Abstract
Background:
Assessment of short-term glycemic control can facilitate monitoring of diabetes development in at-risk individuals and monitoring response to lifestyle modification or medication. We evaluated salivary protein glycosylation levels as a novel, noninvasive, short-term glycemic index in comparison to hemoglobin A1c (HbA1c), fructosamine, 1,5-anhydroglucitol (1,5-AG), and continuous glucose monitoring (CGM).
Methods:
Ten subjects with type 2 diabetes were monitored by CGM and saliva and blood were collected at baseline and days 1, 7, 14, 21, and 28 for determination of salivary protein glycosylation, serum fructosamine, and serum 1,5-anhydroglucitol (1,5-AG) levels, as well as HbA1c (baseline and day 28). Weekly, 14-day, 21-day, and 28-day summary blood glucose measures from CGM were computed and matched to the time of each study visit.
Results:
Salivary protein glycosylation exhibited a moderate correlation with fructosamine (r = .65) and 1,5-AG (r = –.48) at baseline, and weak correlation with HbA1c (r = .3).
Conclusions:
Salivary protein glycosylation exhibited a stronger correlation than fructosamine and 1,5-AG with 7-, 14-, and 21-day average BG (r = .84, .84, and .69, respectively, vs –.37, –.28, and .00 [fructosamine] and .00, –.21, and –.57 [1,5-AG]), maximum BG (r = .79, .76, and .53 vs –.09, –.21, and –.05 [fructosamine] and –.32, –.27, and –.52 [1,5-AG]), and percentage of time over 140 mg/dL (r = .87, .79, and .59 vs –.26, –.32, and .07 [fructosamine] and –.04, –.10, and –.50 [1,5-AG]). Salivary protein glycosylation represents a promising noninvasive technology for monitoring short-term glycemic control.
Keywords: saliva, glycosylation, continuous glucose monitoring, hemoglobin A1c, fructosamine
Monitoring of glycemic status is a cornerstone of diabetes management. In type 1 diabetes and insulin-treated type 2 diabetes, self-monitoring of blood glucose (SMBG) through frequent blood sampling with fingerstick glucose monitors is the norm for adjustment of basal and postprandial insulin dosing. For noninsulin-treated type 2 diabetes, periodic hemoglobin A1c (HbA1c) determinations are the most common method of assessing overall glycemic control. The blood levels of HbA1c represent the average BG level over the previous 3 months, reflecting the life span of the red blood cells that carry hemoglobin. There are a number of issues with using HbA1c to monitor glucose homeostasis, including its inability to reflect shorter-term variations in BG, significant genetic and nonglycemic effects on HbA1c levels, significant age-dependent and ethnic variations in the relationship between HbA1c levels and average BG levels,1-5 and the lack of an association between HbA1c levels and risk of severe hypoglycemia.6 The utility of SMBG for non-insulin-treated type 2 diabetes is a matter of controversy, although this appears to be of benefit in a highly structured setting, although the costs attendant on SMBG are considered a significant additional burden for this group of patients.7-9 The use of glycated albumin10 or fructosamine11 as an alternative offers the advantage of reflecting a intermediate response time (ie, representing the average BG level over the previous 2-4 weeks), with prognostic value in population studies comparable to HbA1c,12 but the effects of the various factors that hamper the utility of HbA1c on the relationship between these glycated proteins and previous BG levels remain to be adequately evaluated,13,14 and the equivalency in diagnostic potential based on epidemiological studies may not extend to individual monitoring.15 The serum metabolite 1,5-anhydroglucitol (1,5-AG) has also been demonstrated to reflect short-term changes in glycemia due to altered renal reabsorption,16 although these measurements will be affected by individual variations in renal function.
The elevated glucose levels seen in prediabetes and poorly controlled established type 2 diabetes affect protein biomarkers found in biological fluids in 2 ways. The first of these is direct, nonenzymatic protein glycation, in which glucose becomes covalently linked to target proteins through the formation of a Schiff base between the aldehyde group of the glucose molecule and the amino group of a lysine residue in a protein. The Schiff base then undergoes an Amadori rearrangement and oxidation to form an advanced glycation end product. HbA1c and glycated albumin (the major constituent of the fructosamine assay) are examples of advanced glycation end products.
