Abstract
Background:
Diabetes mellitus is a noncommunicable disease with a rising prevalence worldwide and in developing countries. The most commonly used diagnostic biofluid for detection of glucose levels is blood, but sample collection is an invasive and painful procedure. Thus, there arises a need for a noninvasive and painless technique to detect glucose levels.
Aims and Objectives:
The objectives of the present study were to estimate the glucose levels of saliva, to assess if any significant correlation existed between the serum and salivary glucose levels, and to correlate salivary glucose levels with regard to duration of diabetes, age, and gender. In the present study, serum and salivary glucose levels of 200 subjects (100 diabetic subjects and 100 nondiabetic subjects) were estimated by glucose oxidase method. Glycosylated hemoglobin levels were also measured in randomly selected 40 diabetic subjects.
Results:
The findings of present study revealed a significant correlation between salivary and serum glucose levels in both diabetic and nondiabetic subjects. No significant relationship was observed between salivary glucose levels and gender or age in both diabetics and nondiabetics and between salivary glucose levels and duration of diabetes in diabetics.
Conclusion:
On the basis of the findings, it was concluded that salivary glucose levels could serve as a potentially noninvasive adjunct to monitor glycemic control in diabetic patients.
Keywords: diabetes mellitus, glucose, noninvasive, postprandial, saliva, serum
Diabetes mellitus is a group of complex multisystem metabolic disorders characterized by relative or absolute insufficiency of insulin secretion and/or concomitant resistance to the metabolic action of insulin on target tissues.1 The worldwide explosion of this chronic ailment is a major health care burden. The number of people globally with diabetes are projected to rise to 439 million (7.7%) by 2030.2 Currently, India has 41 million diabetics, and this number is expected to increase to 70 million by 2025. The increased number of diabetics in India is likely due to unprecedented rates of urbanization and lifestyle changes.3
The increased morbidity and mortality of diabetic patients is mostly attributed to complications of the disease. Hyperglycemia is the immediate metabolic consequence of diabetes, and chronic hyperglycemia leads to several events that promote structural changes in tissues.1,4 A high prevalence of wide spectrum oral alterations associated with diabetes has been described in literature.
To minimize the risk of complications associated with this disease, it is necessary to regularly monitor the glucose levels of diabetic patients. The important aspect in glycemic control is the frequent monitoring of blood glucose levels.5 Various biofluids that are used to monitor glucose levels include blood and urine. The choice of blood as a diagnostic fluid for clinical testing is clear-cut considering its close relationship to the homeostasis of the body. Because blood circulates throughout all organs, its chemical makeup is a composite of nearly all metabolic processes occurring in the individual. But blood collection is an invasive technique and causes momentary discomfort to the subject.6 Thus there arises a need for noninvasive technique for monitoring glycemic control in diabetics.
In the past 2 decades most of the research is centered around establishment of a noninvasive technique for glycemic control.7 An attempt to control blood glucose by monitoring urinary glucose has the advantage of being noninvasive. The control achieved, however, is only approximate and the value of urine tests is restricted by the renal threshold to glucose which varies considerably between patients.8
Since 2002, The National Institute of Dental and Craniofacial Research created opportunities to overcome these limitations by investigating oral fluids as a diagnostic tool for the assessment of health and disease status.9 Saliva fulfills several of the chief diagnostic concerns for a diagnostic biofluid as it is obtained noninvasively, and its collection requires no special skill. Because of the ease, safety, and low cost of saliva collection, its promise for current and future diagnostics warrants special consideration.6
To date few studies have been performed on salivary composition and function in diabetic patients particularly in India. There are conflicting results on the utility of saliva as a diagnostic tool for monitoring diabetes in the English literature. Thus, the aim of the present study was to establish saliva as a diagnostic tool for monitoring glycemic control in diabetic patients.
Aims and Objectives
The objectives of present study were (1) to estimate the glucose levels of saliva to aid in reaching firm conclusions about their alterations in diabetics as compared to healthy nondiabetics, (2) to correlate salivary glucose with regard to duration of diabetes, age, and gender, and (3) to assess if a significant correlation exists between serum and salivary glucose levels.
