Abstract
Previous studies reported impaired cerebral cortical gray matter development and neurodevelopmental impairment following neonatal dexamethasone treatment for chronic lung disease in preterm newborns. No long-term effects on neurocognitive outcome have yet been shown for hydrocortisone treatment. A prospective study was performed to evaluate brain growth at term in preterm infants who did receive neonatal hydrocortisone for chronic lung disease. Thirty-eight preterm infants (n=19 hydrocortisone, n=19 controls) were matched for gestational age at birth. Gestational age and birth weight were 27.0±1.4 vs. 27.6±1.1 weeks (p=ns), and 826±173 vs. 1017±202 gram respectively (p<0.05). Infants were studied at term equivalent age. Hydrocortisone was started with a dose of 5 mg/kg/day for 1 week, followed by a tapering course over 3 weeks. A 3D-MRI technique was used to quantify cerebral tissue volumes: cortical grey matter, basal ganglia/thalami, unmyelinated white matter, myelinated white matter, cerebellum, and cerebrospinal fluid. Infants who were treated with hydrocortisone had more severe respiratory distress. There were no differences in cerebral tissue volumes between the 2 groups at term equivalent age. In conclusion, no effect on brain growth, measured at term equivalent age, was shown following treatment with hydrocortisone for chronic lung disease.
Neonatal corticosteroid exposure for treatment of chronic lung disease in preterm newborns, in particular dexamethasone, is known to have an adverse effect on cerebral development during fetal and early postnatal life. Uno et al (1) reported dexamethasone-induced degeneration and depletion of hippocampal pyramidal and dentate granular neurons in Rhesus monkeys. Subsequently, MRI of the brain at 20 months of age showed a 30% reduction in size and segmental volumes of the hippocampus in dexamethasone treated compared to vehicle treated animals.
The adverse long-term effect of neonatal dexamethasone therapy on neurodevelopmental outcome has also been extensively reported in preterm infants treated for chronic lung disease (CLD) (2,3). Dexamethasone treatment was associated with an increased risk of cerebral palsy (1,2) and an adverse effect on cognitive function (3). Furthermore, it has been shown that neonatal dexamethasone therapy affected regional brain development (1). Advanced quantitative MRI technologies have shown a reduction in total cerebral tissue volume, in particular of the cerebral cortical gray matter volume (6), as well as the cerebellar volume in newborn infants treated with dexamethasone (7).
These findings suggest that there is a need for a safer alternative for the treatment of CLD. Data published so far, using other corticosteroids, such as hydrocortisone for treatment of chronic lung disease are still scarce. In the Wilhelmina Children’s Hospital, hydrocortisone has been used to treat CLD since the late seventies. Hydrocortisone was shown to be equally effective in treatment of CLD in a retrospective observational study (8). This retrospective study also showed that children treated with dexamethasone more often needed special education than matched controls who were not treated with dexamethasone. Moreover, in contrast to dexamethasone, recent studies found no differences between children who were treated with hydrocortisone in the neonatal period as compared to children who did not need hydrocortisone treatment with respect to long-term cognitive and neuromotor outcome (9–11). Furthermore, Lodygensky et al (9) showed no differences in brain tissue volumes and neurodevelopmental outcome in children assessed at the age of 8 years treated with neonatal hydrocortisone compared to children who did not receive any hydrocortisone during their neonatal course. Cortical gray matter and hippocampal volumes were comparable for both groups.
All hydrocortisone studies mentioned above were observational and retrospective. We therefore decided to perform a prospective study to evaluate brain development at term equivalent age in preterm infants, who were treated with hydrocortisone for CLD and compared the results to a group of preterm infants who did not receive hydrocortisone.
PATIENTS AND METHODS
Patients
Twenty-seven preterm infants, born in the Wilhelmina Children’s Hospital, were enrolled into the study at the time they were treated with hydrocortisone for severe respiratory problems. Hydrocortisone was started at a postnatal age of at least one week in an infant who was ventilator dependent with increasing oxygen requirements, which could not be explained by infection or a hemodynamically significant patent ductus arteriosus (PDA), but was considered due to severe infant respiratory distress syndrome (IRDS) and/or development of CLD, despite optimal supportive therapy (diuretics, fluid restriction, pulmonary aerosols). Neonatal hydrocortisone treatment started with a dose of 5 mg/kg/day, divided into 4 doses for one week, followed by a tapering course of 3, 2 and 1 dose(s) every 5 days (a total dose hydrocortisone of 3.75, 2.5 and 1.25 mg/kg/day respectively).
Children with cerebral lesions, more severe than grade 1 germinal matrix hemorrhage-intraventricular hemorrhage (GMH-IVH) or PVL grade I (periventricular echogenicity, being present for more than seven days) on cranial ultrasound were not included in the study. Infants with intra-uterine growth retardation were not eligible for the study. Eight of the 27 infants were excluded because of lack of parental consent (n=5), cerebral pathology visible on cranial ultrasound (n= 2; one was suspected to have pre-cystic PVL on cerebral ultrasound, and the other infant had severe unilateral dilatation of the occipital horn of the lateral ventricle at the moment of inclusion). Furthermore, one patient was excluded because of movement artifacts on MR images. Eventually, 19 hydrocortisone-treated infants were included in the study.
