Abstract
Background:
We assessed the impact of perceived insulin pump usability on attitudes toward insulin pump therapy in diabetic individuals currently treated with multiple daily insulin injections (MDI).
Method:
This comparative, single-arm study recruited 28 adults with type 1 (n = 16) and insulin-treated type 2 diabetes (n = 12) to evaluate 2 current insulin pumps: Medtronic Revel 723 (Pump 1), Asante Snap Insulin Pump (Pump 2). Participants were randomized 1:1 to 1 of 2 assessment sequences: Pump 1 followed by Pump 2; and Pump 2 followed by Pump 1. Structured observational protocols were utilized to assess participants’ ability and time required to learn/perform common tasks associated with pump setup/use. Participants used a modified version of the System Usability Scale (SUS) and investigator-developed questionnaires to rate pump usability and task difficulty; pre-post questionnaires assessed changes in attitudes toward insulin pump therapy.
Results:
All participants completed the study. SUS scores showed Pump 2 to be more usable than Pump 1 on all usability attributes. Participants rated Pump 2 more positively than Pump 1, overall mean SUS scores of 5.7 versus 4.1 respectively, F(1, 52) = 32.7, P < .001, and SUS scores were higher if participants used the Pump 2 last, 5.3 versus 4.4 for Pump 1 last, F(1, 52) = 10.8, P < .01. Pump 2 was preferred for all tasks: manual bolus (86%), bolus calculation (71%), managing basal rates (93%), interpreting alarms (96%), transferring settings (100%), changing insulin and infusion sets (93%), all P < .05.
Conclusions:
Perceptions of pump usability can directly impact acceptance and use of features that may benefit those who wear them. Simpler pump devices that decrease perceptions of complexity may encourage broader use of this technology.
Keywords: CSII, insulin pumps, MDI, usability
Continuous subcutaneous insulin infusion (CSII) with insulin pump devices is considered the “gold standard” of care for diabetic individuals treated basal-bolus or multiple daily insulin injection therapy. Use of insulin pump therapy has been shown to improve glycemic control, glycemic variability, and quality of life in individuals with type 1 and insulin-treated 2 diabetes.1-8
Despite these benefits, associated costs, extensive training requirements and device complexity have restricted broad use of insulin pumps among individuals with insulin-treated diabetes.9 Current use of insulin pump therapy in the United States is estimated to be less than 30% in individuals with type 1 diabetes, and less than 1% in individuals with type 2 diabetes (unpublished data).
Most insurance companies will now reimburse the cost of insulin pump therapy for individuals with type 1 diabetes after prospective approval;10 reimbursement for individuals with type 2 diabetes is improving. Although advances in insulin pump designs have greatly reduced the complexity of insulin pump use, even the latest generation insulin pumps require a minimum level of technical proficiency (eg, programming, response to alerts), critical thinking, and significant lifestyle changes for safe and effective use. It is important that clinicians take these challenges into consideration when selecting potential candidates for insulin pump therapy.
It is likely that willingness to initiate insulin pump therapy may be strongly influenced by individuals’ perceptions of the simplicity, usability and potential benefits of the insulin devices offered to them. We hypothesized that exposure to less complex insulin pump systems may impact patient attitudes toward insulin pump therapy. To explore this hypothesis, we assessed perceptions of the overall usability and ease of use of 2 recent generation insulin pump systems among individuals with type 1 diabetes and insulin-treated type 2 who were naive to insulin pump therapy and currently managed with multiple daily insulin injections (MDI).
Methods
The primary objective of this comparative study was to assess patient perceptions of the usability (eg, simplicity, ease of use) of 2 insulin pump systems and determine whether these perceptions impacted participants’ attitudes toward insulin pump therapy. The 2 devices were the Medtronic Revel 723 insulin pump (Medtronic, Northridge, CA) (Pump 1) and Asante Snap insulin pump (Asante Solutions, Sunnyvale, CA) (Pump 2). Both insulin pump systems had similar operating features.
Participants were randomized 1:1 to 1 of 2 insulin pump assessment sequences: Pump 1 followed by Pump 2, or Pump 2 followed by Pump 1. All participants provided written informed consent prior to study participation.
Participants
Participants were recruited by a market research firm and invited to participate in the study at a single research site in San Francisco, California. Main inclusion criteria were diagnosed diabetes (type 1, type 2, or latent autoimmune diabetes in adults [LADA]), age 21 to 65 years, treated with MDI therapy (minimum 2 injections of human regular or rapid-acting analog per day), blood glucose monitoring frequency ≥ 2 per day, and naive to insulin pump therapy. Participants received a small monetary payment; however, they were unaware of the study sponsor.