Methodology
The present study was submitted to the Ethical Committee for evaluation and ethical clearance was granted to carry out this noninvasive study. The study comprised 200 subjects, which included 100 diabetic subjects (study group) and 100 nondiabetic subjects (control group) reporting to the Department of Oral and Maxillofacial Pathology for evaluation of blood sugar levels. Both diabetic and nondiabetic subjects were grouped on the basis of sex and age (≤40 years, 41-50 years, 51-60 years, and >60 years). The diabetic subjects were further categorized according to the duration of the disease into short-duration diabetics (≤6 years) and long-duration diabetics (>6 years).
Inclusion Criteria
In the present study, subjects suffering from type 2 diabetes (well-known diabetics) comprised the study group, whereas the control group comprised healthy nondiabetic subjects with no systemic disorder. Diagnosis of diabetes was confirmed according to the criterion laid out by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.10
Exclusion Criteria
Subjects with a history of smoking, radiotherapy for head and neck cancer, oral mucosal, or salivary gland disorders, antibiotic or corticosteroid therapy for preceding 3 months or any medication other than those for diabetes, severe diabetic complications, any other systemic illness and pregnant women were excluded from the study.
Collection and Analysis of Sample
The subjects were briefed on the study being undertaken and a written consent was obtained for the procedure to be carried out to obtain the sample. A prestructured questionnaire was prepared and relevant information of all the subjects was recorded. Detailed history revealed that majority of the diabetic subjects were on medication except few who were not taking any medication.
Saliva
Postprandial unstimulated whole saliva was analyzed in the present study. The subject was asked first to rinse his or her mouth thoroughly with water. Each subject was instructed not to swallow for 5 minutes and then expectorate intraorally retained saliva into a sterile container placed on crushed ice. For each subject 1 ml of saliva was collected. Each sample was assayed for glucose immediately or stored at −20°C in case of delay in analysis. Before analyzing, the sample was centrifuged at 3500 rpm for 5 minutes, and then the supernatant clear fluid was used for detection of glucose. Salivary glucose was analyzed by glucose oxidase (Glucose Kit, Crest Biosystems) method using semiautomatic analyzer.
Serum
A postprandial venous blood sample (2.5 ml) was collected in a sterile test tube. The test tube containing blood was centrifuged at 3500 rpm for 5 minutes and then serum was used immediately for the glucose detection or stored at −20°C. Serum glucose was analyzed by glucose oxidase method using semiautomatic analyzer. Blood samples of randomly selected diabetic subjects (n = 40) were assayed for glycosylated hemoglobin (HbA1c) concentration (using NycoCard HbA1c kit). In subjects who were also subjected to the HbA1c test, a total of 5 ml venous blood was collected, 2 ml of which was collected in an ethylenediaminetetraacetic acid (EDTA) containing blood collection tube, and the HbA1c test was performed, and the rest of the blood was collected in a sterilized test tube for serum glucose estimation.
Statistical Analysis
Data obtained from 200 subjects were subjected to statistical analysis. Chi-square test, t test, Pearson’s correlation test, ANOVA test, and means and standard deviations were used for obtaining the P value and to find if there existed any significant relationship between salivary and serum glucose levels in diabetic subjects. P < .05 was considered to be significant. Linear regression coefficient was calculated to form regression line for predicting the values of serum glucose from salivary glucose.
Results
The predominantly affected age group in diabetic subjects (study group) was 51-60 years (32%), followed by >60 years (31%), whereas in nondiabetic subjects (control group) the predominantly affected age group was 41-50 years (37%), followed by ≤40 years (34%). More subjects in the study group were present in the higher age group as compared to the control group, and this difference was significant (P < .001).
In the study group, there were 46 males and 54 females, whereas in the control group there were 54 males and 46 females. This difference in number of males and females in the study group and the control group was not significant (P = .258).
A total of 68 subjects in the study group were short-duration diabetics (≤6 years) and 32 subjects were long-duration diabetics (>6 years). Difference between number of subjects in different age groups in short-duration and long-duration diabetics was significant (P = .006) (Table 1).
Table 1.
Duration and Age Distribution of Subjects in the Study Group.
| Age group (years) |
|||||
|---|---|---|---|---|---|
| Duration (years) | ≤40, n (%) | 41-50, n (%) | 51-60, n (%) | >60, n (%) | Total, n (%) |
| ≤6 years (% within duration group) | 11 (16.2%) | 20 (29.4%) | 23 (33.8%) | 14 (20.6%) | 68 (100.0%) |
| >6 years (% within duration group) | 1 (3.2%) | 5 (15.6%) | 9 (28.1%) | 17 (53.1%) | 32 (100.0%) |
| Total (% within duration group) | 12 (12.0%) | 25 (25.0%) | 32 (32.0%) | 31 (31.0%) | 100 (100.0%) |
| Pearson χ2 | 12.395 | ||||
| P value | .006 | ||||
Mean serum glucose (188.33 ± 50.667) and mean salivary glucose (19.48 ± 5.511) in the study group were higher than the mean serum glucose (101.32 ± 14.405) and mean salivary glucose (7.82 ± 2.423) in the control group and the difference between mean serum glucose and mean salivary glucose in the study group and the control group was highly significant (P < .001) (Table 2).
