Abstract
Macrophages transformed foam cell formation occurs as a result of leukocyte accumulation mediated through intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), and E-selectin, secreted by inflamed or damaged endothelium. The key molecule is the ICAM-1, member of the adhesion immunoglobulin super family that maps to chromosome 19 p13.2-p13.3 codes for 505 amino acids have five extracellular domains including circulatory leukocytes binding site (primarily monocytes) for recruiting it at the sites of inflammation and the tight adhesion with vascular endothelium for the above mentioned pathogenesis as an initial step. Hence the objective of the current paper is to review the Genome Wide Association (GWA) studies and summarizes its understanding of functional Single Nucleotide Polymorphism (SNP's) of ICAM-1 clinical association to provide better guidance for the clinicians and researchers of the merits, demerits of the current results and direct them to do research on larger number of population for better prospective.
Keywords: ICAM, SNP, Genome wide association study
1. Introduction
Endothelium remodeling is a cardinal process for the cause of mortality concerned inflammation related diseases such as Coronary Artery Disease (CAD), Myocardial infarction (MI),1 Diabetic Retinopathy (DR),2 Tumor,3 Rheumatiod Arthritis (RA),4 Primary Sclerosing Cholangitis (PSC),5 Severe Preeclampsia (SPE),6 Fuchs Uveitis (FU), Hashimoto Thyroiditis (HT)7 and Microvascular Complication (MC)8 etc. Deregulated endothelium enhances endothelial permeability to lipoproteins and other plasma components that increases the adhesive capacity with up-regulated leukocyte adhesion molecules (L-selectin, integrins, and platelet endothelial-cell adhesion molecule 1 (PECAM-1)) and endothelial adhesion molecules (including E-selectin, P-selectin, endothelial intercellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule (VCAM-1)). The key molecule is the ICAM-1, member of the adhesion immunolglobulin superfamily9,10 that maps to chromosome 19 p13.2-p13.3 codes for 505 amino acids possess 5 extracellular domains including circulatory leukocytes binding site (primarily monocytes) for recruiting it at the sites of inflammation and the tight adhesion with vascular endothelium for the above mentioned pathogenesis as an initial step. Epidemiological studies are reported from various parts of the world but still the results are controversial11,12 and inconclusive.13,14 Hence the objective of the current paper is to review the Genome Wide Association (GWA) studies and summarizes its understanding of functional Single Nucleotide Polymorphism (SNP's) of ICAM-1 clinical association to provide better guidance for the clinicians and researchers of the merits, demerits of the current results and direct them to perform research on larger number of population for better prospective.
2. Cell adhesion molecular family (CAM)
Selectin family (E, P and L-selectin), immunoglobulin super family (ICAM, PECAM-1 and VCAM-1) and integrins (CD11/18) belongs to CAM that helps the cells interactions. The rapid cycle of attachment, detachment and reattachment underlying rolling of leukocyte through the glycoprotein and glycolipids containing sialyl-Lewisx (sLex, CD 15s) structure11 is found on neutrophils, monocytes, and some lymphocytes that interact with selectins. They are clustered in tandem within a 220-kb region of chromosome 1q23.12 Activated endothelium13 transiently expresses E-selectin (ELAM-1, SELE, CD62E) which supports rolling of neutrophils, monocytes, eosinophils, and some lymphocytes.
There are five members14 of ICAM immunoglobulin super family. Human ICAM-1 gene posses 7 exons and 6 introns making a total of 15.5 kb size in which protein coding region is of 3.3–3.5 kb. ICAM-1 gene structure and its up regulation process at the transcriptional and its post transcriptional have been reported more than a decade before.15 Its regulation is governed by major pathways for the activation of sICAM-1 are NF-kB (TNF-α, IL-1β) and Janus kinases (JAK)-signal transducers signal transduction (STAT) (IFN-γ) pathway through cis-elements in proximal NF-kB binding site and IFN-γ responsive element (IRE) located about 200 bp and 100 bp upstream of the translational start site respectively. ICAM-1 promoter region also contain many AP-1 site in the promoter for its expression via MAP kinase pathways, (through extracellular signal-regulated kinase (ERK), the c-Jun amino-terminal kinase (JNK), and the p38 kinase) important for ICAM-1 expression in endothelial cells. The ICAM-1 variants are present in introns, exons and promoter region. It is well known that intronic variants have the potential to effect a change in mRNA (messenger Ribonucleic Acid) processing and stability that can result in aberrant splicing16 causing altered cell–cell interaction.
