Abstract
Type 1 diabetes is a common chronic disease of childhood and one of the most difficult conditions to manage. Advances in insulin formulations and insulin delivery devices have markedly improved the ability to achieve normal glucose homeostasis. However, hypoglycemia remains the primary limiting factor in achieving normoglycemia and is a frequent complication in children with acute gastroenteritis and/or poor oral intake. In situations of impaired carbohydrate intake or absorption, glucagon therapy is the only out-of-hospital treatment option available to families and caregivers. Glucagon is recommended for the treatment of severe hypoglycemia and rapidly increases blood glucose by increasing hepatic glucose production from glycogenolysis. Mini-dose glucagon is a widely utilized off-label treatment for managing mild or impending hypoglycemia and is administered as a small subcutaneous injection. It was initially described for use in children who were unable to tolerate or absorb oral carbohydrates but not in need of advanced medical care. Yet, mini-dose glucagon may be useful in any individual with relative insulin excess. The regimen aims to prevent severe hypoglycemic episodes and is safe, effective, and easily administered by patients and caregivers in the out-of-hospital setting. By empowering patients and their families, this important tool could help to alleviate the physical, psychosocial, and financial burden evolving from impending hypoglycemia.
Keywords: hypoglycemia, subcutaneous, type 1 diabetes, insulin-treated diabetes
Hypoglycemia is the most common acute complication of insulin therapy in diabetes and the singular factor limiting the achievement of euglycemia.1-3 Every year, up to 10% of children and adults with type 1 diabetes experience a severe hypoglycemic episode associated with marked neurocognitive impairment, coma and/or seizures.4-7 In addition, recurrent and severe episodes may be associated with hypoglycemia unawareness resulting in increased long-term physical and psychosocial morbidity and rarely even death.8-12 This potentially life-threatening condition often requires expensive emergency medical treatment (EMT) and/or transport to an acute care facility.13 And the marked psychological and emotional stress that accompanies severe episodes may profoundly impact the overall quality of life of patients and their families.14,15 Therefore, despite the many advances in insulin formulations and delivery devices, hypoglycemia remains a significant risk for both acute and long-term complications of diabetes.
At present and perhaps into the future, the best approach in preventing episodes of severe hypoglycemia is to be cognizant of situations or conditions that increase the risk of hypoglycemia and to implement anticipatory prevention strategies. Hypoglycemia in individuals with insulin-treated diabetes (type 1 and type 2) is caused by relative and/ or absolute insulin excess. In the patient with type 1 diabetes, this is complicated by a blunted or absent glucagon response to hypoglycemia and/or a defective counterregulatory hormone response as a result of hypoglycemia unawareness.16-20 Young children (< 6 years of age) are most susceptible to severe hypoglycemia,5 which in the past was secondary to the inability to provide appropriately small doses of insulin. This has dramatically improved with the use of insulin pumps in this patient population.21 Yet despite the improvement in insulin delivery devices, increased risk for hypoglycemia remains a problem that is frequently precipitated by an underlying illness, exercise, and, in the very young child, a willful refusal to eat.5 Importantly, and regardless of age, illnesses associated with decreased carbohydrate intake (including nausea and vomiting) can result in hypoglycemia in any patient with insulin-treated diabetes.4,21-23 Therefore, although consumption of carbohydrate containing fluids is the simplest and best mode for preventing hypoglycemia,24 attempting to give oral carbohydrate to an uncooperative child or adult with nausea or vomiting is not ideal.
Currently, there are only 2 alternatives to oral carbohydrate management: (1) glucagon therapy or (2) seeking medical attention via an EMT, urgent care clinic, or emergency room. Out-of-hospital management is generally preferable for the treatment of mild to moderate hypoglycemia.24 The mini-dose glucagon regimen was first described in 200125 and has been internationally accepted for the management of intercurrent illnesses in children.26 Once hypoglycemia is anticipated or documented, glucagon is administered in a small subcutaneous bolus by the patient or caregiver. By preventing severe hypoglycemia, mini-dose glucagon treatment provides a means of empowering the patient’s family and caregivers to take direct action, which may help to reduce emotional distress. In addition, this most likely reduces medical care costs by avoiding expensive use of emergency medical resources. The regimen is well known as a simple and effective tool to manage mild hypoglycemia at home and elsewhere in children, yet it is largely unrecognized in the treatment of adults on exogenous insulin or insulin secretagogue therapy. In this brief review we discuss the off-label use of mini-dose glucagon as an important tool in the management of mild to moderate hypoglycemia in patients with insulin-treated diabetes regardless of age.
