Abstract
Background:
Accurate assessment of diabetes polyneuropathy (DPN) is important in the prevention of foot ulcerations and amputations. Simple screening methods including the 10 g monofilament and the 128-Hz tuning fork are not sensitive enough nor intended for detection of early neuropathy, while more confirmatory tests such as nerve conduction studies are not universally available. We evaluated a rapid, low-cost, point-of-care nerve conduction device (POCD; NC-stat®|DPNCheck™) for the assessment of DPN and compared it with the LDIFLARE technique—an established method for early detection of small fibre dysfunction.
Methods:
A total of 162 patients with diabetes (DM) and 80 healthy controls (HC) were recruited. Based on the 10-point Neuropathy Disability Score (NDS), DPN was categorized into none (<2), mild (3-5) moderate (6-7), and severe (8-10). The LDIFLARE was performed in all patients according to previously described methodology. The associations between POCD outcomes and the LDIFLARE within the NDS categories were evaluated using regression analysis.
Results:
In HC and DM, SNCV measured with the POCD correlated significantly with the LDIFLARE technique (r < 0.90 and r = 0.78, respectively) as did SNAP (r = 0.88 and r = 0.73, respectively); in addition, significance was found in all categories of DPN (r = 0.64 to 0.84; p= ≤ 0.03). ROC curves within each category of DPN showed that the POCD was sensitive in the assessment of DPN.
Conclusion:
We report highly significant linear relationships between the POCD with both comparators—the LDIFLARE technique and clinical neuropathy scores. Thus, the NC-stat|DPNCheck™ system appears to be an excellent adjunctive diagnostic tool for diagnosing DPN in the clinical setting.
Keywords: diabetes neuropathy, early detection, laser doppler imaging, point-of-care device, sural nerve conduction studies
The worldwide prevalence of diabetes mellitus is approximately 382 million and is expected to rise to 592 million by 2035.1 Based on current published data, at least 50%, approximately 300 million, will develop some form of neuropathy, of which diabetes polyneuropathy (DPN) is by far the most common.2,3 DPN, even in asymptomatic patients, can lead to considerable morbidity in the form of foot infection, ulceration and amputation with associated health-care costs.4,5 Hence it is important to detect DPN at its earliest clinical stage so that appropriate preventive measures including patient education and regular surveillance can be initiated to prevent foot disease.6
Of the various methods used to screen for DPN, the most common are the 10 gm Semmes-Wenstein monofilament (MF) and the 128 Hz vibration tuning fork.7 Both can detect patients with established neuropathy and importantly those “at risk” of foot disease. In several large population studies they have been shown to be good predictors of those who later developed food ulceration.8,9 However, they only detect patients with late stage neuropathy. Conventional nerve conduction studies (NCS) remain the best methods for detecting those with early or atypical DPN.10 However, they require referral to specialized neurological laboratories. Their use in screening is neither practical nor possible given the large numbers of patients with the condition and the limited number of specialized centers.11 Thus, the diagnosis of DPN in the majority of patients is based on the clinician’s interpretation of the patient’s symptoms and the bedside clinical examination.
A point-of-care device (POCD) that measures NCS quickly and accurately at the bedside that can be performed by nontechnical personnel with basic training would be of great value in confirming the clinical suspicion of DPN and would be of value in research studies of large populations where formal NCS are not possible. The NC-stat|DPNCheck system (Neurometrix, Waltham, MA) has been developed to fulfill this need; it is a handheld, rapid, low-cost, point-of-care test for the assessment of DPN. It measures sural nerve conduction velocity (SNCV) and sensory nerve action potential (SNAP) amplitude. A previous study demonstrated that it has excellent correlations with conventional NCS and a smaller study of 72 diabetes patients showed reasonable accuracy in diagnosing DPN.12-14 This is the first study to use this POCD, to measure sural nerve amplitude and conduction velocity across a diverse group of diabetes patients to determine how these relate to the severity of DPN.
The laser Doppler (LDI) FLARE technique has been demonstrated in several studies to be a very sensitive and specific method for detecting early small fibre neuropathy in diabetes. It measures the axon-reflex-mediated neurogenic flare after heating the foot skin.15 It has been shown to have an excellent correlation with intraepidermal nerve fibre density and to detect C-fibre dysfunction in early diabetes and in IGT patients when other tests of neuropathy are normal.16,17
The spectrum of DPN includes involvement of large-diameter, myelinated (Aα and Aβ) and small-diameter myelinated (Aδ) and unmyelinated C fibres in varying proportions. Recent studies have suggested that there may be a temporal relationship in which small fibre dysfunction precedes large fibre involvement; hence methods such as the LDIFLARE technique (see below) may be useful in its early detection.18-20 However, some researchers have reported early changes in electrophysiological measures of peroneal and sural nerve action potentials can be predictive of DPN even in the absence of any clinical neuropathy.21,22 DPN is categorized into various stages by using clinical neuropathy scores such as the Neuropathy Disability Score (NDS) and the modified Toronto Clinical neuropathy score (mTCNS).2,23 These are undoubtedly useful when stratifying severity of disease but lack the sensitivity to detect early or subclinical DPN. The POCD used in this study measures large fibre neural function. The aim was to determine whether it is sensitive enough to distinguish between different severities of clinical DPN assessed using the NDS. Furthermore, we aimed to determine the relationship between large fibre function measured by the POCD and small nerve fibre function assessed by the LDIFLARE technique in both people with diabetes and without diabetes.