The second mechanism through which glucose levels can affect protein biomarkers is through modification by intracellular glycosylation as opposed to nonenzymatic glycation. Hyperglycemia can increase the flux of glucose through the hexosamine biosynthetic pathway, which provides the UDP-GlcNAc and GalNAc precursors for the addition of various carbohydrate moieties to proteins through both β-linked O-glycosylation of intracellular proteins as well as α-linked mucin-type O- and β-linked N-glycosylation of cell-surface and secreted proteins.17 O-glycosylation of intracellular proteins has been demonstrated to modulate the ability of various cells to respond to insulin.18,19 The levels of secreted mucin-type O- and N-glycosylated proteins may reflect altered cellular metabolism due to hyperglycemia.20-25 There is also evidence that hyperlipidemia can regulate hexosamine biosynthetic pathway activity.26 Thus, the determination of glycoprotein levels in biological fluids may represent a more sensitive and inherently physiological response to metabolic control than the use of glycated protein biomarkers.
All current methods of assessing glycemic status require blood samples. However, there are many instances in which this is not ideal, either because of patient age, attitude toward fingersticks or venipuncture, or hygiene issues in rural or underdeveloped/resourced areas. Saliva has a number of distinct advantages over blood as a diagnostic fluid. These include being (1) noninvasive; (2) feasible without special training or equipment, which is especially advantageous for pediatric or elderly populations; and (3) amenable to large-scale population assessments, both for screening or epidemiological studies. For these reasons, there has been a significant increase in interest in the development and validation of saliva biomarkers.27-33 We have previously reported the analysis of the salivary proteome in type 2 diabetes, which identified a number of differentially abundant proteins, many of which were glycoproteins whose abundance was altered in prediabetes and diabetes.34 Alterations in the salivary proteome and peptidome have recently been also reported in type 1 diabetes,35 with changes in glycoproteins seen in that study as well. In this study, we combine these 2 concepts and describe the utility of a new technology to measure salivary glycoprotein levels for short-term, noninvasive assessment of glycemia.
Our previous studies demonstrating changes in specific salivary glycoproteins in established type 2 diabetes and prediabetes,34 as well as changes in the overall salivary glycoproteome in type 2 diabetes and prediabetes assessed by 2-dimensional fluorescence difference gel electrophoresis,36 suggested that changes in salivary glycosylation could reflect different levels of glycemia. We therefore undertook an analysis of salivary protein glycosylation in a series of 10 subjects with type 2 diabetes who had undergone 28 days of CGM to ascertain if salivary protein glycosylation levels were correlated with relative glycemia.
Methods
Study Population
For this study, a total of 10 subjects were recruited from a pool of 70 type 2 diabetes patients under care at Nizam’s Institute of Medical Sciences, Hyderabad, India. This study was approved by the Institutional Ethics Committee at Nizam’s Institute of Medical Sciences University, and informed consent was obtained from each subject. Clinical characteristics of the subjects are shown in Table 1.
Table 1.
Baseline Clinical Characteristics of the Study Subjects.
| Participant characteristic | Mean (SD) |
|---|---|
| Male gender (n, %) | 9 (90) |
| Age (years) | 42.6 (16.9) |
| Body mass index (kg/m2) | 26.3 (4.3) |
| Systolic blood pressure (mmHg) | 127 (15) |
| Total cholesterol (mg/dL) | 176 (42) |
| LDL (mg/dL) | 114 (35) |
| HDL (mg/dL) | 32 (7) |
| Triglycerides (mg/dL) | 148 (68) |
| Fasting plasma glucose (mg/dL) | 162 (60) |
| Hemoglobin A1c (%) | 9.3 (2.8) |
| Fasting insulin (mIU/L) | 40.5 (43.4) |
| C-peptide (ng/mL) | 1.46 (0.97) |
Participants underwent continuous glucose monitoring (CGM) using Guardian REAL-Time monitors (Medtronic, Inc, Northridge, CA) for 28 days with weekly study visits for device calibration and sample collection. Subjects’ course of treatment for diabetes was not altered based on CGM results. The average number of BG measures obtained during the study follow-up was 6909 ± 436 (mean ± SD) per subject. Subjects were asked not to eat or smoke for at least 8 hours prior to study visits occurring between 8 am and 9 am on 1, 7, 14, 21, and 28 days following the baseline visit. Unstimulated saliva samples were collected at every study visit and standard blood draws were performed at each visit. In addition, clinical parameters, including height, weight, and blood pressure, were obtained at baseline and at the final study visit.