Glucagon Discovery and Action
Glucagon was serendipitously discovered in 1923 by Murlin et al after a pancreatic extract caused an increase (rather than the anticipated fall) in blood sugar.27 Murlin named the substance “glucagon” because the extract produced a rapid, reproducible, but transient rise in blood glucose concentrations. However, it wasn’t until approximately 20 years later when the 29 amino acid composition was identified,28 and Sutherland et al proposed that glucagon was produced by the alpha cells of the pancreas.29 After porcine glucagon was purified and crystallized, it became an important treatment for insulin-induced hypoglycemia.30 Almost 40 years later, recombinant glucagon was approved in 1998 and is widely used for the emergency rescue of hypoglycemia in patients with diabetes.30-33
Glucagon is now recognized as the primary counterregulatory hormone in individuals without diabetes, which increases blood glucose at the time of insulin induced hypoglycemia by stimulating hepatic glycogenolysis.1,34-36 It is secreted by the alpha cells of the pancreas in response to 3 main stimuli: (1) a decrease in ambient glucose concentrations by a direct effect of low glucose on the alpha cell,37,38 (2) the removal of the insulin inhibitory effect on the alpha cell,39 and less importantly, (3) through stimulation of the autonomic nervous system.35 Blood glucose is the most potent mediator of glucagon secretion.40
It is also well known that glucagon and insulin are both responsible for the regulation and maintenance of normal glucose homeostasis.34,41-44 Following a meal, rising glucose concentrations stimulate insulin secretion, which suppresses hepatic glucose production from glycogen.45,46 As absorption decreases and glucose concentrations fall (even below baseline values), an incremental increase in glucagon stimulates glycogenolysis to maintain blood glucose concentrations within a narrow and defined range.47 However, the glucagon response to hypoglycemia is markedly attenuated in insulin deficient individuals—particularly those with type 1 diabetes, and perhaps in advanced type 2 diabetes as well.34
Hypoglycemia and Glucagon Secretion in Type 1 Diabetes
The defective glucose regulation in type 1 diabetes is characterized by impairment in beta cell secretion of insulin, superimposed on diminished alpha cell responsiveness to hypoglycemia.16-19,38 Beta cell destruction in type 1 diabetes leads to progressive hyperglycemia resulting from a loss of insulin mediated glucose disposal. The intraislet insulin hypothesis proposes that beta cell dysfunction is also the underlying cause of diminished glucagon secretion and there is a fundamental defect in alpha cell sensing for glucagon secretion. With the loss of endogenous insulin regulation there is absence of the decline in intraislet insulin in response to low glucose. Since insulin inhibits glucagon secretion, it is proposed that failure of a decrement in intraislet insulin concentrations results in persistence of the inhibitory signal on the alpha cell which may directly suppress tonic glucagon secretion and compromise the counterregulatory response to hypoglycemia.48-50
This hypothesis is supported by the reciprocal relationship between insulin and glucagon in individuals with and without diabetes. Hyperinsulinemia prevents the glucagon response to hypoglycemia.35,48,49 This possibly occurs via the persistence of the inhibitory signal on the alpha cell, as insulin stimulation by tolbutamide prevents the glucagon response to hypoglycemia.51,52 This mechanism is plausible in patients with insulin-treated diabetes. Relative hyperinsulinemia is not uncommon during insulin therapy as current insulin dosing is based on estimations of carbohydrate intake and relative insulin sensitivity (insulin: carbohydrate ratio, correction factor, and basal insulin infusion rate) and the entry of insulin into the systemic rather than the portal circulation. However, although supportive of the intraislet hypothesis, the presence of hyperinsulinemia does not prove that a loss of a decrement in intraislet insulin is the cause of impaired alpha cell signaling.
Subsequent studies using diazoxide, an agent which decreases insulin secretion, provided more concrete evidence. Individuals without diabetes received oral diazoxide or placebo and subsequently underwent consecutive hyperinsulinemic euglycemic and hypogly-cemic clamps. As expected, diazoxide decreased insulin secretion (C-peptide concentrations) during the hyperin-sulinemic clamp. During the hypoglycemic clamp, C-peptide concentrations continued to fall; but compared to placebo, individuals on diazoxide had a smaller decrease in insulin secretion, which was associated with a smaller increase in plasma glucagon concentrations. Therefore, the compromised glucagon response in diazoxide treated individuals presumably resulted from the reduced decremental change in C-peptide concentrations, that is, reduced intraislet insulin signaling.49 Furthermore, the impairment in glucagon responsiveness to low glucose progresses as beta cell function declines, and appears to be independent of diabetic autonomic neuropathy.39,46,48 Importantly, the relationship of glucagon to insulin signal-ing was independent of other hormonal and autonomic factors (epinephrine, arginine, and sympathetic tone stimu-lation), suggesting that there is a specific insulin-signaling defect in the alpha cell.8,38 Thus impaired alpha cell responsiveness is a likely feature in insulin-treated diabetes, and under these circumstances of relative insulin excess, replacement glucagon would provide a physiologic stimulus to hypoglycemia.
