Abstract
Background:
Commercial glucagon is unstable due to aggregation and degradation. In closed-loop studies, it must be reconstituted frequently. For use in a portable pump for 3 days, a more stable preparation is required. At alkaline pH, curcumin inhibited glucagon aggregation. However, curcumin is not sufficiently stable for long-term use. Here, we evaluated ferulic acid, a stable breakdown product of curcumin, for its ability to stabilize glucagon.
Methods:
Ferulic acid-formulated glucagon (FAFG), composed of ferulic acid, glucagon, L-methionine, polysorbate-80, and human serum albumin in glycine buffer at pH 9, was aged for 7 days at 37°C. Glucagon aggregation was assessed by transmission electron microscopy (TEM) and degradation by high-performance liquid chromatography (HPLC). A cell-based protein kinase A (PKA) assay was used to assess in vitro bioactivity. Pharmacodynamics (PD) of unaged FAFG, 7-day aged FAFG, and unaged synthetic glucagon was determined in octreotide-treated swine.
Results:
No fibrils were observed in TEM images of fresh or aged FAFG. Aged FAFG was 94% intact based on HPLC analysis and there was no loss of bioactivity. In the PD swine analysis, the rise over baseline of glucose with unaged FAFG, aged FAFG, and synthetic native glucagon (unmodified human sequence) was similar.
Conclusions:
After 7 days of aging at 37°C, an alkaline ferulic acid formulation of glucagon exhibited significantly less aggregation and degradation than that seen with native glucagon and was bioactive in vitro and in vivo. Thus, this formulation may be stable for 3-7 days in a portable pump for bihormonal closed-loop treatment of T1D.
Keywords: type 1 diabetes mellitus, closed-loop system, hypoglycemia, glucagon, ferulic acid
In the past decade, there has been great advancement in the field of closed-loop systems for the treatment of type 1 diabetes (T1D). While some investigators use insulin-only systems,1-5 others have reported that, in addition to insulin, the automated delivery of glucagon when glucose is declining and approaching hypoglycemic levels reduces the frequency and duration of hypoglycemia.6-10 However, glucagon is an unstable peptide and forms amyloid fibrils when aged in acidic aqueous solutions.11-14 These fibrils, which can form gels, have been reported to be cytotoxic13,15 and could occlude pump delivery catheters. In addition to its tendency to aggregate, glucagon spontaneously degrades through chemical mechanisms such as deamidation and oxidation.16,17 For these reasons, it is not feasible to use current preparations of glucagon in a portable pump at room or body temperatures over the several days necessary for automated delivery. To avoid these issues, when used in the research setting during closed-loop studies, glucagon is freshly reconstituted from the lyophilized form using commercial glucagon preparations at the point of care.6,7 For future applications outside research, it will be necessary to have a stable preparation of glucagon that does not aggregate, does not chemically degrade, and remains bioactive for at least 3-7 days.
We have previously investigated the effects of curcumin, an active ingredient of turmeric, for its effects on glucagon aggregation, and demonstrated that curcumin reduced glucagon aggregation;18 however, due to its shorter half-life, it is not useful over prolonged periods of time. Although the presence of glucagon and human serum albumin (HSA) improves the half-life of curcumin, it still may not be effective enough over longer intervals. At neutral and basic pH, curcumin breaks down to simpler compounds such as ferulic acid and vanillic acid,19,20 as well as a possible polycyclic oxidation breakdown product.21 We previously reported that alkaline pH was effective in reducing glucagon aggregation.15 To further stabilize glucagon at alkaline pH, we became interested in the potential use of ferulic acid, which has been reported to minimize aggregation of the Alzheimer’s peptide, amyloid-beta.22,23
Ferulic acid is found in various foods and beverages,24-26 and is reported to have neuroprotective,27 antineoplastic,28,29 and antihypertensive30,31 actions due to its antioxidant properties. Due to its beneficial effects on inhibiting peptide aggregation, we investigated ferulic acid for its ability to stabilize glucagon. Here we report the results of several in vitro tests for degradation and aggregation, as well as results of an in vitro cell-based bioassay and in vivo experiments in octreotide-treated Yorkshire swine.