Measures
Investigators used structured observational protocols to determine participants’ ability to use the insulin pumps without instruction (self-exploration) and then learn and perform common tasks associated with insulin pump use. Standardized, investigator-developed questionnaires were used to assess participants’ perceptions of the insulin pumps regarding the difficulty of specific tasks, preferences of the specific features and overall pump preference. Investigators used a modified version of the System Usability Scale (SUS),11 a validated measure of device usability (modified for insulin pump assessment), to assess participant perceptions of pump usability; however, the modified questionnaire was not formally validated (Table 1). Attitudes toward insulin pump therapy were assessed using: an investigator-developed questionnaire (Pump Attitudes—General) to obtain baseline general attitudes toward insulin pump therapy prior to exposure to the study pumps and a second questionnaire (Pump Attitudes—Specific) to determine whether and to what degree exposure to each insulin pump impacted participants’ attitudes toward insulin pump therapy (Table 2).
Table 1.
System Usability Scale (SUS).
| Please indicate the extent to which you agree with the following statements (1 = Strongly Disagree, 7 = Strongly Agree): |
|---|
| 1. I think I would like to use this pump. |
| 2. I found this pump simple. |
| 3. I thought this pump was easy to use. |
| 4. I think that I could use this pump without the support of a technical person. |
| 5. I found the various functions in this pump were well integrated. |
| 6. I thought there was a lot of consistency in this pump. |
| 7. I would imagine that most people would learn to use this pump very quickly. |
| 8. I found this pump very intuitive. |
| 9. I felt very confident using this pump. |
| 10. I could use this pump without having to learn anything new. |
Table 2.
Attitudes Toward Insulin Pump Therapy Assessments: General and Specific.
| Pump Attitudes—General (administered prior to pump evaluation) |
|---|
| 1. Please indicate the extent to which you agree with the following statements (1 = Strongly Disagree, 7 = Strongly Agree) |
| a. An insulin pump is easy to use. |
| b. An insulin pump is convenient to use. |
| c. An insulin pump suits my lifestyle. |
| d. An insulin pump is discrete. |
| e. An insulin pump is safe. |
| f. I would like to use an insulin pump. |
| g. I am confident that I can manage my diabetes using an insulin pump. |
| h. I would recommend an insulin pump to another person with diabetes. |
| 2. If I think about using a pump compared to giving myself injections: |
| a. I would have better control over my diabetes with an insulin pump than with injections. |
| b. I would prefer using an insulin pump over giving myself injections. |
| c. It would be easier to manage my diabetes with an insulin pump than with injections. |
| Pump Attitudes—Specific (administered after evaluation of each pump) |
| 1. Please indicate the extent to which you agree with the following statements (1 = Strongly Disagree, 7 = Strongly Agree) |
| a. The Revel/Snap insulin pump is easy to use. |
| b. The Revel/Snap insulin pump is convenient to use. |
| c. The Revel/Snap insulin pump suits my lifestyle. |
| d. The Revel/Snap insulin pump is discrete. |
| e. The Revel/Snap insulin pump is safe. |
| f. I would like to use the Revel/Snap insulin pump. |
| g. I am confident that I can manage my diabetes using the Revel/Snap insulin pump. |
| h. I would recommend the Revel/Snap insulin pump to another person with diabetes. |
| 2. If I think about using this pump compared to giving myself injections: |
| a. I would have better control over my diabetes with an insulin pump than with injections. |
| b. I would prefer using an insulin pump over giving myself injections. |
| c. It would be easier to manage my diabetes with an insulin pump than with injections. |
Interventions
Prior to arrival at the research site, participants received consent and confidentiality statements for their review and were asked to complete the baseline General Attitudes Assessment Questionnaire included in their packet. At the research site, investigators obtained the completed Attitudes Assessment Questionnaire and signed consent forms from participants.
Pump Therapy Instruction
Prior to exposure to the insulin pumps, participants received training in the basic concepts of insulin pump therapy. The training was facilitated by a certified diabetes educator (CDE) in a group setting and designed to provide a link between the methods and techniques used in insulin pump therapy and MDI therapy (eg, long-acting insulin vs basal insulin rates). General training covered infusion sets, tubing, reservoirs and general pump concepts such as insulin sensitivity factors (ISF), insulin-to carbohydrate ratios (I:CHO) and insulin-on-board. After receiving this instruction, participants were randomly assigned (1:1) to the Pump 1–Pump 2 or Pump 2–Pump 1 pump assessment sequence. Each sequence was conducted in 2 segments: (1) self-exploration and intuitiveness trials and (2) insulin pump task evaluations.
