conformation of glucagon: predictions and consequences. Biochemistry. 1975;14(11):2536-2541. [DOI] [PubMed] [Google Scholar]