Materials and Methods
Reagents
Polysorbate-80 and sodium hydroxide were purchased from Thermo Fisher Scientific, Inc. (San Jose, CA). Ferulic acid, HSA, L-methionine, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (Saint Louis, MO). Native human glucagon was synthesized by AmbioPharm, Inc. (Beech Island, SC). The peptide was purified to >99% and shipped as a lyophilized solid. All reagents were of highest purity available.
Sample Preparation and Aging
A stock of glycine buffer was prepared and adjusted to pH 9 with sodium hydroxide. Stock solutions of HSA, L-methionine, ferulic acid, and polysorbate-80 were also prepared. A small concentration of DMSO (0.5% v/v) was used to assist in solubilizing the ferulic acid. An alkaline solution of ferulic acid (1 mM) was prepared in glycine buffer at pH 9. L-methionine (0.1 mg/ml) and polysorbate-80 (0.5% v/v) were added to the solution. The solution was then brought to the final volume, and glucagon (1 mg/ml) and HSA (1 mg/ml) were added, and passed through a 0.2-µm PVDF filter into an amber-colored glass vial, which was then sealed. All formulations were placed into a stationary 37°C incubator for the aging period. Unaged samples were immediately placed into a −20°C freezer. After aging, the samples were frozen at −20°C until analysis.
UV-Visible Kinetic Analysis
To assess the stability of ferulic acid and curcumin with and without glucagon and HSA, we carried out UV-visible kinetic degradation analysis with ferulic acid and curcumin with or without glucagon and HSA (all at 1 mg/ml). Spectral scanning revealed a peak absorbance of 320 nm for fresh ferulic acid and 425 nm for fresh curcumin. To quantify degradation, absorbance readings were taken at the peak absorbance every 10 minutes for 14 hours. Path-length-corrected absorbance readings were converted to ferulic acid and curcumin concentrations in moles/L using Beer’s law; degradation was found to be first-order. Half-life was determined using linear regression with the Microsoft Excel plug-in XLfit V. 5.3.1.3 (IDBS, Guildford, UK).
Transmission Electron Microscopy (TEM)
A 10-μl sample including 1 mg/ml glucagon with or without 1 mM ferulic acid and 1 mg/ml HSA prepared in glycine buffer at pH 9 was deposited on the top of activated 400-mesh copper grids (01822-F; Ted Pella Inc., Redding, CA). The sample was incubated for 2 minutes on the grid at room temperature and then excess solution was removed by gently wicking the edge of the grid with filter paper. The grid was then stained with freshly prepared 1.33% (w/v) uranyl acetate in water for 45 seconds, wicked with filter paper, air-dried, and imaged at 120 kV on a Tecnai™ Spirit TEM system (FEI, Hillsboro, OR). Images were collected as 2048 × 2048-pixel, 16-bit gray scale using FEI’s TEM Imaging & Analysis (TIA) interface on an Eagle™ 2K CCD multiscan camera. Quantification of protein aggregation using automated coverage analysis through electron microscopy was considered unfeasible to perform due to significant overlap of fibrils and background artifacts. Therefore, in this study, 3 different observers analyzed TEM images in a blinded fashion, grading each image as fibrils being either present or absent. To remove bias from the observations, excipient-only controls were also included in the blinded analysis.