Self-Exploration
During the self-exploration sequence, participants were given 2 minutes to examine the first pump in the sequence without any instructions from the facilitator on pump use. Participants were encouraged to press buttons and examine the various parts of the pump. After 2 minutes, participants were given several tasks to perform without any instruction. Tasks included the following: determine the current time as indicated by the pump, administer a manual bolus of 0.2 units, use the bolus calculator to determine and administer a bolus dose, stop and restart the pump, determine the current basal rate for the pump, set the maximum basal rate, set the automatic off to on, and identify the current date indicated by the pump. Investigators limited the time spent on each task and scored participants’ performance on each task as completed or not completed. The time required to complete each task was recorded or reported as time expired. Participants were asked to rate the difficulty of each task using a standardized questionnaire. The second pump in the sequence was then assessed, following the same protocol.
Pump Tasks
In this segment, investigators provided instruction on how to perform various pump-related tasks, starting with the first pump in the assessment sequence (Pump 1 or Pump 2). Tasks included the following: changing the infusion set, refilling the insulin, manually administering a bolus dose, using the bolus calculator to administer a bolus, changing the basal pattern/profile, inserting a new basal rate into an existing pattern/profile, deleting a basal rate, interpreting alarms, and transferring settings to a replacement pump. Participants were then presented with scenarios that would necessitate performance of these tasks. Investigators measured and documented the number of practice trials required to successfully complete each task, successful completion of each task and time required to complete each task. After all pump-related tasks were been completed, investigators administered the SUS for that pump, followed by the pump-specific attitudes assessment. The second pump in the sequence was then assessed, following the same protocol.
Pump-to-Pump Comparison
At the end of the assessment sequence, participants used a standardized questionnaire to indicate their preferred insulin pump, by function and overall preference. For this assessment, participants first reviewed the specific tasks performed on each pump and then chose the insulin pump they perceived to be the easiest for that task. Participants then completed an overall pump assessment questionnaire by choosing the preferred pump across several user experience and usability dimensions.
Statistical Analysis
ANOVA analyses, 2-sample t tests, and N-1 2-proportion tests were used to assess changes in attitudes toward insulin pump therapy and determine between-pump differences in participant interactions with and attitudes toward each pump. Differences between assessment sequences were also assessed. Change and comparison data are reported as summary statistics.
Results
Twenty-eight individuals with MDI-treated diabetes were enrolled in the study (16 type 1 diabetes, 12 type 2 diabetes; 64% female; average age 42.3 years). All participants completed the evaluation.
Perceptions of Device Usability
Results from the SUS tool showed that participants considered Pump 2 to be more usable than Pump 1 on all usability attributes (Figure 1). Participants rated Pump 2 more positively than Pump 1, overall mean SUS scores of 5.7 versus 4.1, respectively, F(1, 52) = 32.7, P < .001. When device comparisons were randomized 1:1, SUS scores were higher if participants used the Pump 2 last, 5.3 versus 4.4 for Pump 1 last, F(1, 52) = 10.8, P < .01.
Figure 1.
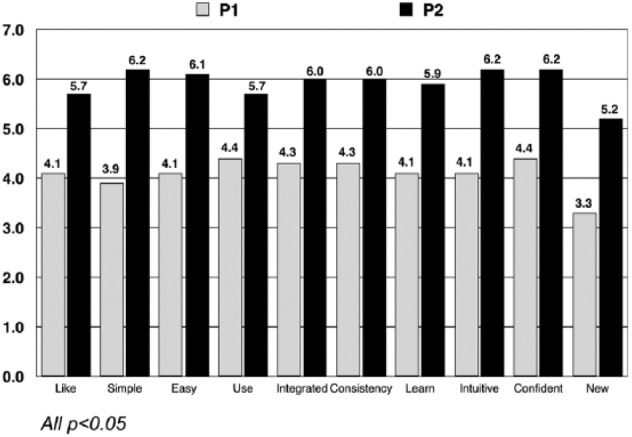
System Usability Scale (SUS) ratings.
Changes in Attitudes Toward Insulin Pump Therapy
With respect to changes in attitude toward pumps, participants became more positive toward insulin pumps after using Pump 2 than Pump 1, mean changes in attitudes were 1.4 and 0.5 respectively, F(1, 52) = 15.4, P < .001, on a 7-point scale. This difference was consistent across all attributes (Figure 2). In addition, participants became more positive toward pumps if they used the Pump 2 last than if used the Pump 1 last, 0.68 versus 0.25, respectively, F(1, 52) = 10.7, P < .01.
Figure 2.