Glucagon Therapy
Glucagon therapy is the treatment of choice in the management of severe hypoglycemia in insulin-treated patients with either type 1 or type 2 diabetes, if intravenous glucose is not immediately available.24 Recombinant crystalline glucagon is available as a lyophilized powder that is mixed with an aqueous diluent to a concentration of 1 mg/ml. There are 2 commercially available glucagon rescue kits in the United States: GlucaGen® HypoKit 1 mg (Novo Nordisk®A/S, Bagsvaerd, Denmark) and Glucagon Emergency Rescue Kit (Eli Lilly and Company, Indianapolis IN, USA ).
Glucagon is a potent and effective agent that can be administered intravenously, intramuscularly or subcutaneously,25,30,53 but the intramuscular route is generally recommended for the treatment of severe hypoglycemia.31,32 The recommended glucagon dosing is weight based: 1 mg for adults and children >20 kg and 0.5 mg or 20 to 30 μg/kg for children <20 kg, (Eli Lilly product/labeling insert), but the evidence for these recommendations is unclear. In normal volunteers, a 1 mg injection of glucagon results in a 1.9-3.1 mmol/l rise in blood glucose within 30 minutes and plasma glucagon concentrations of 6000-7000 pg/ml (Eli Lilly product/labeling insert). This represents a 100- to 200-fold increase in plasma glucagon concentrations above baseline.54 Of note, the hyperglycemic response to glucagon in insulin-treated diabetes can be quite variable and may be dependent on the circulating insulin concentrations at the time of glucagon administration. Therefore, the insulin: glucagon ratio is most likely important and the timing and dosing of the most recent insulin injection is a key factor in assessing glucagon response.
Glucagon has been used for treatment of hypoglycemia for the past 4 decades and has an excellent safety profile.30-32 The primary side effects of rescue doses of glucagon (0.5 to 1.0 mg of glucagon) are nausea and vomiting, which have been reported to be as high as 80%. Children are at highest risk for side effects, and the risk increases with repeat dosing.5 Therefore, rescue doses of glucagon result in side effect profiles that make it a more desirable agent in the management of milder cases of hypoglycemia.
Mini-Dose Glucagon
Subcutaneous mini-dose glucagon was conceptualized because of the observations made during pancreatic clamp studies. In these experiments, replacement glucagon doses of ~7 ng/kg-min resulted in glucagon plasma concentrations of ~40 to 60 pg/ml and relatively small increases in the glucagon infusion rates could result in a rise in blood glucose concentrations.36,42,55 In addition, we rationalized that since subcutaneous insulin pump infusion was effective in controlling the plasma glucose56-58 a similar argument could be made for glucagon.
We first introduced the off-label use of subcutaneous mini-dose glucagon using a U-100 insulin syringe, in children who had impending hypoglycemia secondary to gastroenteritis.25 Children with gastrointestinal illness and/or poor oral carbohydrate intake with a blood glucose ≤ 4.4 mmol/l were given mini-dose glucagon by their caregivers at home. To prepare the mini-dose regimen, the crystallized glucagon was reconstituted with the provided diluent to 1 mg/ml according to pharmaceutical instruct-tions. However, instead of utilizing the intramuscular syringe provided, a U-100 insulin syringe was used to administer the dose.25,59 Each unit on the U-100 insulin syringe would represent ~10 μg of glucagon. As opposed to the 1 cc syringe and the “large” needle for intramuscular injection, caregivers were instructed to use a U-100 insulin syringe, a device with which they had great experience, comfort and confidence. We created an age based dosing of glucagon since most caregivers know their child’s age: 2 “units” (20 μg) for children ≤ 2 years and 1 unit/year for children ≥3-15 years (with a maximum dose of 150 μg or “15 units”). Older patients (adolescents and young adults over 15 years of age) received a maximum dose of 15 units (150 μg). If blood glucose failed to rise over the first 30 minutes, a repeat injection was given using twice the initial dose. The lowest dose of 2 units was chosen to ensure that a reasonably accurate dose of glucagon was actually delivered.
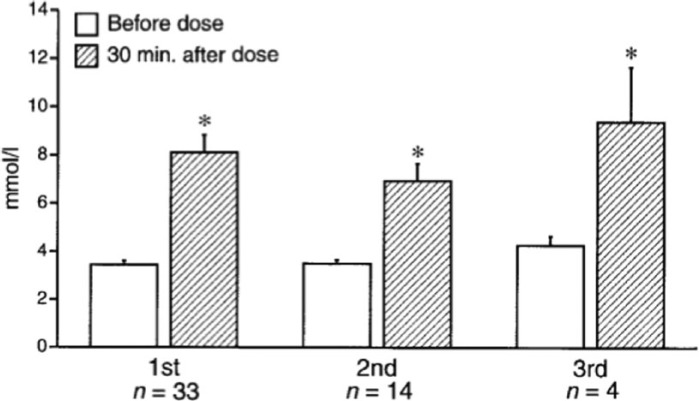
The mini-dose glucagon regimen resulted in an increase of 3.3-5 mmol/l within 30 minutes of administration; average increase was 4.7 mmol/l (Figure 1). All children continued to receive basal insulin dosing as standard sick day management and to prevent the development of ketosis for the duration of their gastrointestinal illness. Over the next 12 to 36 hours of their intercurrent illness, a second and third glucagon dose to treat mild or impending was necessary in ~40% and 12% of children respectively. In all cases, there was an appropriate blood glucose response to the mini-dose glucagon and no patient required repeat dosing within 4 hours of the previous dose (Figure 1). Glucagon dosing did not result in immediate nausea or vomiting.