High-Performance Liquid Chromatography (HPLC)
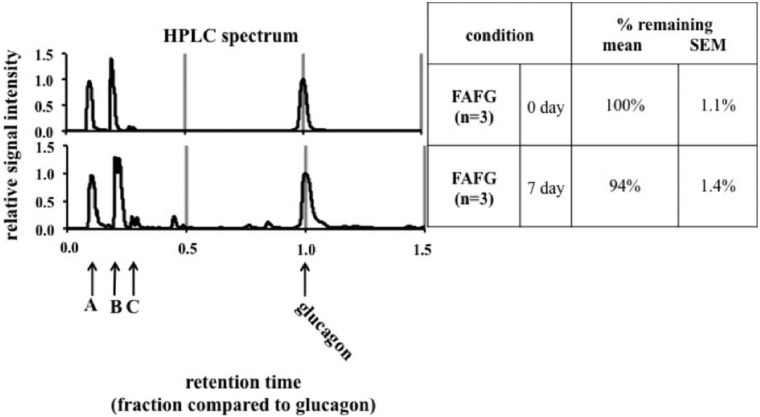
Glucagon degradation was analyzed by reverse-phase HPLC (Surveyor System, Thermo Fisher Scientific) using the method outlined by the United States Pharmacopeial Convention.32 To avoid degradation related to freeze-thaw cycles, we limited all experiments to 1 to 2 freeze-thaw cycles. Briefly, pump A buffer contained 16.3 g of monobasic potassium phosphate in 800 ml of water at pH 2.7, mixed with 200 ml of acetonitrile, while buffer B was a mixture of acetonitrile and water (4:6). A flow rate of 0.5 ml/minute was used with an ACE 3 C18 150x3-mm column (Mac Mod Analytical Inc, Chadds Ford, PA). An isocratic elution was utilized at 59% A/41% B followed by a period of column washing and reequilibration. The X-axis in Figure 3 represents relative retention time. Due to the sensitivity of the method to slight variations in the mobile phase, varied retention times were seen from runs performed on different days. To overcome this variability when analyzing the chromatograms, retention time was transformed to a relative retention time with the glucagon peak as the reference since there was a major glucagon peak in all chromatography performed. The percentage of glucagon remaining was determined by calculating area under the curve (AUC) for the glucagon peak and dividing it by total AUC across the chromatogram. Mean AUC for each unaged and aged FAFG sample was calculated by averaging AUC for glucagon peaks (n = 3). Percentage AUC for aged FAFG was compared to unaged FAFG as the standard (100%).
Figure 3.
HPLC according to United States Pharmacopeia methods. The figures show modifications of unaged and 7-day aged ferulic acid-formulated glucagon (FAFG). (A) injection peak; (B) ferulic acid; (C) a peak that has been identified as glucagon oxidation at Met27 through analysis of an oxidized Met27 glucagon standard. Y-axis represents relative signal intensity. X-axis represents relative retention time (see methods for details).
Fluorescence In Vitro Bioassay
An in vitro cell-based bioassay was used to assess bioactivity of glucagon, as described in Jackson et al.33 This assay utilizes Chinese hamster ovary (CHO)-K1 cells that stably overexpress the human glucagon receptor and also express an enhanced green fluorescent protein-protein kinase A catalytic subunit fusion protein (PKA-EGFP) reporter molecule. Activation of the glucagon receptor causes an increase in intracellular cAMP levels, resulting in release of the PKA catalytic subunit from the PKA regulatory subunit, dispersion of PKA-EGFP from punctate cellular structures, and a dose-dependent decrease in intracellular fluorescence.
Pharmacodynamic (PD) Analysis in Yorkshire Swine
The PD properties of unaged FAFG were compared to those of FAFG aged for 7 days at 37ºC and unaged synthetic human glucagon (all n = 8) in octreotide-treated Yorkshire swine. Overnight-fasted pigs were sedated using Telazol and Atropine, weighed, intubated, and maintained on 1.5-2.0% isoflurane and oxygen. An IV catheter was placed in a superficial abdominal wall vein (lateral epigastric or other, if available) for blood draws. Starting 40 minutes before the glucagon dose, a continuous infusion of octreotide (3 µg/kg /hr) was given to block the endogenous production of insulin and glucagon, mimicking T1D. Venous blood samples were drawn every 10 minutes for 3 hours of study period, and tested for glucose concentration using a Glucose 201 analyzer (HemoCue America, Brea, CA). After a 60-minute stabilization period under anesthesia, a subcutaneous injection of glucagon (2 mcg/kg) was given. This dose was chosen to represent the upper limit of what might be given to humans during treatment with a closed-loop system.
We attempted pharmacokinetic analysis of glucagon as well, although a substantial number of samples showed glucagon levels several orders of magnitude above pharmacologically expected levels thought to be due to contamination, making them uninterpretable. The glucagon injection sites were monitored and photographed to evaluate for any local reaction. In addition, pigs were observed for any systemic reaction to glucagon.