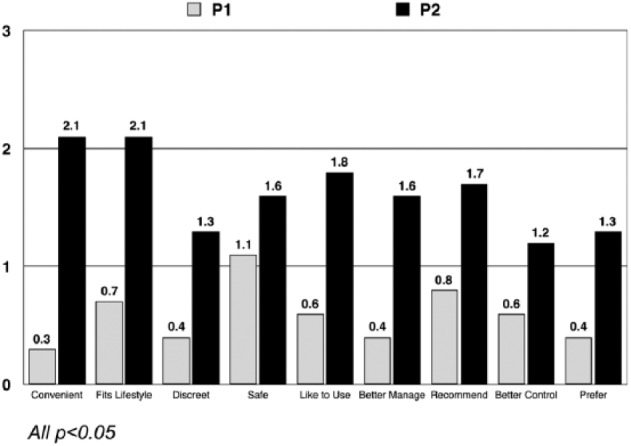
Change from baseline in attitudes toward insulin pump therapy following pump exposure.
Correlations Between Usability and Attitudes
Strong correlations were seen between attitudes toward insulin pump therapy and usability for each device: Pump 1, r = .71, P < .01; Pump 2, r = .86, P < .01. All correlations were also significant when device order was included in the analysis: Pump 1, r = .67, P < .01; Pump 1 after trying Pump 2, r = .76, P < .01; Pump 2, r = .78, P < .01, Pump 2 after trying Pump 1, r = .84, P < .01. Correlations between changes in attitudes toward insulin pump therapy and perceptions of usability were not significant for either pump.
Device Intuitiveness
Without prior instruction, participants were able to more quickly administer a simple bolus and stop the pump using Pump 1 compared with Pump 2: 22 versus 37 seconds, P < .05; and 10 versus 18 seconds, P < .05, respectively. Participants were able to more quickly find the current basal rate, set the auto off to on and find the current date using Pump 2 compared with Pump 1: 53 versus 71 seconds, P < .05; 69 versus 92, P < .05; and 5 versus 34, P < .01, respectively. There were no significant between-pump differences in the time required to find the current time, calculate a bolus, or set the maximum bolus. Participants rated Pump 2 as easier for finding the current basal rate (4.8 vs 3.5, P < .01), turning auto off to on (3.9 vs 2.7, P < .05) and finding the current date (6.8 vs 4.8, P < .01).
Learning Device-Related Tasks
No significant between-pump differences were seen in the number of learning trials required to perform the pump-related tasks. Participants in the Pump 2–Pump 1 assessment sequence administered manual boluses more quickly using Pump 2 than those in the Pump 1–Pump 2 sequence (P < .05).
Time to Perform Device-Related Tasks
In both assessment sequences, participants were able to manually administer insulin more quickly using Pump 1 compared with Pump 2: 8 versus 11 seconds, P < .01. Participants were able to add, insert, and delete basal insulin rates more quickly using Pump 2 compared with Pump 1: 30 versus 40 seconds, P < .05; 26 versus 77 seconds, P < .01; 16 versus 69 seconds, P < .01, respectively. Transferring pump settings and insulin replacement and infusion set changes were performed more quickly with Pump 2 than Pump 1: 17 versus 419 seconds, P < .01; and 261 versus 399 seconds, P < .01, respectively.
Ease of Device Use
After pump task training, participants rated most Pump 2-related tasks as easier than Pump 1: insulin replacement and infusion set changes (5.6 vs 4.3, P < .01); adding, inserting, and deleting basal rates (6.3 vs 5.5, P < .01; 6.5 vs 5.0, P < .01; 6.7 vs 5.2, P < .01, respectively); transferring pump parameters (6.5 vs 3.8, p <0.01); and interpreting alerts and alarms (5.5 vs 3.1, P < .01). Accuracy of participant interpretation of alerts and alarms was higher for Pump 2 compared to Pump 1: 60% versus 11%, P < .01. Participants rated ease of alarm interpretation higher for Pump 2 compared with Pump 1 (5.5 vs 3.1, P < .01).
Insulin Pump Preference
Participants preferred Pump 2 over Pump 1 for all evaluated attributes: administering manual bolus (86%), bolus calculation (71%), managing bolus rates/profiles (93%), interpreting alarms (96%), transferring pump settings (100%), and replacing insulin/changing infusion sets (93%), all P < .05. When choosing which pump they preferred overall, 100% of participants preferred Pump 2.