ICAM-2 gene is located on chromosome 17 region q23-25, codes for a family of type I cell surface glycoprotein which are characterized by immunoglobulin-like extracellular C-type domains that vary in number between two and nine (ICAM–2, -4 have two, and ICAM-1, -3 have five, ICAM-5 have nine domains) followed by a transmembrane region and a cytoplasmic domain. The cell-surface ligands are integrin lymphocyte function-associated antigen-1 LFA-1 (CD18/CD11a, L2), and (ICAM-1, -2, and -4) integrin Mac-1 (macrophage antigen-1) (CD18/CD11b, M2) and (ICAM-3) novel integrin CD18/CD11d, D2.17
Leukocytes, endothelial cells and platelets express ICAM-2 (CD102) constitutively but the expression remains at basal levels by inflammatory cytokines or other treatments under all conditions studies.18
ICAM-3(CD 50) also constitutively expressed highly on the surface of almost resting leucocytes (neutrophils, monocytes, and lymphocytes whereas endothelium ICAM-3 expression is low in inflammatory diseases. It may be important in the generation of immune responses.19 Red Blood.
Cell (RBC) specifically expresses ICAM-4 whereas ICAM-5 (telencephalin, TLN) is expressed only by neurons within the telencephalon of mammalian brains. Platelet or endothelial cell adhesion molecule-1 PECAM-1 (CD31 antigen) mapped to chromosome 17 in the region 17q23 is expressed on the surface of circulating platelets, monocytes, neutrophils, and particular T cell subsets are implicated in transendothelial migration of leukocytes, angiogenesis, and integrin activation. Mononuclear cell-specific adhesion molecule VCAM-1 (CD106) mapped to 1p32-p31 was found only on the surface of mononuclear cells and not on normal endothelium at baseline. Macrophages and dendritic cells, its counter receptor is the integrin very late antigen-4 (VLA-4, CD49d/CD29 and a4b1) is expressed on monocytes, lymphocytes, and eosinophils, but not on neutrophils. Interaction between VLA-4 and VCAM-1 plays major role in binding of this cell to activated endothelial cells.
3. ICAM-1
The ICAM-1 exists as membrane bound and soluble (sICAM-1) glycoprotein. Membrane bound protein belongs to type 1 transmembrane with molecular weight varies on glycosylation pattern (unglycosylated 60 KDa and glycosylated 80–114 KDa). It was demonstrated to be expressed also in leukocytes and endothelial cells (EC). This protein has extracellular portion (attached to a 24 residues single hydrophobic transmembrane region), transmembrane and a 28 residues short cytoplasmic tail. Five extracellular domains (D-1 to D-5) contain 453 amino acids, mostly hydrophobic and its β-sheet structure is stabilized by di-sulfide bond.20 The D1 and D3 interact with their ligands LAF-1 and Mac-1 on leukocytes correspondingly and the tyrosine residue of cytoplasmic tail mediates the signaling