Statistical Methods
All results are presented as mean ± SEM or percentages as appropriate. Interobserver agreement on TEM analyses was calculated by Fleiss’s kappa statistic. Unless otherwise stated, statistical differences were determined by 2-tailed Student’s t test with significance of P < .05.
Results
Ferulic Acid Is More Stable Than Curcumin as Measured by UV-Visible Kinetics
We observed that ferulic acid, with or without HSA and glucagon, was substantially more stable than curcumin. That is, in contrast to curcumin, ferulic acid does not undergo spontaneous degradation (see Figure 1).
Figure 1.
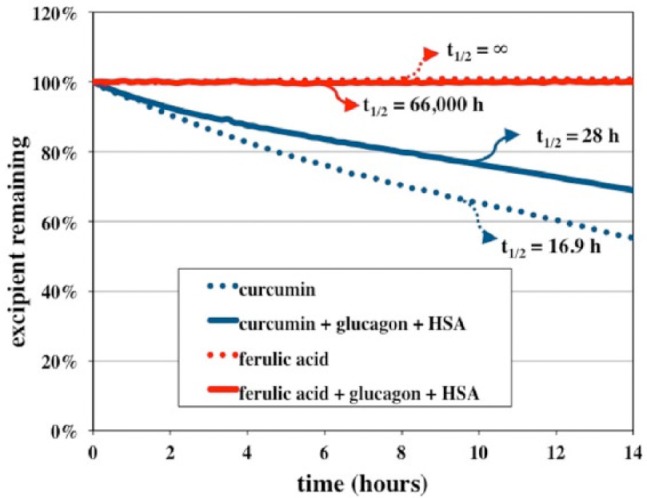
UV-visible kinetic analyses of ferulic acid and curcumin when analyzed alone or with the addition of glucagon (1 mg/ml) and HSA (1 mg/ml). This graph shows that ferulic acid is preserved with or without glucagon and HSA while curcumin degrades rapidly with or without glucagon and HSA.
Ferulic Acid Strongly Inhibits Glucagon Aggregation as Measured by TEM
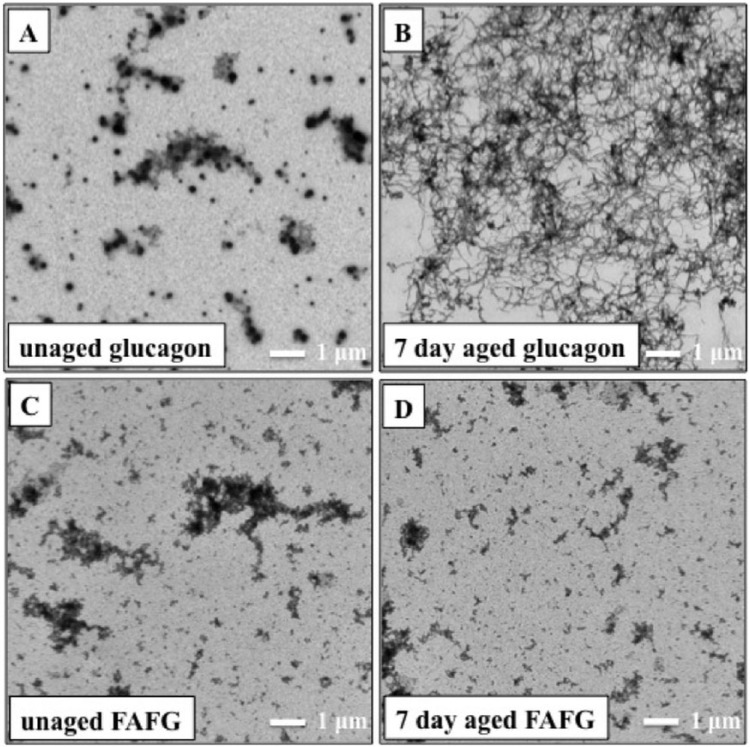
In the absence of ferulic acid, glucagon aged for 7 days at 37ºC formed long stranded fibril-like structures (see Figure 2B), similar to those found in acidic glucagon preparations.34 In the presence of ferulic acid, no fibrils were found in unaged (Figure 2C) or aged (Figure 2D) glucagon formulations. Fisher’s exact probability test was performed comparing all 4 samples. The results were highly significant in favor of ferulic acid (P < .001). In all formulations of glucagon, we observed background artifacts that were easily distinguishable from peptide fibrillation (Figures 2A-2D). Fleiss’s kappa value between all 3 observers was .99, which indicates almost perfect agreement between all 3 observers, with a Z score of 59.46.