Discussion
Despite its proven benefits1-8 insulin pump therapy is underutilized in the treatment of both type 1 and insulin-treated type 2 diabetes. Although cost is often cited as a major contributing factor underutilization, the extensive training requirements and device complexity are also important obstacles that must considered.9
In the current pilot study, we compared 2 current generation insulin pump devices to determine whether participants’ perceptions of device usability impacted their attitudes toward using insulin pump therapy. We found that participants felt more positive toward insulin pump therapy after exposure to the devices compared to baseline attitudes, and the magnitude of attitude changes was associated with their perceptions of usability; the greater the perceived usability, the greater the magnitude of attitude improvement. However, improvements in attitudes were significant only following exposure to the Pump 2 device. Interestingly, the sequence of exposure seemed to impact perceptions of usability. Participants were more positive about Pump 2, and rated Pump 2 usability higher than Pump 1 regardless of the assessment sequence. They rated Pump 2 usability even higher if they were exposed to the Pump 2 device prior to Pump 1 (Pump 2–Pump 1 sequence). Although most of the time differences seen in intuitiveness and task performance phases were statistically significant, we recognize that these differences are not necessarily clinically relevant per se. However, we do believe that the time differences likely contributed to participants’ perceptions of usability, simplicity and ease of use.
An important limitation of the study was the small sample size; a larger number of participants would likely have increased the robustness and generalizability of our findings. However, even with the small sample, we found statistically significant between-pump differences in usability and associated improvements in attitudes. Another limitation of the study was the limited participant demographic data. A larger study population with more in-depth baseline information about the participants would have allowed us to detect relationships between specific participant characteristics and SUS and attitude scores. Another limitation was comparison of only 2 insulin pump devices. Comparison of several insulin pump devices could potentially provide more meaningful information to guide clinicians and candidates for insulin pump therapy in device selection.
It is important to note that our evaluation was not intended as a “head-to-head” comparison of the 2 insulin pumps studied. We recognize the role and utility of insulin pump systems that offer advanced features (eg, augmented with continuous glucose monitoring capability) for patients who have gained expertise and confidence in utilizing insulin pump therapy. Our goal, rather, was to determine whether user perceptions of simplicity and ease of use impacted attitudes toward insulin pump treatment among patients who were naïve to this therapy option.
Conclusions
Perceptions of insulin pump device usability appear to significantly influence attitudes toward insulin pump therapy. This suggests that clinicians should carefully consider the design and operational features of the devices they recommend to their diabetes patients. Large interventional trials, utilizing several insulin devices, are needed to further elucidate our findings and determine whether improved attitudes results in greater uptake and long-term use of insulin pump therapy.
Acknowledgments
The authors wish to thank Christopher G. Parkin (CGParkin Communications, Inc, Boulder City, NV, USA) for editorial assistance in developing this manuscript.
Footnotes
Abbreviations: ANOVA, analysis of variance; CDE, certified diabetes educator; CSII, continuous subcutaneous insulin infusion; I:CHO, insulin-to-carbohydrate ratio; ISF, insulin sensitivity factors; LADA, latent autoimmune diabetes in adults; MDI, multiple daily insulin injections; Pump 1, Medtronic Revel 723 insulin pump; Pump 2, Asante Snap insulin pump; SUS, System Usability Scale.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JC has received honoraria as a member of the Asante advisory board. EG has no disclosures to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for the study was provided by Asante Solutions, Inc, Sunnyvale, CA, USA.
References
- 1. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765-774. [DOI] [PubMed] [Google Scholar]
- 2. Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324(7339):705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008;51(6):941-951. [DOI] [PubMed] [Google Scholar]
- 4. Edelman SV, Bode BW, Bailey TS, et al. Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther. 2010;12(8):627-633. [DOI] [PubMed] [Google Scholar]
- 5. Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598-2603. [DOI] [PubMed] [Google Scholar]
- 6. Herman WH, Ilag LL, Johnson SL, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005;28(7):1568-1573. [DOI] [PubMed] [Google Scholar]
- 7. Wainstein J, Metzger M, Boaz M, et al. Insulin pump therapy vs. multiple daily injections in obese Type 2 diabetic patients. Diabet Med. 2005;22(8):1037-1046. [DOI] [PubMed] [Google Scholar]
- 8. Berthe E, Lireux B, Coffin C, et al. Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res. 2007;39(3):224-229. [DOI] [PubMed] [Google Scholar]
- 9. Bode BW. Insulin pump use in type 2 diabetes. Diabetes Technol Ther. 2010;12(suppl 1):S17-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clin Diabetes. 2007;25(2):50-56. [Google Scholar]
- 11. Bangor A, Kortum PT, Miller JT. An empirical evaluation of the System Usability Scale. Int J Hum Comput Interact. 2008;24(6):574-594. [Google Scholar]