Saliva and Blood Measures
Salivary protein glycosylation levels were measured using a method developed to preferentially detect sialic acid and fucose, as these are major monosaccharides found in secreted glycoproteins and our preliminary studies demonstrated that saliva reactivity to sialic acid and fucose-binding lectins was correlated with hyperglycemia (data not shown). Saliva samples were diluted 1:5 in 2% acetic acid, pH 4.5, and 50 µL were added per well of a 96-well Reacti-Bind polystyrene plate (Thermo Scientific, Rockford, IL) followed by 25 µL of 10 mM sodium metaperiodate (made immediately before use in 2% acetic acid, pH 4.5). The plate was agitated on a rotary shaker for 30 seconds and then covered and incubated for 10 minutes at room temperature. These conditions for metaperiodate oxidation have been previously described to favor oxidation of sialic acid, fucose, and N-acetyl-galactosamine moieties in proteins,37,38 and also to reduce the reactivity of salivary glycoproteins with sialic acid and fucose-specific lectins (data not shown). At the end of the incubation, 150 µl of 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Sigma-Aldrich, St. Louis, MO) solution (175 mg in 35 ml 1N NaOH) was added. The absorbance at 550 nm was determined using an ELx800 plate reader (BioTek, Winooski, VT). Bovine fetuin (Sigma-Aldrich, St. Louis, MO) was used as a standard for glycosylated protein and results were reported in µg/ml fetuin equivalents. Intraassay CVs ranged from 2.6% to 5.4% for low and high calibrators and interassay CVs ranged from 5.2% to 7.5%. Blood samples were processed using an AU400e chemical analyzer (Olympus, Center Valley, PA) for fructosamine quantification using a commercial kit (Glycated Serum Protein; Diazyme Laboratories, Poway, CA) with intra- and interassay CVs of 1.1% and 1.2%, respectively (manufacturer’s data). HbA1c levels were determined on an HLC-723 G8 high-performance liquid chromatograph analyzer (Tosoh Bioscience, King of Prussia, PA) with intra- and interassay CVs of 0.44-0.99% and 0.36-0.61%, respectively (manufacturer’s data). 1,5-anhydroglucitol (I,5-AG) levels were determined using a commercial kit (GlycoMark, Inc, New York, NY) with intra- and interassay CVs of 3.83 and 3.71%, respectively (manufacturer’s data).
Blood Glucose Summary Measures and Statistical Analysis
To quantify glycemic control from the CGM data, BG summary measures were calculated. Daily summary measures were excluded from the analysis if, within a single day, a participant was missing more than 25% of CGM data, and 7-, 14-, 21-, and 28-day summary measures were excluded if the subject was missing more than 2 days within each period. This resulted in 1 subject (number 8) being removed from the analysis. For the final analysis, 3 subjects had adequate 28-day CGM data, 5 subjects had adequate 21-day CGM data, 7 subjects had adequate 14-day CGM data, and 9 subjects had adequate 7-day CGM data. One subject was missing the day-21 saliva sample.
Average, median, and standard deviation (SD) BG were calculated as the mean, median, and SD, respectively, of BG values across each measurement period. CV was calculated as SD BG divided by average BG. Minimum BG, maximum BG, and the number of excursions above 140 mg/dL and below 70 mg/dL were recorded for each 24-hour period. Area under the curve (AUC) was calculated for each 24-hour period for values greater than 140 mg/dL and below 70 mg/dL. Time below 70 mg/dL, time within 70 to 140 mg/dL, and time above 140 mg/dL was obtained for each day. To adjust for missing data, percentage of time was calculated for each category with the total time measured each day as the denominator and the time within each category as the numerator. Mean amplitude of glycemic excursion (MAGE)39 was calculated for each 24-hour period by averaging the mean difference between consecutive BG peaks and troughs which were identified as BG points whose difference was more than the SD BG of that day. All daily values were averaged across 7, 14, 21, and 28 days to match study visits.
Baseline characteristics of the study population were tabulated, as well as the 28-day change in relevant clinical parameters. Due to the small sample size, analyses were primarily descriptive and included plots of salivary protein glycosylation measures across time in comparison with HbA1c, fructosamine, 1,5-AG, and CGM BG summary measures. Pearson’s correlation coefficients were computed and plotted for all measures at baseline and days 1, 7, 14, 21, and 28. Reported P values are 2-sided. Statistical analysis was performed using SAS software, version 9.3, of the SAS System for Windows.