pathway. Trans-endothelial migration of leukocytes to the site of inflammation occurs in four steps through rolling and leukocyte integrin tethering attachment (Endothelial E-and P-selectin binding), activation of integrins by chemokines (increased adhesion to ligands), firm adhesion (LFA-1, Mac-1) and leukocyte migration into sub-endothelial surface. ICAM-1 causes leukocyte transmigration by EC junctional adhesion molecules weakening and contraction via inducing intracellular Ca2+ levels leading to the activation of p38 and Rho.21
Like membrane bound ICAM-1, it possess extracellular domain except transmembrane and cytoplasmic region. sICAM-1 is expressed on the wide variety of human cells such as vascular EC, saphenous vein EC, aortic smooth muscle cell, astrocytes, keratinocytes and carcinoma cells. It released in to circulation through the proteolytic cleavage of extracellular region of membrane bound ICAM-1 by Matrix Metalloproteinases (MMP), human leukocyte elastase and TNF-α converting enzyme (TACE)22 and its level may be detected in blood and other body fluids. The advantages of sICAM-1 binding with its ligands have been suggested explored to utilize it as a potential agent to block interaction of leukocyte and endothelial.23 Recent studies suggested that circulating serum sICAM-1 concentration can be used predict the risk of post transplant ischemic events or cardiac graft failure and CAD.24
4. ICAM-1 and its molecular pathogenesis mechanism
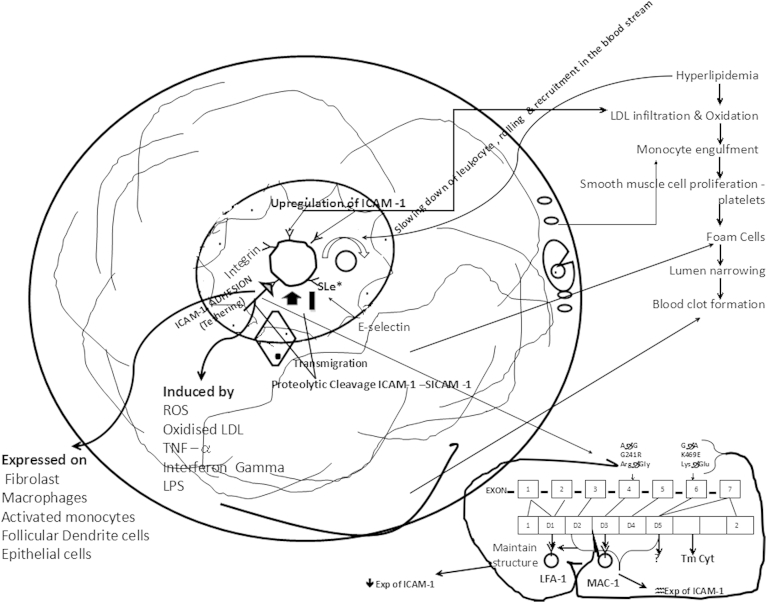
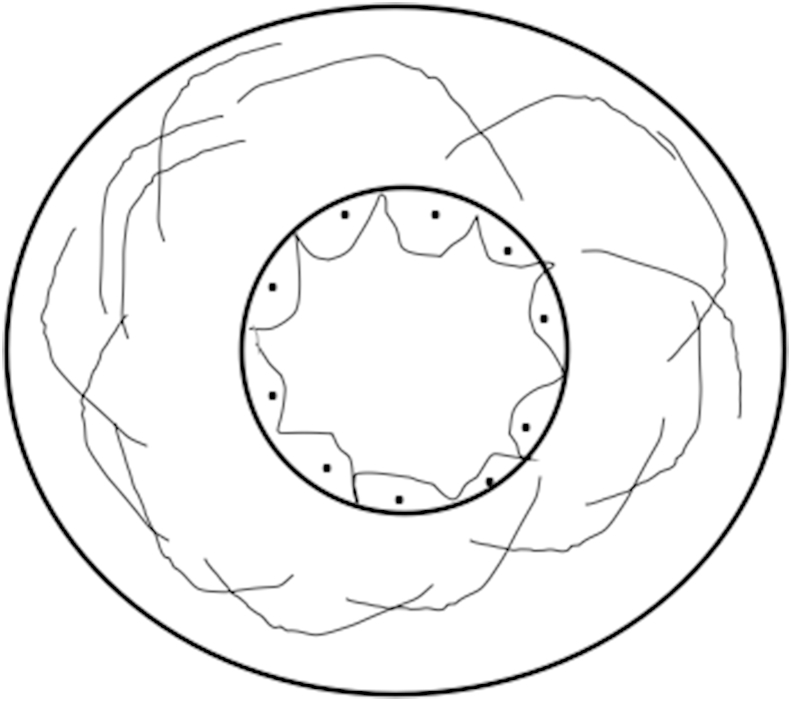
The interaction between lipids, the endothelium, circulating and tissue inflammatory cells (leukocytes), platelets and vascular smooth muscle cells and extracellular matrix in the intima of large arteries results in pathogenesis of disease, are one of the earliest and critical events. In case of atherosclerosis, hyperlipidemia causes slowing down of circulating leukocytes in the streaming blood by rolling and tethering of circulating leukocytes in the monocytes and lymphocytes along the arterial endothelial lining, mediated by the selectin class of adhesion molecules and subsequent firm attachment to endothelial cells and transendothelial migration of these adherent leukocytes across the endothelial surface to the intima of the blood vessels wall. Its accumulation in the intima is mediated by β2-integrins by interacting with counter ligands from the Ig superfamily including the ICAM-1 in activated endothelial cells, where monocytes transform into lipid engorged “foam cells” by the uptake of lipids that which results of fatty streak lesions formation. The secretion of cytokines and growth factors are also important events in the initiation and progression of atherosclerotic plaques25 as shown in Fig. 2 incontrast to normal endothelium as shown in Fig. 1.