Table 2.
Mean Serum and Salivary Glucose Levels in the Study Group and Control Group.
| Serum glucose (mg/dl) |
Salivary glucose (mg/dl) |
|
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Study group | 188.33 ± 50.667 | 19.48 ± 5.511 |
| Control group | 101.32 ± 14.405 | 7.82 ± 2.423 |
| P value | <.001 | <.001 |
In diabetic subjects, salivary glucose levels ranged from 10.00 mg/dl-32.00 mg/dl. In nondiabetic subjects, salivary glucose levels ranged from 4.30 mg/dl to 12.90 mg/dl. In both diabetic and nondiabetic subjects salivary glucose levels increased with an increase in serum glucose levels. Difference between mean salivary glucose levels in each group was significant both in diabetics and nondiabetics (P < .001) (Table 3).
Table 3.
Salivary Glucose Levels in the Study Group and Control Group.
| Salivary glucose (mg/dl) |
||||
|---|---|---|---|---|
| Serum glucose (mg/dl) | n | Range | Mean ± SD | |
| Study group | 120-200.90 | 65 | 10.00-20.60 | 16.12 ± 2.993 |
| 201-260.90 | 23 | 21.00-25.90 | 23.89 ± 1.385 | |
| 261-above | 12 | 26.00-32.00 | 29.26 ± 2.368 | |
| Total | 100 | 10.00-32.00 | 19.48 ± 5.511 | |
| P value | <.001 | |||
| Control group | 75-100.90 | 46 | 4.30-7.00 | 5.78 ± 0.887 |
| 101-120.90 | 46 | 7.10-12.00 | 9.06 ± 1.609 | |
| 121-140 | 8 | 12.10-12.90 | 12.40 ± 0.251 | |
| Total | 100 | 4.30-12.90 | 7.82 ± 2.423 | |
| P value | <.001 | |||
A significant correlation existed between serum and salivary glucose levels in both diabetics and nondiabetics (P < .001) (Figures 1 and 2). In diabetics a significant correlation also existed between serum glucose and HbA1c levels and salivary glucose and HbA1c levels (P < .001) (Figure 3).
Figure 1.
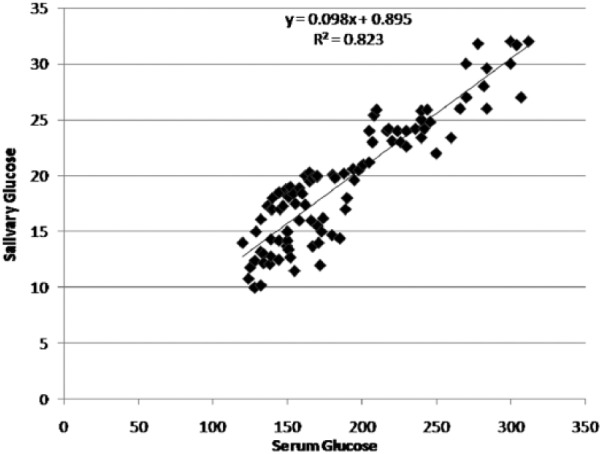
Correlation between serum glucose and salivary glucose levels in the study group.
Figure 2.
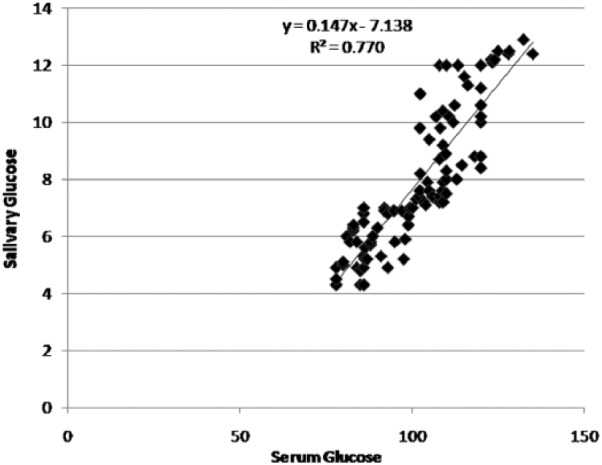
Correlation between serum glucose and salivary glucose levels in the control group.