Results
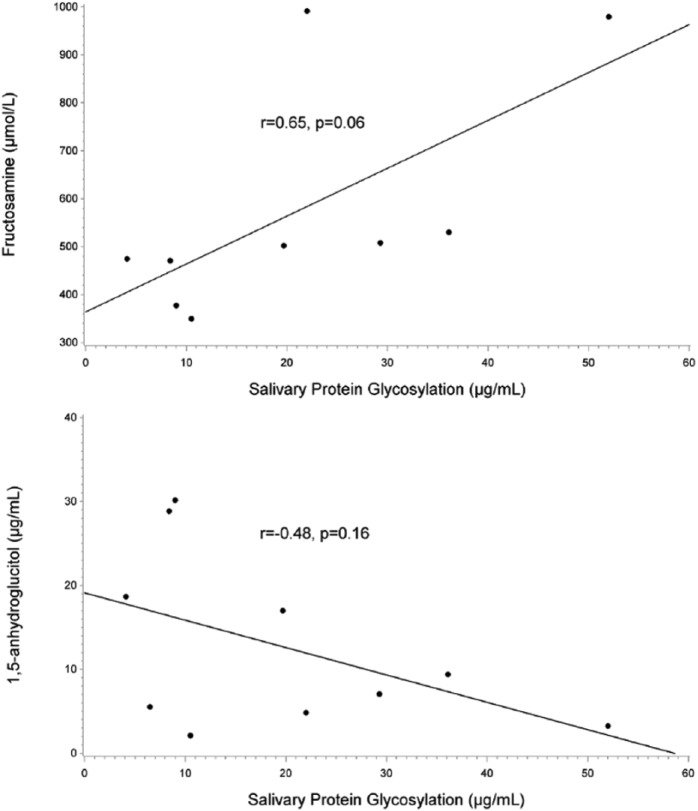
As shown in Figure 1, baseline salivary protein glycosylation measures were moderately correlated with baseline fructosamine and 1,5-AG values (r = .65, P = .06; r = –.48, P = .16). Salivary protein glycosylation was weakly correlated with HbA1c (r = .30, P = .40; data not shown).
Figure 1.
Relationship of baseline salivary protein glycosylation to fructosamine and 1,5-AG. Baseline salivary protein glycosylation was correlated with baseline fructosamine (top panel) and 1,5-AG (bottom panel). Salivary protein glycosylation levels are expressed as μg/ml of bovine fetuin equivalents.
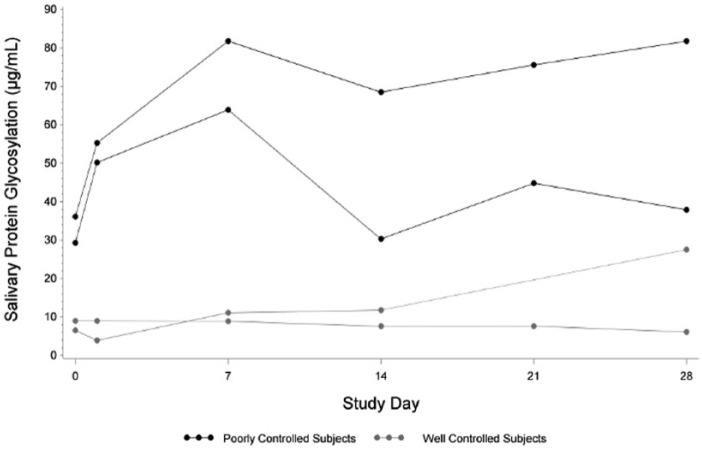
Longitudinal values of salivary protein glycosylation were plotted over time to analyze the range and variability in these subjects. Figure 2 shows this time plot for 2 well controlled and 2 poorly controlled subjects. Across 28 days, well-controlled subjects had an average maximum daily BG of 196 and were above 140 mg/dL for 34% of the day, while poorly controlled subjects had an average maximum daily BG of 289 and were above 140 mg/dL for 65% of the day. Salivary glycosylation mean, SD, and range were calculated for each subject and are shown in Table 2.
Figure 2.
Salivary protein glycosylation in subjects over time. Weekly salivary protein glycosylation was plotted against study day for 2 representative subjects with well-controlled glycemia and 2 representative subjects with poorly controlled glycemia (classified as described in the main text). Salivary protein glycosylation levels are expressed as μg/ml of bovine fetuin equivalents.