Figure 1.
Blood glucose at baseline and 30 minutes after mini-dose glucagon rescue in children with mild or impending hypoglycemia (blood glucose < 4.4 mmol/l) associated with gastroenteritis or refusal to eat. Data are means ± SE. 1st, 2nd, and 3rd refer to the first, second, and third doses of glucagon given over the course of the children’s episodes. n refers to the number of children receiving the dose of glucagon. *P < .01 from a paired Student t test. Reproduced with permission of the American Diabetes Association.25
The rise in blood glucose after mini-dose glucagon administration was larger than described in normoglycemic children who received glucagon.54 In the normoglycemic children who received glucagon 0.03 mg/kg intravenously (4-6 times the mini-dose glucagon regimen), the rise in blood glucose was only 1-3 mmol/l, 30 minutes after administration. Although direct comparisons were not made between the children with and without diabetes, the blunted response in the children without diabetes was most likely the result of their increased insulin secretion attenuating the glycogenolytic effect of glucagon.54 In contrast, children with type 1 diabetes have an absolute insulin deficiency that may result in unopposed glucagon action and a greater rise in blood glucose.
This mini-dose regimen is currently available to our patients and parents to be used as necessary and is part of their standard education (Figure 2). The regimen has been recognized nationally and internationally as a safe and reliable tool to manage both mild and impending hypoglycemia in the out-of-hospital setting.25,26,59,60 The International Society for Pediatric and Adolescent Diabetes (ISPAD) advises the use of mini-dose glucagon for the management of mild hypoglycemia (<3.5-4 mmol/l) when nausea and food refusal are prominent.26 Mini-dose glucagon is also used worldwide by several children’s hospitals and organizations; an Internet search for mini-dose glucagon returns 15 website links to patient education guidelines and materials using this tool.60-75
Figure 2.
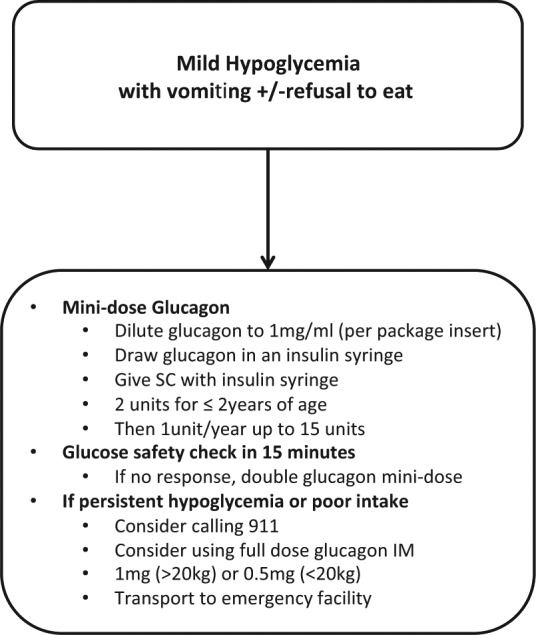
Algorithm for management of mild or impending hypoglycemia with gastroenteritis and/or refusal to eat. IM, intramuscular; SC, subcutaneous injection.
The mini-dose glucagon mode of administration and dosing has 2 main advantages. First, the subcutaneous injections using an insulin syringe are a route with which the patients and their caregivers are familiar—which avoids the stress of using a different syringe and an intramuscular injection. In our experience and that of the Brisbane group,59 the mini-dose regimen may help to alleviate the anxiety associated with administering intramuscular injections and would encourage anticipatory management to avoid or prevent a potentially serious hypoglycemic event.
Second, the mini-dose regimen is effective in a variety of out-of-hospital situations. In the Brisbane pediatric series from 2002 to 2004, the mini-dose glucagon regimen was implemented for the outpatient management of mild or impending hypoglycemia in patients with type 1 diabetes with an inability or refusal to take oral carbohydrates.59 Twenty-five children were treated with mini-dose glucagon on 38 occasions over the 2 year period. The vast majority of cases (~85%) were successfully managed at home and the parents “expressed relief” at the additional treatment option. Of the 15% of cases (n = 6) that required hospital treatment, only 1 child experienced persistent hypoglycemia, but the mini-dose glucagon was not doubled as per their study protocol and our recommendations.25,59 In the remaining 5 children, all had appropriate responses to mini-dose glucagon but required in-hospital treatment for continued vomiting and/or ketonuria.