Figure 2.
Transmission electron micrographs (4800× magnification) comparing (A) unaged glucagon, (B) 7-day aged glucagon, (C) unaged ferulic acid-formulated glucagon (FAFG), and (D) 7-day aged FAFG. Fibrils are absent in unaged glucagon but fibrils are clearly seen after 7 days of aging without any excipient. When glucagon is formulated in FAFG, no fibrils were seen in unaged or aged formulations. There were nonfibrillar artifacts of TEM analysis as seen in A, B, C, and D, identical to artifacts found by TEM analysis of non-glucagon-containing samples.
To analyze the effects of HSA on glucagon aggregation, we carried out TEM on aged and unaged glucagon samples with HSA alone (ie, the formulation was the same as the ferulic acid stabilized formulation but without the ferulic acid). In this formulation, we observed significantly greater fibril formation in 7 day aged glucagon compared to unaged glucagon (P < .001).
Ferulic Acid Maintains the Glucagon Peak After 7 Days of Aging at 37°C as Analyzed by HPLC
Figure 3 shows 94% ± 1.4% retention of glucagon in the 7-day aged FAFG formulation as compared to the fresh formulation.
In the PKA Bioassay, Ferulic Acid Helps Retain Aged Glucagon Potency
Figure 4 shows the dose response relationship between unaged and aged (37°C) FAFG. There were no significant differences (n = 7) in glucagon potency (0.5-fold increase in EC50) between the 2 samples.
Figure 4.
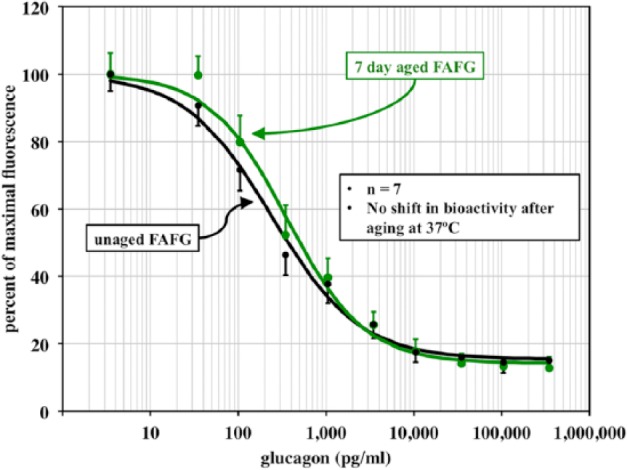
Cell-based bioassay dose-response curves for unaged glucagon and FAFG after aging for 7 days at 37°C. No loss of potency or power was observed with the aged vs unaged FAFG.
Ferulic Acid Preserves Glycemic Response to Glucagon After 7 Days of Aging in Yorkshire Pigs
All 3 formulations led to robust and similar glycemic responses to glucagon (Figure 5). For PD metrics, see Table 1. In some cases (early time to half maximal response), the FAFG formulation (fresh and aged) had slightly more rapid onset of action than the fresh synthetic glucagon. There were no noted injection site reactions in any of the animals.
Figure 5.