Table 2.
Descriptive Statistics of 6 Salivary Protein Glycosylation Measurements Over 28 Days by Subject.
| Subject | Salivary Protein Glycosylation (µg/mL), Mean (SD) | Range |
|---|---|---|
| 1 | 33.1 (20.2) | 2.2-52.0 |
| 2 | 42.7 (13.2) | 29.3-63.9 |
| 3 | 8.0 (1.2) | 6.1-9.0 |
| 4 | 66.5 (17.9) | 36.1-81.8 |
| 5a | 12.2 (9.2) | 3.9-27.5 |
| 6 | 26.3 (14.6) | 8.4-44.8 |
| 7 | 21.7 (15.2) | 5.3-43.2 |
| 8 | 24.0 (22.6) | 9.4-64.4 |
| 9 | 16.9 (7.0) | 10.4-28.6 |
| 10 | 5.9 (2.6) | 3.5-10.6 |
Salivary protein glycosylation levels are expressed as µg/ml of bovine fetuin equivalents.
Subject 5 was missing day 21 salivary protein glycosylation data.
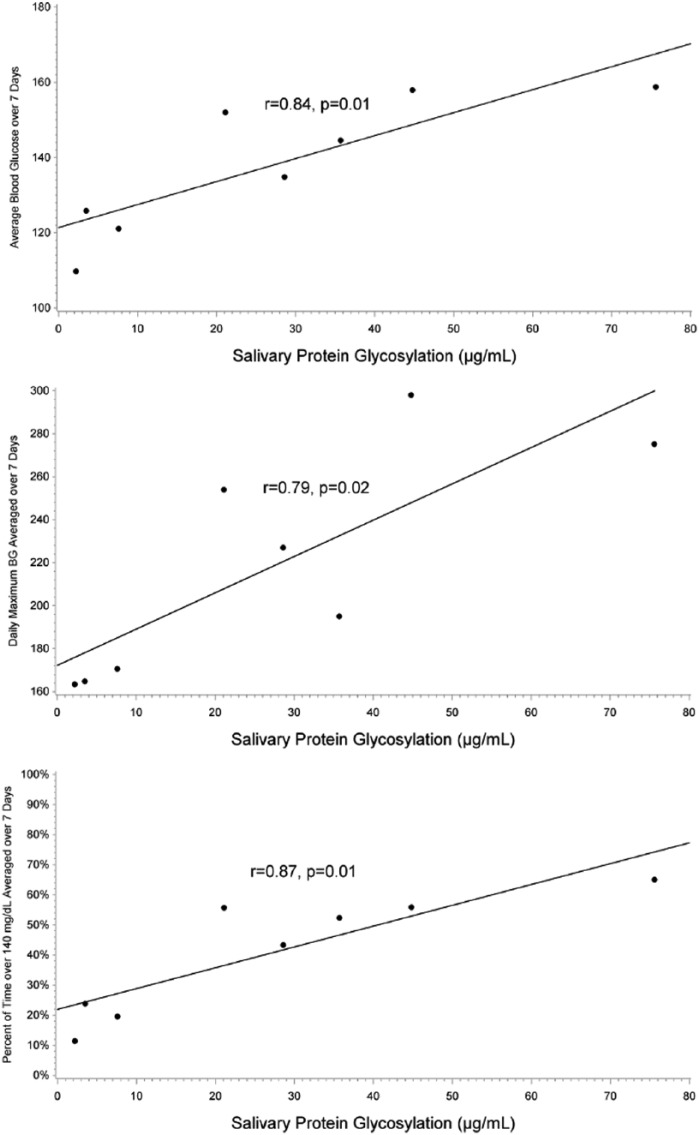
Pearson’s correlation coefficients and corresponding P values for correlation of salivary protein glycosylation measurements with BG summary measures across 7, 14, 21, and 28 days indicated that the strongest relationship between salivary protein glycosylation and BG summary measures was between 7 and 21 days. Average BG, maximum BG, and percentage of time above 140 mg/dL over 7, 14, and 21 days were strongly correlated with salivary glycosylation at day 21. Results from this analysis are shown in Table 3 and Figure 3.
Table 3.