Methods
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the NRES Committee East of England, Norfolk, UK (REC reference: 13/EE/0162). All patients provided a written informed consent.
Study Population
A total of 162 patients—80 with type 1 and 82 with type 2 diabetes—were accrued from the Diabetes outpatient clinics at Ipswich hospital, a secondary care referral center in Suffolk, UK. A total of 80 healthy controls (HC) were also recruited by invitation from our institutional staff and through local press.
Inclusion Criteria
Diabetes subjects aged between 18 and 80 years of age and defined as per criteria by the American Diabetes Association were considered for this study. To eliminate selection bias, potential patients were randomly identified from our database and thereafter invited to take part in the study which was conducted prospectively. Prior to the study, there was no attempt to alter their treatment apart from usual standards of care and none of them were part of any other diabetes trial study.
Exclusion Criteria
Subjects with history of minor or major amputation, limb deformities, open ulcers, or injuries to legs and those unable to give consent were excluded.
Clinical Neurological Assessment
All subjects underwent detailed neurological examination using the modified Neurology Disability Score (NDS).2 The reason for choosing NDS over other composite scores was its practicality; being easily performed in the clinic setting and taking only around 2 minutes. It is based on 4 criteria: vibration perception threshold, temperature perception, pin-prick and Achilles reflex. The maximum deficit score is 10, which would indicate complete loss of sensation to all sensory modalities and absent reflexes. Based on the NDS score, patients were categorized into 4 groups of severity, namely no DPN (NDS score 0-2), mild DPN (3-5), moderate DPN (6-8), and severe DPN (9-10). In a longitudinal European community-based study, an NDS of ≥ 6 was equated with a 2.32 relative risk of insensate foot ulceration.24
Point-of-care Nerve Conduction Device—NC-stat|DPNCheck System
This POCD is an automated electrophysiological device that operates on the same principles as conventional nerve conduction devices. It is a hand-held instrument which is applied to the skin posterior to the lateral malleolus of the testing leg in the area which overlies the distribution of the sural nerve. It comprises of a single-use biosensor pad which is applied to the skin surface and 2 metal stimulating probes placed 9.22 cm from the biosensor which discharge a 100 mA current to be detected orthodromically by the biosensor. The device has an inbuilt infrared thermometer within the probes that automatically corrects for skin temperature between 23 and 30°C. There is a LCD panel for readouts and a port for connection for optional PC software that can be used for hard copy retrieval and electronic medical record archiving. Recent studies have shown that this point-of-care system demonstrates excellent reliability and acceptable accuracy.14,25 The device was operated as per procedure already described and does not need calibration apart from change of battery source.19
However, it must be highlighted that this portable hand-held system has minor technical limitations. First, the device is not powered to detect sensory nerve amplitude potentials <1.5 µV and automatically calibrates them as zero; thus for example a reading of 1.3 µV would be registered as ‘zero’. In this study all such zero results were recorded as 1.5 µV as the lowest reading.13 In patients where a device error prevented a reading in the first attempt, the testing protocol was repeated. If a second error was also observed, then an additional attempt was allowed. The test was unsuccessful in only a small number of patients—4 diabetes patients (2. 7%) and 2 HC’s (2.5%). To eliminate observer bias, these patients were excluded since it is not possible to differentiate between those with absent readings due to either severe DPN or due to anatomical absence of the sural nerve. Second, compared to the antidromic stimulation of conventional nerve conduction devices, it stimulates the sural nerve orthodromically which theoretically can lower sensory amplitude potential. Third, since it is arbitrarily placed on the assumed anatomical location of the sural nerve, there is a potential for error due to repeated stimulation of the nerve till a valid response is detected by the biosensor; this however is dependent on the expertise of the operator. These limitations are overcome by configuring the electrode spacing, fixed conduction distance and filter settings, all of which maximize amplitude and improve signal to noise ratio.