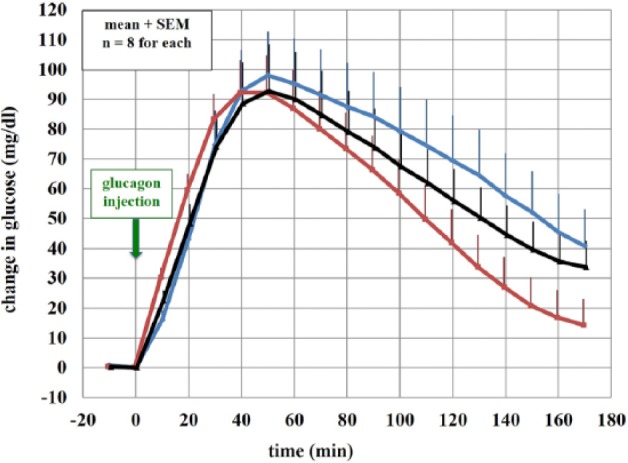
Rise in blood glucose from the baseline of fresh, no-excipient synthetic glucagon (blue), fresh FAFG (red), and 7-day aged FAFG (black) at 37º C.
Table 1.
Mean ± SEM Pharmacodynamic Data.
| Metric (n = 8) | Formulation |
|||
|---|---|---|---|---|
| No excipient glucagon 0d | FAFG 0d | FAFG 7d | ||
| Venous glucose (PD) | max Δ (mg/dl) | 99 ± 15 | 87 ± 18 | 89 ± 24 |
| early t50% (min) | 23 ± 1 | 17 ± 1* | 19 ± 1* | |
| tmax (min) | 51 ± 3 | 45 ± 5 | 48 ± 3 | |
| late t50% (min) | 144 ± 10 | 107 ± 14* | 130 ± 10 | |
| 60 min AUCinc (min*mg/dl) | 3667 ± 551 | 3571 ± 664 | 3444 ± 800 | |
| 90 min AUCinc (min*mg/dl) | 6323 ± 966 | 5632 ± 1231 | 5751 ± 1483 | |
| 160 min AUCinc (min*mg/dl) | 10 871 ± 1871 | 7805 ± 2570 | 9146 ± 263 | |
There were no significant differences between parameters with the exception of time to early and late half max between FAFG 0d and fresh, no-excipient synthetic glucagon.
P < .05 vs no excipient glucagon 0d.
Discussion
The potential importance of inhibiting glucagon aggregation has been recognized for several years, and several methods have been investigated to block aggregation with some success, but no published articles have demonstrated efficacy for inhibiting the aggregation while simultaneously preventing degradation (i.e., deamidation, oxidation, and isomerization) of glucagon.
It is difficult to develop a stable formulation of glucagon at physiologic pH because glucagon has an isoelectric point of about 7.35 In addition, we have shown that pH < 9 worsens glucagon aggregation15,18 and that pH > 9 favors glucagon degradation;17 therefore, we chose a working pH of 9 for the glucagon formulation. Although pH 9 helps prevent chemical degradation,17 it does not stop peptide aggregation.18
To be used as an excipient, it is very important for the compound to be stable. In the current study, we observed that ferulic acid is more stable and has a longer half-life compared to curcumin, and its half-life is unaffected by glucagon and HSA. Although unclear, it is possible that the beneficial effects of curcumin reported by Caputo et al.18 may be due to its breakdown product, ferulic acid.
Various methods have been proposed to assess glucagon aggregation, one of which is the Thioflavin T (ThT) fluorescence assay. Although it is a very sensitive assay, it is not specific,18 and therefore absorbance could be falsely elevated in the presence of soluble aggregates and sample contamination. Instead, we used TEM to evaluate glucagon fibrils. TEM is frequently used to characterize the microstructure of aggregates, with magnification up to 1 million times. Therefore, it is more specific than ThT for insoluble fibrils, as these can be easily visualized. No fibrils were observed in any of 120 images in unaged or aged FAFG samples Figures 2C and 2D, indicating inhibition of peptide aggregation by ferulic acid. However, it could be assumed that HSA (not ferulic acid) was a beneficial excipient in the FAFG formulation. Nonetheless, when we carried out TEM on glucagon samples with HSA alone, we identified fibrils; unlike the FAFG formulation, it did not completely block fibril formation.