Correlation Between Salivary Protein Glycosylation at Day 21 and 7-, 14-, and 21-Day BG Summary Measures.
| Summary measure | 7-day CGM Measures | 14-day CGM Measures | 21-day CGM Measures |
|---|---|---|---|
| Average BG | |||
| r | .84 | .90 | .70 |
| P | .01 | .04 | .19 |
| n | 8 | 5 | 5 |
| Daily maximum BG | |||
| r | .79 | .76 | .50 |
| P | .02 | .05 | .31 |
| n | 8 | 7 | 6 |
| Daily percentage of time above 140 mg/dL | |||
| r | .87 | .79 | .66 |
| P | .01 | .03 | .16 |
| n | 8 | 7 | 6 |
r is Pearson’s correlation coefficient.
Figure 3.
Relationship between salivary protein glycosylation and average BG, maximum BG, and percentage of time above 140 mg/dL. Salivary protein glycosylation was plotted against 7-day average BG (top panel), average daily maximum BG (middle panel), and average daily percentage of time above 140 mg/dL (bottom panel). Salivary protein glycosylation was strongly correlated with all 3 measures. Salivary protein glycosylation levels are expressed as μg/ml of bovine fetuin equivalents.
CGM measures of glycemic control were correlated with measures of salivary protein glycosylation and fructosamine. Table 4 provides the Pearson’s correlation coefficients for 7-, 14-, and 21-day average BG, maximum BG, and percentage of time above 140 mg/dL for salivary protein glycosylation and fructosamine at day 21. Salivary protein glycosylation exhibited a stronger correlation with all BG summary measures than did fructosamine or 1,5-AG.
Table 4.
Correlation of 7-, 14-, and 21-Day BG Summary Measures With Salivary Protein Glycosylation, Fructosamine, and 1,5-AG at Day 21.
| Over 7 days |
Over 14 days |
Over 21 Days |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure of glycemia | Average BG | Maximum BG | % time above 140 mg/dL | Average BG | Maximum BG | % time above 140 mg/dL | Average BG | Maximum BG | % time above 140 mg/dL |
| Salivary glycosylation | |||||||||
| r | .84 | .79 | .87 | .84 | .76 | .79 | .69 | .53 | .59 |
| P | .01* | .02* | .01* | .02* | .05* | .03* | .19 | .36 | .29 |
| n | 8 | 8 | 8 | 7 | 7 | 7 | 5 | 5 | 5 |
| Fructosamine | |||||||||
| r | −.37 | −.09 | −.26 | −.28 | −.21 | −.32 | .00 | −.05 | .07 |
| P | .36 | .83 | .53 | .54 | .64 | .48 | .99 | .93 | .92 |
| n | 8 | 8 | 8 | 7 | 7 | 7 | 5 | 5 | 5 |
| 1,5-AG | |||||||||
| r | .00 | −.32 | −.04 | −.21 | −.27 | −.10 | −.57 | −.52 | −.50 |
| P | .99 | .43 | .93 | .66 | .55 | .84 | .31 | .37 | .39 |
| n | 8 | 8 | 8 | 7 | 7 | 7 | 5 | 5 | 5 |
r is Pearson’s correlation coefficient.
P < .05.
Discussion
The array of approaches to determine glycemic control ranges from purely glucose-based parameters such as random BG testing routinely employed by individuals with type 1 diabetes and fasting BG and oral glucose tolerance tests to assess impaired fasting glucose or impaired glucose tolerance in prediabetes and type 2 diabetes, to indices of hyperglycemia-induced protein glycation (HbA1c, fructosamine, and glycated albumin) and reabsorption of 1,5-AG. A number of studies have compared the existing assays for long- and short-term average glycemia, with each approach having advantages and disadvantages;40-45 especially noteworthy is the inability of HbA1c to reflect intrinsic variability in glucose levels in individual patients and discrepancies in the average glucose levels derived from different measurements (the so-called glycation gap).46
In this study, we describe our initial results evaluating an alternative parameter of glycemia (salivary protein glycosylation) that is potentially driven by cellular metabolism and that is discernible in saliva. Specifically, we found that baseline salivary protein glycosylation measures were moderately correlated with fructosamine (r = .65) and 1,5-AG (r = –.48), measures of 2- to 4-week glycemic control; however, compared to fructosamine and 1,5-AG, salivary protein glycosylation measures were better correlated with average BG, maximum BG, and time above 140 mg/dL.