The LDIFLARE Technique
The LDIFLARE methodology involves sequential heating of the dorsal foot skin to 47°C for 6 minutes. The resultant axon reflex mediated hyperaemic response (“flare”) is measured quantitatively using a 630 nm mono wave laser scanner (moor LDI1 laser Doppler imager™, Moor Instruments, Exeter, UK), which generates a map of the flare area the size of which is measured in cm2.26 We have shown that LDIFLARE technique is sensitive in both type 1 and type 2 diabetes in diagnosing early small fibre neuropathy and can also detect incipient neuropathy in impaired glucose tolerant subjects.17 The LDI1 laser Doppler machine is calibrated according to industry standards set by the manufacturer.
In all participants, the above assessments were undertaken by masked observers to eliminate bias. The POCD assessment was performed prior to the LDIFLARE technique since the latter entails warming the foot to achieve a skin temperature of at least 30°C prior to applying the heating probe.
Statistical Analysis
Estimating the prevalence of DPN in unselected diabetes population to be 45%, our power calculations demonstrated that we needed at least 75 subjects in each category of both type 1 and type 2 diabetes as well as an equal number of HCs.
Statistical analysis was performed using SPSS version 22 for Windows (IBM Corp, Armonk, NY) and StatsDirect version 3 (StatsDirect, Cheshire, UK). Clinical characteristics in nominal values were expressed as mean ± SD. Difference in categorical variables including nerve conduction parameters and LDIFLARE outcomes were assessed using the paired-samples t test while differences in continuous variables were assessed using ANOVA. Linear associations between POCD parameters and LDIFLARE outcomes were assessed using linear regression and correlation methods, and we report both linear regression and the correlation coefficients. Receiver operating characteristic (ROC) curves were used to calculate the sensitivity and specificity of the POCD in different clinical stages of DPN. Youden’s index was calculated as equal to (sensitivity + specificity) – 1. The CVs for LDIFLARE were conservatively estimated at 20% (actual CVs for LDIFLARE 8.7%)
Results
Clinical characteristics of all study subjects are shown in Table 1. Both HC and diabetes cohort (DM) were similar in age (p = 0.22) and sex (p = 0.57). Compared to HC, DM were significantly overweight (p = 0.004) and had significantly higher systolic (p < 0.0001) and diastolic blood pressure (p < 0.0001). The LDIFLARE was significantly smaller in DM when compared to HC (5.81 ± 2.09 vs 9.11 ± 2.17 cm2; p < 0.0001), and similarly both SNAP (42.04 ± 9.11 vs 50.22 ± 5.69 m/s; p < 0.0001) and SNCV (10.13 ± 3.12 vs 18.49 ± 4.13 µV; p < 0.0001) were significantly lower in the DM cohort in comparison to HC.
Table 1.
Clinical Characteristics of All Study Subjects Including Healthy Controls (HC) and Diabetes Subjects (DM).
| Characteristic | Healthy controls (HC) | Diabetes group (DM) | p for DM vs HC | p for LDIFLARE vs POCD in HC (DM) |
|---|---|---|---|---|
| N | 80 | 162 | — | — |
| Age (years) | 39.67 ± 15.17 | 47.96 ± 13.98 | 0.22 | — |
| Female/male sex | 38/42 | 70/92 | 0.57 | — |
| Duration of diabetes (years) | - | 11.4 ± 9.4 | — | — |
| BMI (kg/m2) | 26.07 ± 4.37 | 29.26 ± 3.45 | 0.004 | — |
| Systolic blood pressure (mm Hg) | 118 ± 19 | 133 ± 15 | <0.0001 | — |
| Diastolic blood pressure (mm Hg) | 67 ± 6 | 83 ± 11 | <0.0001 | — |
| Vibration perception threshold (mV) | 6.08 ± 2.09 | 19.4 + 11.1 | <0.0001 | — |
| LDIflare (cm2) | 9.11 ± 2.17 | 5.81 ± 2.09 | <0.0001 | — |
| POCD outcomes | ||||
| Sural nerve conduction velocity (SNCV, m/s) | 50.22 ± 5.69 | 42.04 ± 9.11 | <0.0001 | <0.0001 (0.002) |
| Sural nerve amplitude potential (SNAP, µV) | 18.49 ± 4.13 | 10.13 ± 3.12 | <0.0001 | <0.0001 (0.003) |
BMI, body mass index; LDIflare, laser Doppler imager flare; N, number of patients; POCD, point-of-care device; SNAP, sural nerve amplitude potential; SNCV, sural nerve conduction velocity. Significance = p < .05.