Mini-dose glucagon has also proven to be extremely effective in the diabetes camp setting where there is increased physical activity, and more frequent risk for hypoglycemia despite reductions in insulin dosing.76 In this circumstance, recurrent hypoglycemia may occur because of ongoing effects exercise and increased insulin sensitivity. In a series of 220 campers, with an average of 28 hypoglycemic episodes/week, 5 children were treated for mild hypoglycemia (2.4 ± 0.5 mmol/l) with the mini-dose glucagon protocol25 after attempts at oral carbohydrate resuscitation failed. Blood glucose concentrations rose immediately after mini-dose glucagon administration and increased by 6.0 ± 0.5 mmol/l within 30 minutes. There were no adverse events associated with the mini-dose regimen and all 5 campers had complete recovery of their mental status changes within 30 minutes of receiving the dose. Of note, the regimen may even be a valuable adjunct to in-hospital management of mild to moderate hypoglycemia77 but is largely unrecognized and /or untaught by emergency room personnel and adult endocrinologists. Thus, it has not been systematically studied in adults but may be of benefit in treating mild hypoglycemia in adults prescribed insulin or insulin secretagogues. Although there are no reports of mini-glucagon use in older adults, from our clinical experience, the mini-dose regimen is quite effective in adolescents and young adults.
Considerations of Mini-Dose Glucagon Therapy and Future Treatment Options
Currently, glucagon is only available as a crystallized powder that must be reconstituted prior to use. Although the mixing process is simple, especially for patients with insulin-treated diabetes who are accustomed to mixing and dosing insulin, it can be overlooked in situations of panic associated with moderate or severe hypoglycemia. Glucagon is also an unstable peptide in the aqueous form because it dimerizes and is prone to spontaneous amyloid polymerization and rapid degradation. Thus, reconstituted glucagon should be used immediately and must be discarded within 24 hours. Significant amyloid formation has been reported to occur within 24 hours and is potentially toxic to cells.78-80 Finally, a 1 mg glucagon emergency kit costs ~$80-100 at current prices; therefore frequent use of mini-dose glucagon to correct mildly low glucose values could add significant financial burden to the disease. Nevertheless, the increased cost of glucagon therapy should be considered alongside the potential savings from a decline in severe hypoglycemic episodes requiring emergency treatment.81
Alternative glucagon preparations and diluents, which stabilize glucagon, are urgently needed. A stable nonaqueous liquid glucagon is in clinical trials at this time (G-Pen Mini, Xeris Pharmaceuticals, NCT02081014 Clinicaltrials.gov). The intranasal powder form of glucagon is another alternative because it does not require reconstitution and can be easily administered in children and adults.53,82,83 Side effects of intranasal administration are common and include rhinorrhea, sneezing, nasal congestion, watery and/or itchy eyes, ears or throat.84,85 However, there are limited data on the efficacy and use of intranasal glucagon in treating mild hypoglycemia. At present, a randomized clinical trial is ongoing to determine the safety and tolerability of intranasal glucagon in children and adults (NCT01997411, NCT01994746 Clinical-trials.gov).
Conclusion
Hypoglycemia is a common yet serious event that may be associated with significant neurocognitive and physical impairment. Mini-dose glucagon is an important therapeutic tool for treating and/or preventing mild or impending hypoglycemia in specific situations of relative insulin excess. However, glucagon is prone to spontaneous polymerization, and ongoing research is focused on identifying stable formulations of glucagon that can be safely and easily administered.
Footnotes
Abbreviations: EMT, emergency medical treatment; IM, intramuscular; ISPAD, International Society for Pediatric and Adolescent Diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MWH is the co–principal investigatoron an NIH SBIR grant to study the nonaqueous glucagon preparation produced by Xeris Pharmaceuticals and is on their advisory committee for non-acqueous glucagon studies. This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, TX). The contents of this publication do not necessarily reflect the views of policies of the US Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement from the US government. STC has no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: STC is supported by intramural program of NIDDK, NIH, and MWH is supported by USDAARS 6250-5100 grant and NIH 1R44-DK096715.