Our findings suggest that salivary protein glycosylation is a potential alternative biomarker for recent hyperglycemia, as it has better ability to predict 7 to 21-day blood glucose measures than fructosamine or 1,5-AG. In addition, the ability to use saliva rather than blood constitutes a separate, significant advantage for salivary protein glycosylation. Two specific situations in which salivary protein glycosylation assessments may be advantageous would be large-scale screening for diabetes and for determining changes in glycemia following initiation of or alterations in therapy.
The major limitation of this study is the small number of predominantly male subjects. Future studies will include a larger number of subjects of both sexes to further validate the current findings. These studies will aim to further establish salivary protein glycosylation as a measure of recent hyperglycemia as well as to enhance the under-standing of the relationship between blood glucose and salivary protein glycosylation.
Acknowledgments
We thank Austin Gower for expert technical assistance.
Footnotes
Abbreviations: AUC, area under the curve; BG, blood glucose; CGM, continuous glucose monitoring; CV, coefficient of variation; HbA1c, hemoglobin A1c; MAGE, mean amplitude of glycemic excursion 1,5-AG, 1,5-anhydroglucitol; SD, standard deviation; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AL, ESB, and SRN are employees of and PVR, CTR, and SRN are shareholders in DiabetOmics, Inc.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grant R43 DE020973 to SRN and by DiabetOmics, Inc.
References
- 1. Dagogo-Jack S. Pitfalls in the use of HbA1c as a diagnostic test: the ethnic conundrum. Nat Rev Endo. 2010;6:589-593. [DOI] [PubMed] [Google Scholar]
- 2. Cohen RM, Haggerty S, Herman WH. HbA1c for the diagnosis of diabetes and prediabetes: is it time for a midcourse correction? J Clin Endo Metab. 2010;95:5203-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1c. Diabetes Care. 2011;34:S184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hinzmann R, Schlaeger C, Tran CT. What do we need beyond hemoglobin A1c to get the complete picture of glycemia in people with diabetes? Int J Med Sci. 2012;9:665-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilpatrick ES. The rise and fall of HbA1c as a risk marker for diabetes complications. Diabetologia. 2012;55:2089-2091. [DOI] [PubMed] [Google Scholar]
- 6. Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes. The Diabetes and Aging Study. Diabetes Care. 2013;36:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bosi E, Scavini M, Ceriello A, et al. Intensive structured self-monitoring of blood glucose and glycemic control in non-insulin-treated type 2 diabetes. The PRISMA randomized trial. Diabetes Care. 2013;36:2887-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malanda UL, Bot SD, Nijpels G. Self-monitoring of blood glucose in noninsulin-using type 2 diabetes. It is time to face the evidence. Diabetes Care. 2013;36:176-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polonsky WH, Fisher L. Self-monitoring of blood glucose in noninsulin-using type 2 diabetes. Right answer, but wrong question: self-monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care. 2013;36:179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim KJ, Lee BW. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J. 2012;36:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem. 1987;33:2153-2163. [PubMed] [Google Scholar]
- 12. Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2014;2:279-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tahara Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care. 1995;18:440-447. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi S, Uchino H, Shimizu T, et al. Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short-term changes in glycemic control. Endocr J. 2007;54:139-144. [DOI] [PubMed] [Google Scholar]
- 15. Buse JB, Freeman JL, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark): a short-term glycemic marker. Diabetes Technol Ther. 2003;5:355-363. [DOI] [PubMed] [Google Scholar]
- 16. Cohen RM, Herman WH. Are glycated serum proteins ready for prime time? Lancet Diabetes Endocrinol. 2014;2:264-265. [DOI] [PubMed] [Google Scholar]
- 17. Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R-56R. [DOI] [PubMed] [Google Scholar]
- 18. Yang X, Ongusaha PP, Miles PD, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964-969. [DOI] [PubMed] [Google Scholar]
- 19. Issad T, Masson E, Pagesy P. O-GlcNAc modification, insulin signaling and diabetic complications. Diabetes Metabol. 2010;36:423-435. [DOI] [PubMed] [Google Scholar]