Fig. 2.
Schematic detailed representation of ICAm-1 molecular mechanism for CAD and MI; LPS Lipopolysachride, TNF Tumor Necrosis Factor, ROS Reactive Oxygen Species, LDL Low Density Lipoproteins.
Fig. 1.
Normal vascular lumen with intact endothelium.
5. Discussion
ICAM-1 is a well-known identified critical molecule secreted by endothelium during vascular inflammation and is responsible for formation, growth and rupture of atheroma.26 Table 1 provides the ICAM-1 gene SNP's details that are investigated for its clinical association with cardiac diseases. Among the SNP's, G241R and K469E gene are studied in quite considerable number of population globally and are implicated for different phenotypes. The studies of McGlinchey PG et al27 (2004), Milutinovic A et al28 (2006) and Aminian B et al (2007)1 showed the lack of ICAM-1 gene association with Ischemic Heart Disease (IHD) (K469E) and MI (G24R1) patients. In contrast, a recent study29 on atherosclerosis risk communities concluded that ICAM-1 241RR genotype is associated with increased risk of incident Ischemic Stroke (IS) in both whites and African Americans. The same study also demonstrated the non-association of K469Egenotype. Mohammed AA et al30 proved that K469E polymorphism is positively associated with CAD and negatively associated with the circulating levels of sICAm-1 in Egyptian population. For ACS recurrence and cardiac mortality, Liu Lz et al31 proposed that K allele could be independent risk factor. In 2014, Zou S32 et al performed a control study meta-analysis on large Chinese and established that individuals with KK genotype carries CAD risk by 80% in similar to Lid et al,33 Jin Yn et al34 and Yanan Y et al.35 Clinical studies also suggested that ICAM-1 genotypes were not related to CAD severity,36 intima medial thickness and coronary artery calcium.11
Table 1.
ICAM-1 molecule SNP's information.
| S. No | SNP | Allele change | Amino acid change | Function | Region |
|---|---|---|---|---|---|
| 2 | rs5491 | A > T | Lys56Met (M56K) | Missense | Exon2 |
| 1 | rs1799969 | G > A | Gly241Arg (G241R) | Missense | Exon4 |
| 4 | rs1801714 | C > T | Pro352Leu (P352L) | Coding non-synonymous | Exon5 |
| 3 | rs5498 | A > G | Lys469Glu (K469E) in domain5 | Missense upstream variant | Exon 6 |
| 5 | rs 281428 | C > T | – | Introns variant | Introns |
| 6 | rs281432 | C > G | – | Intron variant | Intron |
| 7 | rs281437 | C > T | – | Upstream variant (3′-UTR) | Intron |
Classical risk factors such DM, HTN, hyperlipidemia and smoking parameters, either alone or in combination provides invaluable information in predicting the risk of CAD due to the disease complexity. Hence, the search for biomarkers to validate risk factor was necessitated. Studies attaining this objective have shown the synergistic effect of rs5498 polymorphisms with smoking and triglycerides (G allele) posess threats to CAD64 risk like other risk factors such DM2 (AA genotype: K469E), HTN and low HDL concentration.65 GWA investigation studied four novel loci and showed that ICAM-1 (K469E) polymorphism determines the circulating concentration of sICAM-166 apart from G241R.
Circulating levels of sICAM-1 showed positive and negative associations30 with atherosclerosis particularly with CAD and unstable angina patients. Studies67 were carried to determine whether polymorphism affects the sICAM-1 levels or cell cell–cell binding ability. It concluded that G241R/K469E polymorphisms are responsible for expression pattern and not concerned with binding abilities. Several studies had shown that sICAM-1 may be used to predict post operative LCO event68 in pulmonary artery hypertensive patients, MI progress and recurrence after thrombolysis.69 It was also found to have inverse relation with left ventricular ejection fraction.70 Clinical studies have shown that pitavastatin stimulated urocortin-1,71 belongs to corticotrophin releasing factor/Urotensin I family to be safe and effective anti-inflammatory and may be used as a new line of atherosclerosis related diseases treatment in future.