Table 2 depicts the distribution of results according to the severity of DPN as indicated by NDS (none, mild, moderate, and severe). Using multiple regression analysis, age, sex, and systolic blood pressure were not associated with progression of severity of DPN. In contrast, longer duration of diabetes (p = 0.03), higher BMI (p = 0.008), and higher diastolic blood pressure (p = 0.001) were associated with greater severity of DPN. Furthermore, with progression to higher NDS scores, LDIFLARE size was significantly smaller (p < 0.0001), and similarly both SNCV and SNAP were significantly lower (p < 0.0001 for both).
Table 2.
Distribution of POCD Outcomes in Various Severities of DPN as Categorized by NDS and Their Correlations to LDIFLARE in Each Stage of DPN: Correlation Between POCD Outcomes and LDIFLARE in Healthy Controls (HC).
| Characteristic | HC | Categorization of diabetes patients according to severity of DPN |
Significance with increasing severity of DPN (p) | ||||
|---|---|---|---|---|---|---|---|
| No DPN (NDS 0-2) | Mild DPN (NDS 3-5) | Moderate DPN (NDS 6-8) | Severe DPN (NDS 9-10) | ||||
| N | 80 | 60 | 38 | 46 | 18 | 0.47 | |
| Age (years) | 39.67 ± 15.17 | 42.97 ± 15.21 | 54.60 ± 15.03 | 54.25 ± 6.59 | 58.23 ± 8.98 | 0.06 | |
| Female/male sex | 38/42 | 28/32 | 15/23 | 24/22 | 7/11 | 0.12 | |
| Duration of diabetes (years) | — | 9.8 ± 5.9 | 8.8 ± 7.8 | 12.2 ± 8.7 | 14.6 ± 11.6 | 0.03 | |
| BMI (kg/m2) | 26.07 ± 4.37 | 27.54 ± 4.57 | 27.22 ± 6.68 | 34.98 ± 5.69 | 36.88 ± 4.77 | 0.008 | |
| SBP (mm Hg) | 118 ± 19 | 133 ± 6 | 132 ± 11 | 136 ± 15 | 141 ± 11 | 0.43 | |
| DBP (mm Hg) | 67 ± 6 | 80 ± 8 | 82 ± 13 | 85 ± 6 | 93 ± 6 | 0.001 | |
| Vibration perception threshold (mV) | 6.08 ± 2.09 | 14.22 ± 4.39 | 18.89 ± 6.67 | 24.33 ± 4.45 | 29.54.08 ± 7.07 | 0.009 | |
| LDIflare (cm2) | 9.11 ± 2.17 | p=<0.0001* | 7.52 ± 2.59 | 5.98 ± 2.07 | 5.01 ± 0.69 | 3.25 ± 1.12 | <0.0001 |
| POCD outcomes | |||||||
| Sural nerve conduction velocity (SNCV, m/s) | 50.22 ± 5.69 | p=0.11* | 48.95 ± 12.70 | 40.16 ± 8.43 | 32.16 ± 7.74 | 26.49 ± 8.07 | <0.0001 |
| Sural nerve amplitude potential (SNAP, µV) | 18.49 ± 4.13 | p=0.15* | 16.61 ± 8.45 | 11.32 ± 5.16 | 6.52 ± 3.84 | 2.87 ± 1.91 | <0.0001 |
BMI, body mass index; DBP, diastolic blood pressure; DPN, diabetes polyneuropathy; LDIflare, laser Doppler imager flare; N, number of patients; NDS, Neuropathy Disability Score; POCD, point-of-care device; SBP, systolic blood pressure. Significance = p < 0.05.
Significance between HC and DPN (0-2) only.