References
- 1. Cryer PE. Hypoglycemia—the limiting factor in the management of iddm. Diabetes. November 1994;43(11):1378-1389. [DOI] [PubMed] [Google Scholar]
- 2. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type i and type ii diabetes. Diabetologia. July 2002;45(7):937-948. [DOI] [PubMed] [Google Scholar]
- 3. Hepburn DA, MacLeod KM, Pell AC, Scougal IJ, Frier BM. Frequency and symptoms of hypoglycaemia experienced by patients with type 2 diabetes treated with insulin. Diabetic Med. April 1993;10(3):231-237. [DOI] [PubMed] [Google Scholar]
- 4. Maltoni G, Zucchini S, Scipione M, et al. Severe hypoglycemic episodes: a persistent threat for children with Type 1 diabetes mellitus and their families. J Endocrinol Invest. September 2013;36(8):617-621. [DOI] [PubMed] [Google Scholar]
- 5. Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr Diabetes. September 2013;14(6):447-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cengiz E, Connor CG, Ruedy KJ, et al. Pediatric diabetes consortium T1D New Onset (NeOn) study: clinical outcomes during the first year following diagnosis. Pediatr Diabetes. June 2014;15(4):287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab. August 2013;98(8):3411-3419. [DOI] [PubMed] [Google Scholar]
- 8. Aman J, Wranne L. Hypoglycaemia in childhood diabetes. I. Clinical signs and hormonal counterregulation. Acta Paediatrica Scandinavica. July 1988;77(4):542-547. [DOI] [PubMed] [Google Scholar]
- 9. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. September 2012;35(9):1897-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacLeod KM, Hepburn DA, Frier BM. Frequency and morbidity of severe hypoglycaemia in insulin-treated diabetic patients. Diabetic Med. April 1993;10(3):238-245. [DOI] [PubMed] [Google Scholar]
- 11. Brodovicz KG, Mehta V, Zhang Q, et al. Association between hypoglycemia and inpatient mortality and length of hospital stay in hospitalized, insulin-treated patients. Curr Med Res Opin. February 2013;29(2):101-107. [DOI] [PubMed] [Google Scholar]
- 12. Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care. September 2012;35(9):1814-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leese GP, Wang J, Broomhall J, et al. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. April 2003;26(4):1176-1180. [DOI] [PubMed] [Google Scholar]
- 14. Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14(5):646-655. [DOI] [PubMed] [Google Scholar]
- 15. Rombopoulos G, Hatzikou M, Latsou D, Yfantopoulos J. The prevalence of hypoglycemia and its impact on the quality of life (QoL) of type 2 diabetes mellitus patients (the HYPO Study). Hormones (Athens). Oct-Dec 2013;12(4):550-558. [DOI] [PubMed] [Google Scholar]
- 16. Hoffman RP, Arslanian S, Drash AL, Becker DJ. Impaired counterregulatory hormone responses to hypoglycemia in children and adolescents with new onset IDDM. J Pediatr Endocrinol. Jul-Sep 1994;7(3):235-244. [DOI] [PubMed] [Google Scholar]
- 17. Liu DT, Adamson UC, Lins PE, Kollind ME, Moberg EA, Andreasson K. Inhibitory effect of circulating insulin on glucagon secretion during hypoglycemia in type I diabetic patients. Diabetes Care. January 1992;15(1):59-65. [DOI] [PubMed] [Google Scholar]
- 18. Sjoberg S, Ahren B, Bolinder J. Residual insulin secretion is not coupled to a maintained glucagon response to hypoglycaemia in long-term type 1 diabetes. J Int Med. October 2002;252(4):342-351. [DOI] [PubMed] [Google Scholar]
- 19. Liu D, Adamson U, Lins PE, Clausen-Sjobom N. An analysis of the glucagon response to hypoglycaemia in patients with type 1 diabetes and in healthy subjects. Diabetic Med. April 1993;10(3):246-254. [DOI] [PubMed] [Google Scholar]
- 20. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. December 2005;54(12):3592-3601. [DOI] [PubMed] [Google Scholar]
- 21. Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. October 2004;27(10):2293-2298. [DOI] [PubMed] [Google Scholar]
- 22. Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. January 1997;20(1):22-25. [DOI] [PubMed] [Google Scholar]
- 23. Rewers A, Chase HP, Mackenzie T, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. May 2002;287(19):2511-2518. [DOI] [PubMed] [Google Scholar]
- 24. Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. September 2009;10(suppl 12):134-145. [DOI] [PubMed] [Google Scholar]
- 25. Haymond MW, Schreiner B. Mini-dose glucagon rescue for hypoglycemia in children with type 1 diabetes. Diabetes Care. 2001;24(4):643-645. [DOI] [PubMed] [Google Scholar]
- 26. Brink S, Laffel L, Likitmaskul S, et al. Sick day management in children and adolescents with diabetes. Pediatr Diabetes. September 2009;10(suppl 12):146-153. [DOI] [PubMed] [Google Scholar]
- 27. Murlin J, Clough HD, Gibbs CBF, Stokes AM. Aqueous extracts of pancreas. I influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem. 1923;56:253-296. [Google Scholar]
- 28. Bromer W, Staub A, Diller ER, Bird HL, Sinn LG, Behrens OK. The amino acid sequence of glucagon. i. amino acid composition and terminal amino acid analyses. 1957. 1956;79(11):2794-2798. [Google Scholar]
- 29. Sutherland EaDDC. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. J Biol Chem. 1948;175:663-674. [PubMed] [Google Scholar]
- 30. Carson MJ, Koch R. Clinical studies with glucagon in children. J Pediatr. August 1955;47(2):161-170. [DOI] [PubMed] [Google Scholar]
- 31. Vukmir RB, Paris PM, Yealy DM. Glucagon: prehospital therapy for hypoglycemia. Ann Emerg Med. April 1991;20(4):375-379. [DOI] [PubMed] [Google Scholar]
- 32. Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndrome Obes. 2011;4:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed July 11, 2014.