The results of genotypes are still debatable except in Chinese population than the rest of the world, as the publication's and sample size are less. Even the limited number of publication doesn't permit us for meta-analysis of data in that particular region. This might be attributable to high cost involved in genotyping techniques such Polymerase Chain Reaction-Fragment Length Polymorphism (PCR-RFLP),30 sequencing or real time RCR29 which are commonly employed by the researchers. Hence, we suggest the use of reliable, convenient and less time consuming novel genotyping method of Polymerase Chain Reaction with Confronting Two Pair Primer (PCR-CTPP).72 This method involves normal PCR reaction with four primers (a pair forward and reverse primer) followed by agarose gel electrophoresis and has been demonstrated in clinical samples.73 The designing of primers could be done using prim-SNPing online software tool.
In general beyond ethnicity, ICAM-1 K469 E and G241R genotype is found to be common variant in the diseased population and may be promising molecule particularly for clinical and sub-clinical atherosclerosis. Thus, we conclude that future studies should focus on adopting reliable, cost effective and less time consuming methods to perform K469E and G241R genotyping on large number of similar ethnic groups and thereby making it possible to establish the real fate of genotype for the benefit of the society.
Conflicts of interest
All authors have none to declare.
Acknowledgment
I thank my wife A. Vasugi for preparing table and Ms. Valli Pannerselvam for providing technical support in preparing this manuscript. My sincere and honest gratitude to Mrs. Revathy Vijayakumar, Doctors Secretary for spending her precious time in drawing the schematic representation of ICAM-1 molecular mechanism picture.
References
- 1.Aminian B., Ardekani A.R.A., Arandi N. ICAM-1 polymorphisms (G241R, K469E) in coronary artery disease and myocardial infarction. Iran J Immunol. 2007;4:227–235. doi: 10.22034/iji.2007.17202. [DOI] [PubMed] [Google Scholar]
- 2.Vinita K., Sripriya S., Prathiba K. ICAM-1 K469E polymorphism is a genetic determinant for the clinical risk factors of T2D subjects with retinopathy in Indians: a population based case–control study. BMJ Open. 2012:e001036. doi: 10.1136/bmjopen-2012-001036. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q.L., Li B.H., Liu B. Polymorphisms of the ICAM-1 exon 6 (E469K) are associated with differentiation of colorectal cancer. J Exp Clin Cancer Res. 2009;28:139. doi: 10.1186/1756-9966-28-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimiuk P.A., Sierakowski S., Latosiewicz R. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann Rheum Dis. 2002;61:804–809. doi: 10.1136/ard.61.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lus C.B., Karlsen T.H., Broom U. Analysis of MAdCAM-1 and ICAM-1 polymorphisms in 365 Scandinavian patients with primary sclerosing cholangitis. J Hepatol. 2006;45:704–710. doi: 10.1016/j.jhep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Tabatabai E., Salimi S., Khorasani M.M. KE and EE genotypes of ICAM-1 gene K469E polymorphism is associated with severe preeclampsia. Dis Markers. 2014;124941:1–5. doi: 10.1155/2014/124941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emin A.F., Muge K., Pervin V. 241R and K469E polymorphisms of intercellular adhesion molecule 1 (ICAM-1) could predispose to Hashimoto thyroiditis. Mol Biol Rep. 2012;39:10723. doi: 10.1007/s11033-012-1963-7. [DOI] [PubMed] [Google Scholar]
- 8.Su X., Chen X., Liu L., Chang X., Yu X., Sun K. Intracellular adhesion Molecule-1 K469E gene polymorphism and risk of diabetic microvascular complications: a meta-analysis. Plos One. 2013;8:e69940. doi: 10.1371/journal.pone.0069940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebedeva T., Dustin M.L., Sykulev Y. ICAM-1 co-stimulate target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–258. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins A.M., Baird A.W., Nusrat A. ICAM-1: targeted docking for exogenous as well as endogenous ligands. Adv Drug Deliv Rev. 2004;56:763–778. doi: 10.1016/j.addr.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Bielinski S.J., Reiner A.P., Nickerson D. Polymorphisms in the ICAM1 gene predict circulating soluble intercellular adhesion molecule-1(sICAM-1) Atherosclerosis. 