HPLC showed that 94% of the glucagon parent peak was preserved after 7 days of aging when formulated as FAFG. The mechanism by which ferulic acid interacts with the peptide and inhibits aggregation is unclear. This chemical stability of FAFG presumably contributes to preservation of bioactivity. Figure 3 shows a very small glucagon oxidation peak in the chromatogram. This finding is consistent with what has been shown previously, that is, that some minor modifications do not produce any major potency shifts.17
PD properties in swine showed no significant difference in the maximal rise in venous blood glucose after glucagon injection when comparing aged FAFG with either unaged FAFG or unaged synthetic glucagon. Time to early half max was significantly different comparing unaged synthetic glucagon vs unaged FAFG with the latter being faster. It has been reported that ferulic acid has a vasodilatory effect31 and such an effect could increase the absorption speed of the formulation. Table 1 references all evaluated parameters comparing all 3 formulations.
A limitation of the current study is that FAFG was only aged for 7 days, so longer-term stability studies will be important. Prior to being used as a drug, it will be necessary to demonstrate long-term stability during storage and distribution at the intended temperature. Although no adverse events were observed in the swine, formal toxicology studies will be necessary before any human trials.
Conclusion
An alkaline formulation of glucagon with ferulic acid is very effective in reducing glucagon aggregation and degradation, even after 7 days of aging. In addition, it retains bioactivity in both cellular assays and in animal studies. This formulation may be sufficiently stable for its potential use in a bihormonal, closed-loop system. Formal toxicology and long-term stability studies are needed prior to human trials.
Acknowledgments
We thank Claudia López for her help with transmission electron microscopy and Deborah Branigan for her help with the swine study.
Footnotes
Abbreviations: AUCinc, incremental area under the curve; cAMP, cyclic adenosine monophosphate; CCD, charge coupled device; CHO, Chinese hamster ovary; DMSO, dimethyl sulfoxide; EGFP, enhanced green fluorescent protein; FAFG, ferulic acid-formulated glucagon; HPLC, high-performance liquid chromatography; HSA, human serum albumin; IACUC, Institutional Animal Care and Use Committee; max Δ, maximum change; PD, pharmacodynamics; PKA, protein kinase A; PVDF, polyvinylidene fluoride; TEM, transmission electron microscopy; t50%, half life; ThT, thioflavin T; TIA, TEM imaging and analysis; tmax, time to reach maximum concentration; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: WKW, JRC, PAB, and NC have filed provisional patent applications regarding methods of stabilizing glucagon. JRC has a financial interest in Pacific Diabetes Technologies, Inc, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the Juvenile Diabetes Research Foundation (grant 17-2012-15) and NIH (NIDDK) (grant K23DK090133).
References
- 1. Del Favero S, Bruttomesso D, Di Palma F, et al. First use of model predictive control in outpatient wearable artificial pancreas. Diabetes Care. 2014;37(5):1212-1215. [DOI] [PubMed] [Google Scholar]
- 2. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5): 1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013;36(7):1851-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buckingham BA, Cameron F, Calhoun P, et al. Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther. 2013;15(8):622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33(6):1282-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haidar A, Legault L, Dallaire M, et al. Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ. 2013;185:297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JB, Devries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014;16:131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen CB, Otzen D, Christiansen G, Rischel C. Glucagon amyloid-like fibril morphology is selected via morphology-dependent growth inhibition. Biochemistry. 2007;46(24):7314-7324. [DOI] [PubMed] [Google Scholar]
- 12. Beaven GH, Gratzer WB, Davies HG. Formation and structure of gels and fibrils from glucagon. Eur J Biochem. 1969;11(1):37-42. [DOI] [PubMed] [Google Scholar]