Figure 3.
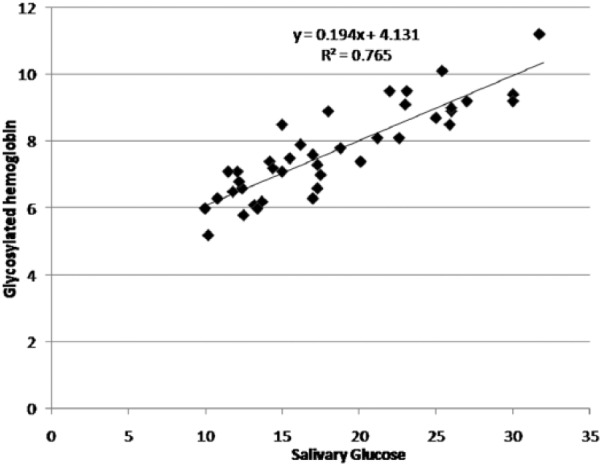
Correlation between salivary glucose and glycosylated hemoglobin levels in the study group.
In the study and control groups, difference between mean salivary glucose in different age groups was not significant, and no significant relationship was observed between salivary glucose and age (P = .439 and P = .537, respectively).
In the study and control groups, the difference between mean salivary glucose in males and females was not significant, and there was no significant relationship between salivary glucose and gender (P = .876 and P = .924, respectively).
In the study group, difference between mean salivary glucose in short-duration diabetics (≤ 6 years), and long-duration diabetics (>6 years) was not significant and no significant relationship existed between salivary glucose and duration of diabetes (P = .272).
Discussion
Diabetes mellitus is characterized by insulin deficiency, cellular resistance to insulin action or both, resulting in hyperglycemia and other related metabolic disturbances. It represents one of the major chronic health problems facing the world today.11 Diagnostic tests for diabetes generally uses blood and urine samples. Blood as a diagnostic tool has an advantage because of its close relationship to the homeostasis of the body.6 However, blood collection is an invasive procedure and is more costly as it requires the help of a trained technician and the use of sharps.12 Thus a noninvasive technique is required to monitor glycemic control. Saliva is the easiest sample that can be collected noninvasively with minimal armamentarium and is associated with fewer compliance problems as compared to blood.13
Investigators have reported an altered salivary composition in diabetics.14 Diabetes is often associated with increased basement membrane permeability, which can be attributed to the increased passage of molecules from exocrine glands into their secretions leading to an enhanced leakage of serum derived components into whole saliva via gingival crevices.15 Glucose, a small molecule can easily diffuse through semipermeable membranes thus increasing the salivary glucose levels, which ultimately results in consequent loss of homeostasis and greater susceptibility to diseases in the oral cavity.14,16-17
There is lack of consensus among different authors on the utility of saliva for monitoring glycemic control. Thus the aim of the present study was to establish saliva as a diagnostic tool for monitoring glycemic control in diabetic patients.
In the present study, glucose concentration in unstimulated whole saliva was analyzed. Whole saliva is most frequently studied for salivary analysis of systemic disorders.13 Saliva can be collected with or without stimulation. Unstimulated whole saliva has been used in the majority of diagnostic studies because stimulated whole saliva is less suitable for diagnostic applications as the foreign substances used to stimulate saliva tend to modulate the fluid pH and generally stimulate the water phase of saliva secretion, resulting in a dilution in the concentration of molecules of interest.18
In the present study, glucose was found in the saliva of both diabetic and nondiabetic subjects. This was in accordance with the observations by Ben-Aryeh et al,19 Aydin,20 Bernardi et al,21 and Naik et al,5 who also found glucose in saliva of both the diabetic and nondiabetic subjects. However, few researchers like Amer et al22 did not find glucose in the saliva of nondiabetic (healthy) subjects.
Mean salivary glucose levels (19.48 ± 5.511) in diabetic subjects were found to be significantly higher than the levels in nondiabetic subjects (7.82 ± 2.423) (P < .001) in the present study. This was in agreement with studies conducted by Aydin,20 Jurysta et al,23 Vasconcelos et al,16 and Naik et al.5 But Sharon et al24 found no significant difference in diabetics and controls.