2011;216:390–394. doi: 10.1016/j.atherosclerosis.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGlinchey P.G., Spence M.S., Patterson C.C. The ICAM-1 gene K469E polymorphism is not associated with ischaemic heart disease: an investigation using family-based tests of association. Eur J Immunogenet. 2004;31:201–206. doi: 10.1111/j.1365-2370.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 13.Bielinski S.J., Pankow J.S., Li N. ICAM1 and VCAM1 polymorphisms, coronary artery calcium, and circulating levels of soluble ICAM-1: the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;201:339–344. doi: 10.1016/j.atherosclerosis.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang W., Pankow J.S., Carr J.J. Association of sICAM-1 and MCP-1 with coronary artery calcification in families enriched for coronary heart disease or hypertension: the NHLBI Family Heart Study. BMC Cardiovasc Disord. 2007;7:30. doi: 10.1186/1471-2261-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roebuck K.A., Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 16.Baralle D., Baralle M. Splicing in action: assessing disease causing sequence changes. J Med Genet. 2005;42:737–748. doi: 10.1136/jmg.2004.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foxall C., Watson S.R., Dowbenko D., Fennie C., Lasky L.A. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson M.L., Kingsmore S.F., Johnston G.I., Siegelman M.H., Le Beau M.M. Genomic organization of the selectin family of leukocyte adhesion molecules on human and mouse chromosome 1. J Exp Med. 1990;172:263–272. doi: 10.1084/jem.172.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings R.D., Smith D.F. The selectin family of carbohydrate binding proteins: structure and importance of carbohydrate ligands for cell adhesion. Bioessays. 1992;14:849–856. doi: 10.1002/bies.950141210. [DOI] [PubMed] [Google Scholar]
- 20.Staunton D.E., Dustin M.L., Erickson H.P., Springer T.A. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- 21.Lawson C., Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 22.Tsakadze N.L., Sen U., Zhao Z. Signals mediating cleavage of intercellular adhesion molecule-1. Cell Physiol. 2004;287:C55–C63. doi: 10.1152/ajpcell.00585.2003. [DOI] [PubMed] [Google Scholar]
- 23.Marlin S.D., Staunton D.E., Springer T.A., Stratowa C., Sommergruber W., Merluzzi V.J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990;344:70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- 24.Labarrere C.A., Nelson D.R., Miller S.J. Value of serum-soluble intercellular adhesion molecule-1 for the noninvasive risk assessment of transplant coronary artery disease, posttransplant ischemic events, and cardiac graft failure. Circulation. 2000;102:1549–1555. doi: 10.1161/01.cir.102.13.1549. [DOI] [PubMed] [Google Scholar]
- 25.Gahmberg C.G., Tolvanen M., Kotovuori P. Leukocyte adhesion – structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur J Biochem. 1997;245:215–232. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 26.Ley K., Miller Y.I., Hedrick C.C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGlinchey P.G., Spence M.S., Patterson C.C. The intercellular adhesion molecule-1 (ICAM-1) gene K469E polymorphism is not associated with ischaemic heart disease: an investigation using family-based tests of association. Eur J Immunogenet. 2004;31:201–206. doi: 10.1111/j.1365-2370.2004.00474.x. [DOI] [PubMed] [Google Scholar]
- 28.Milutinović A., Petrovic D. The K469E polymorphism of the intracellular adhesion molecule 1 (ICAM-1) gene is not associated with myocardial infarction in Caucasians with type 2 diabetes. Folia Biol (Praha) 2006;52:79–80. doi: 10.14712/fb2006052030079. [DOI] [PubMed] [Google Scholar]
- 29.Volcik K.A., Ballantyne C.M., Hoogeveen R., Folsom A.R., Boerwinkle E. Intercellular adhesion molecule-1 G241R polymorphism predicts risk of incident ischemic stroke: atherosclerosis Risk in Communities study. Stroke. 2010;41:1038–1040. doi: 10.1161/STROKEAHA.109.575563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed A.A., Rashed L., Amin H., Abu-Farha M., Fadl S.A.E., Pakhoum S. K469E polymorphism of the intercellular adhesion molecule-1 gene in Egyptians with coronary heart disease. Ann Saudi Med. 2010;30:432–436. doi: 10.4103/0256-4947.71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L.Z., Wu E.P., Liu H.L. Relation between K469E gene polymorphism of ICAM-1 and recurrence of ACS and cardiovascular mortality. Asian Pac J Trop Med. 2013;6:916–920. doi: 10.1016/S1995-7645(13)60164-9. [DOI] [PubMed] [Google Scholar]