- 13. Onoue S, Ohshima K, Debari K, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloi-dogenic fibrils. Pharm Res. 2004;21(7):1274-1283. [DOI] [PubMed] [Google Scholar]
- 14. Pedersen JS. The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol. 2010;4(6):1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ward WK, Massoud RG, Szybala CJ, et al. In vitro and in vivo evaluation of native glucagon and glucagon analog (MAR-D28) during aging: lack of cytotoxicity and preservation of hyperglycemic effect. J Diabetes Sci Technol. 2010;4(6):1311-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joshi AB, Rus E, Kirsch LE. The degradation pathways of glucagon in acidic solutions. Int J Pharm. 2000;203(1-2):115-125. [DOI] [PubMed] [Google Scholar]
- 17. Caputo N, Castle JR, Bergstrom CP, et al. Mechanisms of glucagon degradation at alkaline pH. Peptides. 2013;45:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caputo N, Jackson MA, Castle JR, et al. Biochemical stabilization of glucagon at alkaline pH [published online ahead of print June 26, 2014]. Diabetes Technol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen L, Ji H-F. Low stability remedies the low bioavailability of curcumin. Trends Mol Med. 2012;18(7):363-364. [Google Scholar]
- 20. Wang YJ, Pan MH, Cheng AL, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15(12):1867-1876. [DOI] [PubMed] [Google Scholar]
- 21. Gordon ON, Schneider C. Vanillin and ferulic acid: not the major degradation products of curcumin. Trends Mol Med. 2012;18(7):361-363; author reply 3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ono K, Hirohata M, Yamada M. Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem Biophys Res Commun. 2005;336(2):444-449. [DOI] [PubMed] [Google Scholar]
- 23. Sgarbossa A, Monti S, Lenci F, Bramanti E, Bizzarri R, Barone V. The effects of ferulic acid on beta-amyloid fibrillar structures investigated through experimental and computation-nal techniques. Biochimica et biophysica acta. 2013;1830(4):2924-2937. [DOI] [PubMed] [Google Scholar]
- 24. D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R. Polyphenols, dietary sources and bioavailability. Annali dell’Istituto superiore di sanita. 2007;43(4):348-361. [PubMed] [Google Scholar]
- 25. Rechner AR, Pannala AS, Rice-Evans CA. Caffeic acid derivatives in artichoke extract are metabolised to phenolic acids in vivo. Free Radic Res. 2001;35(2):195-202. [DOI] [PubMed] [Google Scholar]
- 26. Bourne LC, Rice-Evans C. Bioavailability of ferulic acid. Biochem Biophys Res Commun. 1998;253(2):222-227. [DOI] [PubMed] [Google Scholar]
- 27. Mancuso C, Santangelo R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem Toxicol. 2014;65C:185-195. [DOI] [PubMed] [Google Scholar]
- 28. Janicke B, Hegardt C, Krogh M, et al. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr Cancer. 2011;63(4):611-622. [DOI] [PubMed] [Google Scholar]
- 29. Jayaprakasam B, Vanisree M, Zhang Y, Dewitt DL, Nair MG. Impact of alkyl esters of caffeic and ferulic acids on tumor cell proliferation, cyclooxygenase enzyme, and lipid peroxidation. Journal of agricultural and food chemistry. J Agric Food Chem. 2006;54(15):5375-5381. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki A, Kagawa D, Fujii A, Ochiai R, Tokimitsu I, Saito I. Short- and long-term effects of ferulic acid on blood pressure in spontaneously hypertensive rats. Am J Hypertens. 2002;15(4 pt 1):351-357. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki A, Yamamoto M, Jokura H, et al. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontane-ously hypertensive rats. Am J Hypertens. 2007;20(5):508-513. [DOI] [PubMed] [Google Scholar]
- 32. USP Organization; United States Pharmacopoeia (USP 36-NF 31). May 1, 2013:3741-2. [Google Scholar]
- 33. Jackson M, Stonex T, Caputo N, et al. Development of a stable formulation of liquid glucagon for use in a bihormonal pump. Paper presented at: 2012 Scientific Session, American Diabetes Association; Philadelphia, PA. [Google Scholar]
- 34. Pedersen JS, Dikov D, Flink JL, Hjuler HA, Christiansen G, Otzen DE. The changing face of glucagon fibrillation: structural polymorphism and conformational imprinting. J Mol Biol. 2006;355(3):501-523. [DOI] [PubMed] [Google Scholar]
- 35. Chabenne JR, DiMarchi MA, Gelfanov VM, DiMarchi RD. Optimization of the native glucagon sequence for medicinal purposes. J Diabetes Sci Technol. 2010;4(6):1322-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]