In our study it was observed that salivary glucose levels increased with an increase in serum glucose levels both in diabetic and nondiabetic subjects. In diabetics and nondiabetics, salivary glucose levels showed a significant correlation with serum glucose levels. These findings were in accordance with those of the Naik et al5 and Abhikshyeet et al,12 who also found a highly significant correlation between salivary glucose level and serum glucose levels in both groups. But Hegde et al25 and Darwazeh et al26 found a significant correlation only in diabetic subjects.
No significant relation of salivary glucose and gender in diabetic and nondiabetic subjects was found in the present study. There have been varying reports on the salivary glucose levels based on gender. Darwazeh et al26 found higher levels of salivary glucose in males as compared to females, and Soares et al27 did not find any significant relationship with gender.
No significant relationship between salivary glucose levels and age in diabetic subjects and nondiabetic subjects was observed in our study. Darwazeh et al26 and Sashikumar and Kannan28 did not find a statistically significant effect of age on the observed value of salivary glucose levels in their studies.
In the analysis of effect of duration of diabetes on salivary glucose levels, no significant relationship was observed in diabetic subjects (P = .272). This result was similar to the findings by Darwazeh et al.26
In the present study a significant correlation was found in salivary glucose levels and HbA1c levels in diabetic subjects (P < .001). Abhikshyeet et al12 also reported a significant correlation between HbA1c levels and salivary glucose hence substantiating our findings. But Sashikumar and Kannan28 did not find a correlation between the same.
The regression coefficient gives the amount of increase or decrease in the serum glucose for a unit change in the salivary glucose. Hence, from a given value of salivary glucose, serum glucose level could be predicted by using the regression equation. We devised a linear regression equation to determine serum glucose if salivary glucose is known.
salivary glucose = 0.895 + 0.099 × serum glucose (study group)
salivary glucose = −7.139 + 0.148 × serum glucose (control group)
Conclusion
Frequent monitoring of glycemic control in diabetes is required to reduce the complications associated with it. Thus, there arises a need for a noninvasive and painless technique to estimate glucose levels. But there are varying results regarding the utilization of saliva as a diagnostic tool to evaluate glucose levels. So, from the observations of the present study it can be inferred that saliva could act as a potential noninvasive adjunct to monitor glycemic control in diabetic patients.
Footnotes
Abbreviations: EDTA, ethylenediaminetetraacetic acid; HbA1c, glycosy-lated hemoglobin.
Authors’ Note: This original research was submitted as thesis in the partial fulfillment of the requirements for the degree of Master of Dental Surgery of the Baba Farid University of Health Sciences, Faridkot, Punjab. Part of the research was presented at the 21st National IAOMP Conference held in Goa, from October 26-28 2012.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Manfredi M, McCullough MJ, Vescovi P, Al-Kaarawi ZM, Porter SR. Update on diabetes mellitus and related oral diseases. Oral Dis. 2004;10(4):187-200. [DOI] [PubMed] [Google Scholar]
- 2. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus-present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228-236. [DOI] [PubMed] [Google Scholar]
- 3. Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1(1):18-28. [DOI] [PubMed] [Google Scholar]
- 4. Bastos AS, Leite AR, Spin-Neto R, Nassar PO, Massucato EM, Orrico SR. Diabetes mellitus and oral mucosa alterations: prevalence and risk factors. Diabetes Res Clin Pract. 2011;92(1):100-105. [DOI] [PubMed] [Google Scholar]
- 5. Naik VV, Satpathy Y, Pilli GS, Mishra MN. Comparison and correlation of glucose levels in serum and saliva of patients with diabetes mellitus. Indian J Pub Health Res Dev. 2011;2(1):103-105. [Google Scholar]
- 6. Srivastava S, Krueger KE. Diagnostics other than blood. In: Wong DT, ed. Salivary Diagnostics. New York, NY: Wiley-Blackwell; 2008:94-103. [Google Scholar]