- 32.Zou S., Pan X., Chen Z., Wei C., He B., Zhang H. Intercellular adhesion molecule-1 K469E polymorphism and risk of coronary artery disease: a meta-analysis. Med Sci Monit. 2014;15:2677–2682. doi: 10.12659/MSM.891235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D., Qu C., Dong P. The ICAM-1 K469E polymorphism is associated with the risk of coronary artery disease: a meta-analysis. Coron Artery Dis. 2014;25:665–670. doi: 10.1097/MCA.0000000000000136. [DOI] [PubMed] [Google Scholar]
- 34.Ji Y.N., Wang Q., Zhan P. Intercellular adhesion molecule 1 gene K469E polymorphism is associated with coronary heart disease risk: a meta-analysis involving 12 studies. Mol Biol Rep. 2012;39:6043–6048. doi: 10.1007/s11033-011-1418-6. Epub 2011 Dec 28. [DOI] [PubMed] [Google Scholar]
- 35.Yanyan L. Intercellular adhesion molecule-1 E469K gene polymorphism and coronary artery disease in the Chinese population: a meta-analysis involving 3065 subjects. Clin Cardiol. 2012;35:55–60. doi: 10.1002/clc.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M., Fu Z., Zhang Q., Xin Y., Chen Y., Tian Y. Association between the polymorphisms in intercellular adhesion molecule-1 and the risk of coronary atherosclerosis: a case-controlled study. PLoS One. 2014;9:e109658. doi: 10.1371/journal.pone.0109658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarecka-Hujar B., Zak I., Krauze J. Interactions between rs5498 polymorphism in the ICAM1 gene and traditional risk factors influence susceptibility to coronary artery disease. Clin Exp Med. 2009;9:117–124. doi: 10.1007/s10238-008-0022-0. [DOI] [PubMed] [Google Scholar]
- 65.Tang L., Peng H., Xu T. Association of biomarkers of inflammation with dyslipidemia and its components among Mongolians in China. PLoS One. 2014;9:e89023. doi: 10.1371/journal.pone.0089023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paré G., Ridker P.M., Rose L. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7:e1001374. doi: 10.1371/journal.pgen.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai R., Yi S., Zhang X., Liu H., Fang X. Role of ICAM-1 polymorphisms (G241R, K469E) in mediating its single-molecule binding ability: atomic force microscopy measurements on living cells. Biochem Biophys Res Commun. 2014; 13;448:372–378. doi: 10.1016/j.bbrc.2014.04.113. [DOI] [PubMed] [Google Scholar]
- 68.Yıldırım A., Güzelmeriç F., Öner C.N. Prognostic significance of sICAM-1 and sVCAM-1 molecules for cardiac surgery in pediatric patients with pulmonary hypertension. Anadolu Kardiyol Derg. 2014;14:274–279. doi: 10.5152/akd.2013.4543. [DOI] [PubMed] [Google Scholar]
- 69.Saidi H., Vakilian M., Noori G.H., Ghafouri H.B., Abazarian N. Alterations in circulating adhesion molecules in acute myocardial infarction before and after thrombolysis with Streptokinase. J Cardiovasc Thorac Res. 2013;5:139–141. doi: 10.5681/jcvtr.2013.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hulok A., Sciborski K., Marczak J., Bańkowski T., Poręba Rand Negrusz-Kawecka M. Soluble cell adhesion molecules – does estimating sVCAM-1 and sICAM-1 concentration provide additional information about cardiovascular risk in patients with coronary artery disease? Adv Clin Exp Med. 2014;23:735–741. doi: 10.17219/acem/37232. [DOI] [PubMed] [Google Scholar]
- 71.Hasegawa A., Sato K., Shirai R. December 2, 2014. Vasoprotective Effects of Urocortin 1 Against Atherosclerosis in Vitro and in Vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin G., Mitsuda Y., Ezaki T., Hamajima N.A. New PCR method: one primer amplification of PCR-CTPP products. Mol Biotechnol. 2012;52:180–183. doi: 10.1007/s12033-011-9485-4. [DOI] [PubMed] [Google Scholar]
- 73.Kilic U., Gok O., Bacaksiz A., Izmirli M., Elibol-Can B. SIRT1 gene polymorphisms affect the protein expression in cardiovascular diseases. PLoS One. 2014;9:e90428. doi: 10.1371/journal.pone.0090428. [DOI] [PMC free article] [PubMed] [Google Scholar]