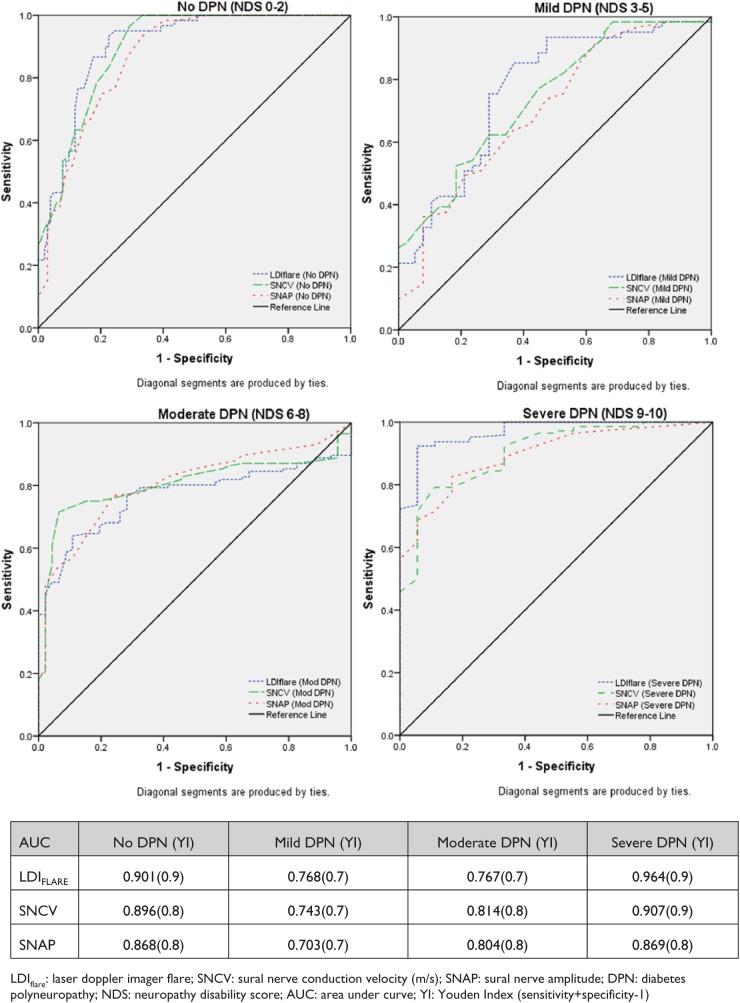
Figure 1 show the ROC curves and Youden’s Index for each of the 3 assessments, namely the LDIFLARE and POCD outcomes of SNCV and SNAP within each the NDS severity groups. The area under each of the curves suggests that irrespective of the stage of neuropathy, the POCD can detect the presence of neuropathy with high sensitivity and specificity.
Figure 1.
ROC curves for LDIFLARES, SNCV, and SNAP measured by the POCD in different grades of severity of DPN as categorized by NDS. DPN, diabetes polyneuropathy; LDI, laser Doppler imaging; NDS, Neuropathy Disability Score; POCD, point-of-care device; ROC, receiver operating characteristic; SNAP, sural nerve amplitude potential; SNCV, sural nerve conduction velocity.
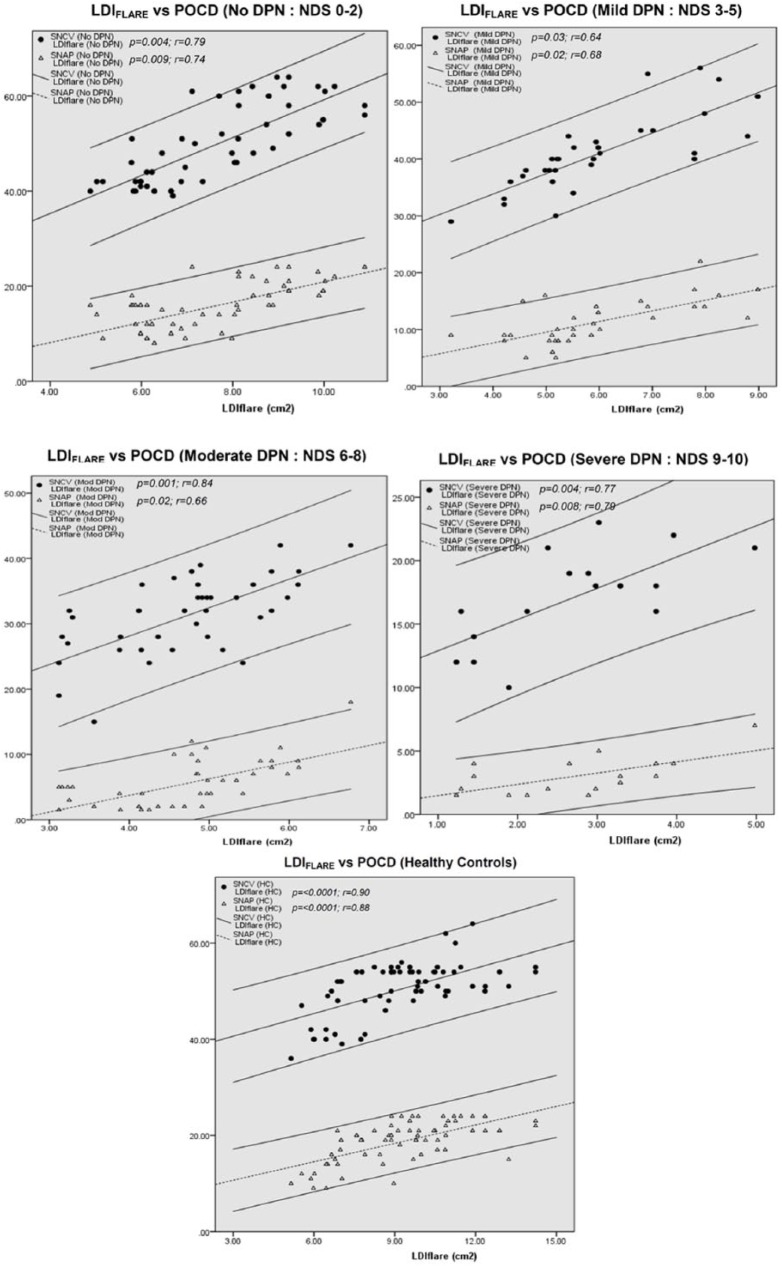
Figure 2 depicts the correlation between the LDIFLARE outcome and the POCD outcomes in various stages of neuropathy. In the absence of DPN (NDS 0-2), both SNCV (p = 0.004) and SNAP (p = 0.009) correlated significantly with LDIFLARE, and the same could also be seen in mild, moderate, and severe DPN groups. Interestingly, in HC, there was a significantly strong correlation between the LDIFLARE and POCD outcomes (SNCV: p < 0.0001 and SNAP: p < 0.0001) since they are all inversely correlated with age (SNCV: p < 0.0001, SNAP: p < 0.0001, and LDIFLARE: p < 0.0001) as shown in Figure 3.