- 34. Tse TF, Clutter WE, Shah SD, Cryer PE. Mechanisms of postprandial glucose counterregulation in man. Physiologic roles of glucagon and epinephrine vis-a-vis insulin in the prevention of hypoglycemia late after glucose ingestion. J Clin Invest. 1983;72(1):278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahren B, Nobin A, Schersten B. Insulin and C-peptide secretory responses to glucagon in man: studies on the dose-response relationships. Acta Medica Scandinavica. 1987;221(2):185-190. [DOI] [PubMed] [Google Scholar]
- 36. Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. July 1979;64(1):62-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cryer PE. Minireview: glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology. March 2012;153(3):1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. October 1973;182(4108):171-173. [DOI] [PubMed] [Google Scholar]
- 39. Bolli G, de Feo P, Compagnucci P, et al. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. February 1983;32(2):134-141. [DOI] [PubMed] [Google Scholar]
- 40. Ferrannini E, DeFronzo RA, Sherwin RS. Transient hepatic response to glucagon in man: role of insulin and hyperglycemia. Am J Physiol. 1982;242(2):E73-E81. [DOI] [PubMed] [Google Scholar]
- 41. Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metab Clin Exp. November 1978;27(11):1691-1709. [DOI] [PubMed] [Google Scholar]
- 42. Gerich J, Davis J, Lorenzi M, et al. Hormonal mechanisms of recovery from insulin-induced hypoglycemia in man. Am J Physiol. April 1979;236(4):E380-385. [DOI] [PubMed] [Google Scholar]
- 43. Rizza R, Verdonk C, Miles J, Service FJ, Gerich J. Effect of intermittent endogenous hyperglucagonemia on glucose homeostasis in normal and diabetic man. J Clin Invest. June 1979;63(6):1119-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rizza RA, Gerich JE. Persistent effect of sustained hyperglucagonemia on glucose production in man. J Clin Endocrinol Metab. February 1979;48(2):352-355. [DOI] [PubMed] [Google Scholar]
- 45. Kaplan W, Sunehag AL, Dao H, Haymond MW. Short-term effects of recombinant human growth hormone and feeding on gluconeogenesis in humans. Metab Clin Exp. June 2008;57(6):725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tigas S, Sunehag A, Haymond M. Metabolic adaptation to feeding and fasting during lactation in humans. J Clin Endocrinol Metab. 2002;87(1):302-307. [DOI] [PubMed] [Google Scholar]
- 47. Cherrington AD, Williams PE, Shulman GI, Lacy WW. Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes. 1981;30(3):180-187. [DOI] [PubMed] [Google Scholar]
- 48. Gosmanov NR, Szoke E, Israelian Z, et al. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care. May 2005;28(5):1124-1131. [DOI] [PubMed] [Google Scholar]
- 49. Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes. March 2005;54(3):757-764. [DOI] [PubMed] [Google Scholar]
- 50. Raju B, Cryer PE. Maintenance of the postabsorptive plasma glucose concentration: insulin or insulin plus glucagon? Am J Physiol Endocrinol Metab. August 2005;289(2):E181-186. [DOI] [PubMed] [Google Scholar]
- 51. Banarer S, McGregor VP, Cryer PE. Intraislet hyperinsulinemia prevents the glucagon response to hypoglycemia despite an intact autonomic response. Diabetes. April 2002;51(4):958-965. [DOI] [PubMed] [Google Scholar]
- 52. Kawamori D, Kurpad AJ, Hu J, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. April 2009;9(4):350-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vermeulen M, Klompas M, Ray J, Mazza C, Morrison L. Subcutaneous glucagon may be better than oral glucose for prehospital treatment of symptomatic hypoglycemia. Diabetes Care. 2003;26(8):2472-2473. [DOI] [PubMed] [Google Scholar]
- 54. Pagliara AS, Kari IE, De Vivo DC, Feigin RD, Kipnis DM. Hypoalaninemia: a concomitant of ketotic hypoglycemia. J Clin Invest. 1972;51(6):1440-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rizza R, Verdonk C, Miles J, Service FJ, Haymond M, Gerich J. Somatostatin does not cause sustained fasting hyperglycemia in man. Horm Metab Res. November 1979;11(11):643-644. [DOI] [PubMed] [Google Scholar]
- 56. Service FJ, Rizza RA, Gerich JE. Infusion-pump treatment of diabetes mellitus. N Engl J Med. August 1979;301(5):267-268. [PubMed] [Google Scholar]
- 57. Felig P, Tamborlane W, Sherwin RS, Genel M. Insulin-infusion pump for diabetes. N Engl J Med. November 1979;301(18):1004-1005. [PubMed] [Google Scholar]
- 58. Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med. March 1979;300(11):573-578. [DOI] [PubMed] [Google Scholar]
- 59. Hartley M, Thomsett MJ, Cotterill AM. Mini-dose glucagon rescue for mild hypoglycaemia in children with type 1 diabetes: the Brisbane experience. J Paediatr Child Health. March 2006;42(3):108-111. [DOI] [PubMed] [Google Scholar]
- 60. Committee CDACPGE. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37(1):S1-S212. [DOI] [PubMed] [Google Scholar]
- 61.http://www.hamiltonhealthsciences.ca/documents/Patient%20Education/GlucagonMiniDose-lw.pdf. Accessed May 24, 2014.