- 7. Tura A, Maran A, Pacini G. Non-invasive glucose monitoring: assessment of technologies and devices according to quantitative criteria. Diabetes Res Clin Pract. 2007;77(1):16-40. [DOI] [PubMed] [Google Scholar]
- 8. Forbat LN, Collins RE, Maskell GK, Sonksen PH. Glucose concentrations in parotid fluid and venous blood of patients attending a diabetic clinic. J R Soc Med. 1981;74(10):725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee YH, Wong DT. Saliva: an emerging biofluid for early detection of diseases. Am J Dent. 2009;22(4):241-248. [PMC free article] [PubMed] [Google Scholar]
- 10. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1998;21(suppl 1):5-19. [DOI] [PubMed] [Google Scholar]
- 11. Murrah VA. Diabetes mellitus and associated oral manifestations: a review. J Oral Pathol. 1985;14(4):271-281. [DOI] [PubMed] [Google Scholar]
- 12. Abhikshyeet P, Ramesh V, Oza N. Glucose estimation in the salivary secretion of diabetes mellitus patients. Diabetes Metab Syndr Obes. 2012;5:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaufman E, Lamster IB. The diagnostic applications of saliva- a review. Crit Rev Oral Biol Med. 2002;13(2):197-212. [DOI] [PubMed] [Google Scholar]
- 14. Harrison R, Bowen WH. Flow rate and organic constituents of whole saliva in insulin-dependent diabetic children and adolescents. Pediatr Dent. 1987;9(4):287-291. [PubMed] [Google Scholar]
- 15. Tenovuo J, Lehtonen OP, Vikari J, Larjava H, Vilja P, Tuohimaa P. Immunoglobulins and innate antimicrobial factors in whole saliva of patients with insulin-dependent diabetes mellitus. J Dent Res. 1986;65(2):62-66. [DOI] [PubMed] [Google Scholar]
- 16. Vasconcelos AC, Soares MS, Almeida PC, Soares TC. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J Oral Sci. 2010;52(3):293-298. [DOI] [PubMed] [Google Scholar]
- 17. Panchbhai AS, Degwekar SS, Bhowte RR. Estimation of salivary glucose, salivary amylase, salivary total protein and salivary flow rate in diabetics in India. J Oral Sci. 2010;52(3):359-368. [DOI] [PubMed] [Google Scholar]
- 18. Miller CS, Foley JD, Bailey AD, et al. Current development in salivary diagnostics. Biomark Med. 2010;4(1):171-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ben-Aryeh H, Cohen M, Kanter Y, Szargel R, Laufer D. Salivary composition in diabetic patients. J Diabet Complications. 1988; 2(2):96-99. [DOI] [PubMed] [Google Scholar]
- 20. Aydin S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol. 2007;40(1):29-35. [DOI] [PubMed] [Google Scholar]
- 21. Bernardi MJ, Reis A, Loguercio AD, Kehrig R, Leite MF, Nicolau J. Study of the buffering capacity, pH and salivary flow rate in type 2 well-controlled and poorly controlled diabetic patients. Oral Health Prev Dent. 2007;5(1):73-78. [PubMed] [Google Scholar]
- 22. Amer S, Yousuf M, Siddiqui PQ, Alam J. Salivary glucose concentrations in patients with diabetes mellitus—a minimally invasive technique for monitoring blood glucose levels. Pak J Pharm Sci. 2001;14(1):33-37. [PubMed] [Google Scholar]
- 23. Jurysta C, Bulur N, Oguzhan B, et al. Salivary glucose concentration and excretion in normal and diabetic subjects. J Biomed Biotechnol. 2009;2009:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharon A, Ben-Aryeh H, Itzhak B, Yoram K, Szargel R, Gutman D. Salivary composition in diabetic patients. J Oral Med. 1985;40(1):23-26. [PubMed] [Google Scholar]
- 25. Hegde A, Shenoy R, D’Mello P, Smitha A, Tintu A, Manjrekar P. Alternative markers of glycemic status in diabetes mellitus. Biomed Res. 2010;21(3):252-256. [Google Scholar]
- 26. Darwazeh AM, MacFarlane TW, McCuish A, Lamey PJ. Mixed salivary glucose levels and candidal carriage in patients with diabetes mellitus. J Oral Pathol Med. 1991;20(6):280-283. [DOI] [PubMed] [Google Scholar]
- 27. Soares MS, Batista-Filho MM, Pimentel MJ, Passos IA, Chimenos-Kustner E. Determination of salivary glucose in healthy adults. Med Oral Patol Oral Cir Bucal. 2009;14(10):e510-e513. [DOI] [PubMed] [Google Scholar]
- 28. Sashikumar R, Kannan R. Salivary glucose levels and oral candidal carriage in type II diabetics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):706-711. [DOI] [PubMed] [Google Scholar]