Figure 2.
Correlation between the LDIFLARE size and the POCD measurements in various stages of DPN and HC. DPN, diabetes polyneuropathy; HC, healthy controls; LDI, laser Doppler imaging; POCD, point-of-care device.
Figure 3.
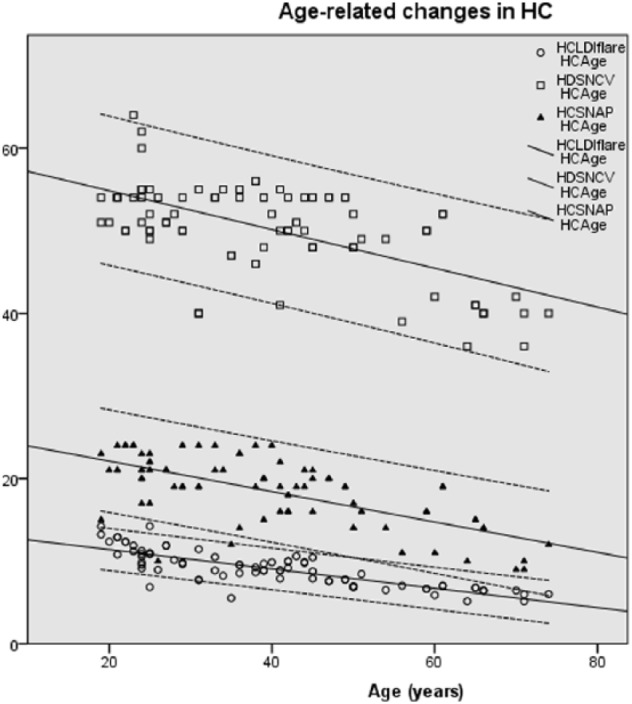
Highly significant inverse correlations between the age and LDIFLARE size and the POCD measured SNCV and SNAP. LDI, laser Doppler imaging; POCD, point-of-care device; SNAP, sural nerve amplitude potential; SNCV, sural nerve conduction velocity.
Discussion
The gold standard for confirming DPN is an abnormal nerve conduction test performed in an established and accredited neurological center.10 However, as this is not feasible in all patients with diabetes, such tests remain either confirmatory or research tools. The POCD evaluated in this study being handheld, portable, and quick to perform enables bedside measurement, thus extending NCS to a larger group of people with diabetes. It has previously been shown to provide accurate neurophysiological assessments with minimal personnel training.13
Ours is the first study to evaluate the device in a large cohort of diabetes subjects with different severities of DPN together with 80 healthy subjects. All subjects were clinically evaluated using the well-established NDS clinical neuropathy score. We assessed the sensitivity and specificity of the device in determining SNCV and SNAP within each of the severity groups and found that the POCD consistently provided a high sensitivity and specificity throughout all clinical stages of DPN.
The NDS, though an extremely valuable clinical tool, is open to subjective differences in perception, for example different patients may perceive a painful stimulus differently and their perception may also be influenced by the degree of their attention.27 In addition, interobserver variation in eliciting of ankle deep tendon reflexes is another variable which can also hinder prospective follow up when more than 1 individual is involved in determining the DPN. The POCD in comparison has been shown to have a low interobserver variability and provides an objective measurement that can complement the NDS or be used on its own. The latter is relevant in those diabetic subjects with painful feet in whom there are no clinical signs (NDS 0), where a diagnosis of painful neuropathy may be supported by the use of this device. The same benefit may also arise in patients with very early neuropathy where the NDS may be normal (0-2). Finally, the relatively less precise NDS with its inherent subjective variability may not demonstrate progression of DPN for several years, whilst the POCD is more likely to detect such progression with the added advantage over conventional NCS of its use in the outpatient setting.