- 62.http://www.childrenwithdiabetes.com/d_0j_20w.htm. Accessed May 24, 2014.
- 63.www.nationwidechildrens.org. Accessed May 24, 2014.
- 64.www.seattlechildrens.org/pdf/PE566.pdf. Accessed May 24, 2014.
- 65.www.schn.health.nsw.gov.au/policies/pdf/2010-8034.pdf. Accessed May 24, 2014.
- 66.http://www.rch.org.au/uploadedFiles/Main/Content/diabetes/Minidose%20Glucagon%20Guideline%20glucagon%20rescue.pdf. Accessed May 24, 2014.
- 67.http://www.fvfiles.com/521147.pdf. Accessed May 24, 2014.
- 68.http://www.bcchildrens.ca/NR/rdonlyres/8954ADE9-2B5B-45-24-9715-93F1E0CD5ED0/64812/minigluc.pdf. Accessed May 24, 2014.
- 69.http://www.uwhealth.org/healthfacts/pediatric-diabetes/7481.pdf. Accessed May 24, 2014.
- 70.http://www.hoteldieu.com/dec/MinidoseGlucagonProtocol.pdf. Accessed May 24, 2014.
- 71.http://www.health.qld.gov.au/caru/networks/docs/parentsguide.pdf. Accessed May 24, 2014.
- 72.http://diabetes.ucsf.edu/sites/diabetes.ucsf.edu/files/Sick%20Day%20Final%2011%2023%2009_0.pdfhwjofp. Accessed May 24, 2014.
- 73.www.adea.com.au/wp-content/uploads/2013/08/sickday_sum-mary.pdf. Accessed May 24, 2014.
- 74.http://www.childrensmn.org/images/PDF/diabetesmanual.pdf. Accessed May 24, 2014.
- 75.http://choosehealth.utah.gov/documents/dpr/udpr_section4_children_sep07.pdf. Accessed May 24, 2014.
- 76. Hasan KS, Kabbani M. Mini-dose glucagon is effective at diabetes camp. J Pediatr. June 2004;144(6):834. [PubMed] [Google Scholar]
- 77. Busselo J, Domino AJ, Marra EM. Mini-dose of glucagon: great efficacy avoiding admission of insulin-dependent-diabetic children with decreasing normoglycemia or hypoglycemia due to vomiting or gastroenteritis. Pediatr Res. 2010;68:363-364. [Google Scholar]
- 78. Staub AaB OK. The glucagon content of crystalline insulin preparations. J Clin Invest. 1954;33(12):1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Onoue S, Iwasa S, Kojima T, et al. Structural transition of glucagon in the concentrated solution observed by electrophoretic and spectroscopic techniques. J Chromatogr A. 2006;1109(2):167-173. [DOI] [PubMed] [Google Scholar]
- 80. Onoue S, Ohshima K, Debari K, et al. Mishandling of the therapeutic peptide glucagon generates cytotoxic amyloidogenic fibrils. Pharm Res. 2004;21(7):1274-1283. [DOI] [PubMed] [Google Scholar]
- 81. Elliott J, Jacques RM, Kruger J, et al. Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with Type 1 diabetes. Diabetic Med. July 2014;31(7):847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pontiroli AE, Calderara A, Pajetta E, Alberetto M, Pozza G. Intranasal glucagon as remedy for hypoglycemia. Studies in healthy subjects and type I diabetic patients. Diabetes Care. October 1989;12(9):604-608. [DOI] [PubMed] [Google Scholar]
- 83. Stenninger E, Aman J. Intranasal glucagon treatment relieves hypoglycaemia in children with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. October 1993;36(10):931-935. [DOI] [PubMed] [Google Scholar]
- 84. Rosenfalck AM, Bendtson I, Jorgensen S, Binder C. Nasal glucagon in the treatment of hypoglycaemia in type 1 (insulin-dependent) diabetic patients. Diabetes Res Clin Pract. July 1992;17(1):43-50. [DOI] [PubMed] [Google Scholar]
- 85. Pontiroli AE, Calderara A, Pozza G. Intranasal drug delivery. Potential advantages and limitations from a clinical pharmacokinetic perspective. Clin Pharmacokinet. November 1989;17(5):299-307. [DOI] [PubMed] [Google Scholar]