- 20. Lau KS, Partridge EA, Grigorian A, et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123-134. [DOI] [PubMed] [Google Scholar]
- 21. Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139:1229-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riedl E, Koeppel H, Pfister F, et al. N-glycosylation of carnosinase influences protein secretion and enzyme activity. Implications for hyperglycemia. Diabetes. 2010;59:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Park K, Comer F, et al. Site-specific GlcNAcylation of human erythrocyte proteins. Potential biomarkers for diabetes. Diabetes. 2009;58:309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flintegaard TV, Thygesen P, Rahbek-Nielsen H, et al. N-glycosylation increases the half-life of human growth hormone. Endocrinology. 2010;151:5326-5336. [DOI] [PubMed] [Google Scholar]
- 25. Lee C-L, Chiu PCN, Pang PC, et al. Glycosylation failure extends to glycoproteins in gestational diabetes mellitus. Evidence from reduced 2-6 sialylation and impaired immunomodulatory activities of pregnancy-related glycodelin-A. Diabetes. 2011;60:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weigert C, Klopfer K, Kausch C, et al. Palmitate-induced activation of the hexosamine pathway in human myotubes. Diabetes. 2003;52:650-656. [DOI] [PubMed] [Google Scholar]
- 27. Streckfus CF, Bigler LR. Salivary glands and saliva, number 3: saliva as a diagnostic fluid. Oral Dis. 2002;8:69-76. [DOI] [PubMed] [Google Scholar]
- 28. Malamud D. Salivary diagnostics: the future is now. J Am Dental Assoc. 2006;137:284-286. [DOI] [PubMed] [Google Scholar]
- 29. Tabak LA. Point-of-care diagnostics enter the mouth. Ann NY Acad Sci. 2007;1098:7-14. [DOI] [PubMed] [Google Scholar]
- 30. Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am. 2011;55:159-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amado F, Lobo MJC, Domingues P, Duarte JA, Vitorino R. Salivary peptidomics. Expert Rev Proteomics. 2010;7:709-721. [DOI] [PubMed] [Google Scholar]
- 32. Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57:675-677. [DOI] [PubMed] [Google Scholar]
- 33. Ai J-Y, Smith B, Wong DTW. Bioinformatics advances in saliva diagnostics. Int J Oral Sci. 2012;4:85-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao PV, Reddy AP, Lu X, et al. Proteomic identification of salivary biomarkers of type-2 diabetes. J Proteome Res. 2009;8:239-245. [DOI] [PubMed] [Google Scholar]
- 35. Cabras T, Pisano E, Mastinu A, et al. Alterations of the salivary secretory peptidome profile in children affected by type 1 diabetes. Mol Cell Proteomics. 2010;9:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paturi VR, Reddy AP, Lu X, et al. Salivary biomarkers of type-2 diabetes: proteomic profiles for non-invasive diagnostics. Paper presented at: American Association of Clinical Endocrinologists; 2009; Houston, TX. [Google Scholar]
- 37. Woodward MP, Young WW, Jr, Bloodgood RA. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143-153. [DOI] [PubMed] [Google Scholar]
- 38. Norgard KE, Han H, Powell L, Kriegler M, Varki A, Varki NM. Enhanced interaction of L-selectin with the high endothelial venule ligand via selectively oxidized sialic acids. Proc Natl Acad Sci USA. 1993;90:1068-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baghurst PA. Calculating the mean amplitude of glycemic excursion from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther. 2011;13:296-302. [DOI] [PubMed] [Google Scholar]
- 40. Herman WH, Dungan KM, Wolffenbuttel BH, et al. Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1689-1694. [DOI] [PubMed] [Google Scholar]
- 41. Beck R, Steffes M, Xing D, et al. The interrelationships of glycemic control measures: HbA1c, glycated albumin, fructosamine, 1,5-anhydroglucitol, and continuous glucose monitoring. Pediatr Diabetes. 2011;12:690-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cohen RM, Sacks DB. Comparing multiple measures of glycemia: how to transition from biomarker to diagnostic test? Clin Chem. 2012;58:1615-1617. [DOI] [PubMed] [Google Scholar]
- 43. Juraschek SP, Steffes MW, Miller ER, III, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care. 2012;35:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem. 2012;58:1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wright LA-C, Hirsch IB. The challenge of the use of glycemic biomarkers in diabetes; reflecting on hemoglobin A1c, 1,5-anhydroglucitol, and the glycated proteins fructosamine and glycated albumin. Diabetes Spectrum. 2012;25:141-148. [Google Scholar]
- 46. Sacks DB, Nathan DM, Lachin JM. Gaps in the glycation gap hypothesis. Clin Chem. 2010;57:150-152. [DOI] [PubMed] [Google Scholar]