There is increasing evidence that small fibre damage precedes large fibre neuronal pathology in both type 1 and type 2 diabetes.20,28,29 For this reason, there is increasing interest in techniques which can precisely measure small fibre function and/or structure.29 The former include the LDIFLARE technique and quantitative sensory thresholds of warm and cold perception; and the structural methods include in vivo corneal confocal microscopy and IENFD.20 In this study, we used the LDIFLARE technique in the same cohort of patients to determine whether there is a correlation between small and large fibre function across the whole group as well as within the individual groups. We found that both SNCV and SNAP are significantly correlated with LDIFLARE sizes with increasing severity of DPN (p < 0.0001). Of interest, there was a good correlation between the POCD parameters and the LDIFLARE sizes in all the diabetic groups including those without clinical neuropathy (NCS 0-2) suggesting that the device will be helpful in assessing progression in individuals even at the early stages of neuropathy. It is of interest that in the HC, the POCD showed an age-related decline in nerve function similar to that found with the LDIFLARE which explains their good correlation in this group. This age-related decline in nerve conduction velocity and amplitude is well described as is the decline in LDIFLARE size.30-32
However, it should be noted that although there was a highly significant difference (p < 0.0001) in the LDIFLARE sizes between the HC and the diabetic group without clinical neuropathy (NDS 0-2), no such significance difference for SNCV and SNAP was seen between the same groups, that is, HC and diabetic group with NDS 0-2 (p = 0.11 and .15, respectively). This supports the increasing evidence that small fibre dysfunction precedes large fibre neuropathy in diabetes.
It should be noted that despite these positive findings, the NC-stat|DPNCheck™ is not without its limitations. The device is dependent on the presence of an accessible sural nerve which can be anatomically absent in up to 9% of healthy subjects.33,34 In our study, as explained in the methods, to eliminate observer bias, all subjects who had undetectable read-outs were excluded and those as “zero” were assigned as 1.5 µV since this is the lowest reading possible by the POCD. These are obvious shortcomings but of the 162 subjects we had only 4 diabetes patients (2.7%) and 2 HC (2.5%) in whom the sural nerve could not be recorded. Incidentally, all 4 diabetes subjects who were excluded due to unrecordable POCD measures had DPN 0-2, and hence it can be assumed that the inability to obtain readings is probably due to variance in the anatomy of the sural nerve. However, in subjects who present clinically with symptoms and signs of severe DPN, if electro-physiological confirmation is required, conventional NCS in a neurophysiology laboratory would be necessary if the POCD gives a “zero” reading as it is not possible to differentiate a pathologically absent sural nerve response from an anatomical variant. In our study, none of the subjects found the test procedure any more than mildly discomforting.
Conclusions
To conclude, we report a POCD that has good sensitivity and specificity at the various stages of DPN and correlates well with the LDIFLARE technique. These findings suggest that the device has considerable potential for assessing DPN in diabetes clinics. Furthermore, it may have substantial value in large population-based research where time may be critical factor and therefore a rapid, objective, and sensitive method is required. This may be particularly the case in prospective studies in rural settings.
Acknowledgments
The authors would like to thank Neurometrix Inc for providing the point-of-care devices used in this study.
Footnotes
Abbreviations: DM, diabetes mellitus; DPN, diabetes polyneuropathy; LDI, laser Doppler imaging; NDS, Neuropathy Disability Score; POCD, point-of-care device; ROC, receiver operating characteristic; SNAP, sural nerve amplitude potential; SNCV, sural nerve conduction velocity.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was self-funded by the Diabetes Research Unit, The Ipswich Hospital NHS Trust.
References
- 1. Federation ID. IDF Diabetes Atlas. 2013. Available at: http://www.idf.org/diabetesatlas. Accessed March 12, 2014.
- 2. Boulton AJ. Management of diabetic peripheral neuropathy. Clin Diabetes. 2005;23(1):9-15. [Google Scholar]
- 3. Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817-824. [DOI] [PubMed] [Google Scholar]
- 4. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513-521. [DOI] [PubMed] [Google Scholar]
- 5. Bokan V. Risk factors for diabetic foot ulceration-foot deformity and neuropathy. Acta Medica Medianae. 2010;49:19-22. [Google Scholar]
- 6. Kumar S, Ashe HA, Parnell LN, et al. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994;11(5):480-484. [DOI] [PubMed] [Google Scholar]
- 7. Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660-663. [DOI] [PubMed] [Google Scholar]
- 8. Feng Y, Schlosser FJ, Sumpio BE. The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50(3):675-682, 82e1. [DOI] [PubMed] [Google Scholar]
- 9. Perkins BA, Olaleye D, Zinman B, Bril V. Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250-256. [DOI] [PubMed] [Google Scholar]
- 10. England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199-207. [DOI] [PubMed] [Google Scholar]
- 11. Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480-1481. [DOI] [PubMed] [Google Scholar]
- 12. Perkins BA, Grewal J, Ng E, Ngo M, Bril V. Validation of a novel point-of-care nerve conduction device for the detection of diabetic sensorimotor polyneuropathy. Diabetes Care. 2006;29(9):2023-2027. [DOI] [PubMed] [Google Scholar]
- 13. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy. PLOS ONE. 2014;9(1):e86515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perkins BA, Orszag A, Grewal J, Ng E, Ngo M, Bril V. Multi-site testing with a point-of-care nerve conduction device can be used in an algorithm to diagnose diabetic sensorimotor polyneuropathy. Diabetes Care. 2008;31(3):522-524. [DOI] [PubMed] [Google Scholar]
- 15. Krishnan STM, Rayman G. The axon-reflex flare assessed by the laser Doppler Imaging (the “LDIflare”) demonstrates early neuropathy in type 2 diabetes. Diabetes. 2004;53:A211-A. [Google Scholar]
- 16. Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fibre density in patients with painful diabetic neuropathy. Diabetes Care. 2009;32(3):451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green AQ, Rayman G. Impaired glucose tolerance is associated with a physiological defect in small fibre function. Diabetes. 2007;56:A210-A. [Google Scholar]
- 18. Loseth S, Stalberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255(8):1197-1202. [DOI] [PubMed] [Google Scholar]
- 19. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fibre density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35(5):591-598. [DOI] [PubMed] [Google Scholar]
- 20. Breiner A, Lovblom LE, Perkins BA, Bril V. Does the prevailing hypothesis that small-fibre dysfunction precedes large-fibre dysfunction apply to type 1 diabetic patients? Diabetes Care. 2014;37:1418-1424. [DOI] [PubMed] [Google Scholar]
- 21. Hyllienmark L, Alstrand N, Jonsson B, Ludvigsson J, Cooray G, Wahlberg-Topp J. Early electrophysiological abnormalities and clinical neuropathy: a prospective study in patients with type 1 diabetes. Diabetes Care. 2013;36(10):3187-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perkins BA, Ngo M, Bril V. Symmetry of nerve conduction studies in different stages of diabetic polyneuropathy. Muscle Nerve. 2002;25(2):212-217. [DOI] [PubMed] [Google Scholar]
- 23. Bril V, Tomioka S, Buchanan RA, Perkins BA, m TSG. Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med. 2009;26(3):240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbott CA, Carrington AL, Ashe H, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377-384. [DOI] [PubMed] [Google Scholar]
- 25. Neurometrix. NC-stat DPNcheck Normative Database: Collection, Analysis and Recommended Normal Limits. 2013. [Google Scholar]
- 26. Vas PR, Rayman G. Validation of the modified LDIFlare technique: a simple and quick method to assess C-fibre function. Muscle Nerve. 2013;47(3):351-356. [DOI] [PubMed] [Google Scholar]
- 27. Jia WP, Shen Q, Bao YQ, Lu JX, Li M, Xiang KS. [Evaluation of the four simple methods in the diagnosis of diabetic peripheral neuropathy]. Zhonghua Yi Xue Za Zhi. 2006;86(38):2707-2710. [PubMed] [Google Scholar]
- 28. Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57(9):1701-1704. [DOI] [PubMed] [Google Scholar]
- 29. Jimenez-Cohl P, Grekin C, Leyton C, Vargas C, Villaseca R. Thermal threshold: research study on small fibre dysfunction in distal diabetic polyneuropathy. J Diabetes Sci Technol. 2012;6(1):177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001;24(9):1134-1341. [DOI] [PubMed] [Google Scholar]
- 31. Vas PR, Rayman G. The rate of decline in small fibre function assessed using axon reflex-mediated neurogenic vasodilatation and the importance of age related centile values to improve the detection of clinical neuropathy. PLOS ONE. 2013;8(7):e69920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stetson DS, Albers JW, Silverstein BA, Wolfe RA. Effects of age, sex, and anthropometric factors on nerve conduction measures. Muscle Nerve. 1992;15(10):1095-1104. [DOI] [PubMed] [Google Scholar]
- 33. Burke D, Skuse NF, Lethlean AK. Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry. 1974;37(6):647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Killian JM, Foreman PJ. Clinical utility of dorsal sural nerve conduction studies. Muscle Nerve. 2001;24(6):817-820. [DOI] [PubMed] [Google Scholar]