These infants were matched for gestational age and whenever possible also for gender and respiratory status. Three matched controls were born in Utrecht and 16 in Geneva. These children did not receive postnatal corticosteroids and had no intracranial lesions on their cranial ultrasounds (worse than grade I GMH-IVH, PVL grade II–III) or MRI at term equivalent age. All these children had sequential cerebral ultrasound examinations after birth according to similar ultrasound protocols in Utrecht and Geneva.
The study was approved by the local ethics committees. Written informed parental consent was always obtained for the children included in the study.
Data acquisition
Most infants were imaged with sedation to prevent image degradation from motion artifacts. A neonatologist was always present during the examination. During MR examination, the neonates were placed in a vacuum fixation pillow (Med Vac Infant Immobilizer Bag, CFI Medical Solutions, Fenton, MI). Monitoring was performed using pulse oximetry (Nonin, Minneapolis, MN) and respiration rate was observed using the standard Philips equipment (Philips Medical Systems, Best, Netherlands). For hearing protection Minimuffs® (Natus Medical Incorporated, San Carlos, CA) were used.
Both in Utrecht and Geneva, we used a 1.5T Philips MR scanner (Philips Medical Systems, Best, The Netherlands). The protocol consisted of a 3D T1 fast-gradient echo sequence (repetition time (TR) = 15 ms, echo time (TE) = 4.4 ms, flip angle 25°), field of view (FOV) 18 cm and a double echo (T2-and proton-density) fast-spin echo sequence (TR = 3500 ms, TE = 30/150 ms, interleaved, no gap acquisition). Both sequences were acquired with coronal slices of 1.5 mm and in plane resolution of 0.7 × 0.7 mm2, (field of view 18×18 cm2, matrix 256×256).
Data postprocessing
3D Volumetric MRI: Post acquisition processing was carried out on a workstation (Sun Microsystems, Mountain View, CA) with specially developed software (12,13). The T2-weighted and proton density-weighted images were registered to the T1 weighted images. Segmentations of the intra-cranial cavity (ICV), cerebro-spinal fluid (CSF), and four tissue classes (cortical gray matter (CGM), subcortical gray matter (SGM), unmyelinated (UMWM) and myelinated (MWM) white matter) were obtained using a semi-automatic segmentation method (14,15). Sample voxels were selected interactively and optimal estimation of the distribution of MRI signal intensities associated with each type of tissue was estimated. Initial estimates for the tissue classifiers were manually defined by a single investigator (MB). An anatomical template was aligned with the ICV of the subject and utilized to disambiguate the segmentation of tissues that have overlapping signal intensity characteristics but non-overlapping spatial distribution (figure 1). The volume of each tissue class was quantified. The sum of CGM, SGM, UMWM, and MWM defined the total brain volume (TBV). Total brain volume and CSF represented the intracranial volume (ICV).
Figure 1.
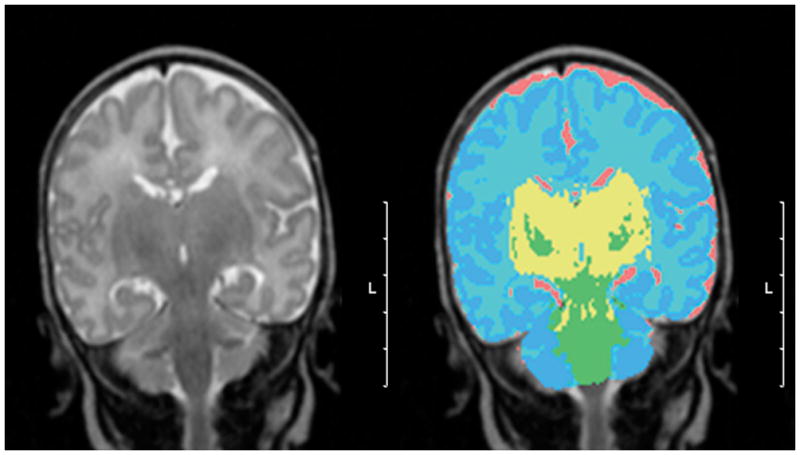
A. T2-weighted MR image in coronal plane. B. Final segmented image depicting CGM in dark blue, UNMW in light blue, CSF in pink, MWM in green, and SGM (basal ganglia) in yellow. (Scale bar = 5 cm, L = left).
Cerebellar volumes were measured by manual outlining on the T2 weighted images of the registered 3D MRI data using Slicer (www.slicer.org) (16). The segmentation was started on coronal slices and then corrected on sagittal slices. Three-dimensional reconstruction of the cerebellum was performed to check the quality of the segmentation (figure 2). A single investigator (R.H.V.) performed all manual outlining of the cerebellum, with an intra-rater reliability coefficient (5 MRI scans) of {alpha} = .96.
Figure 2.
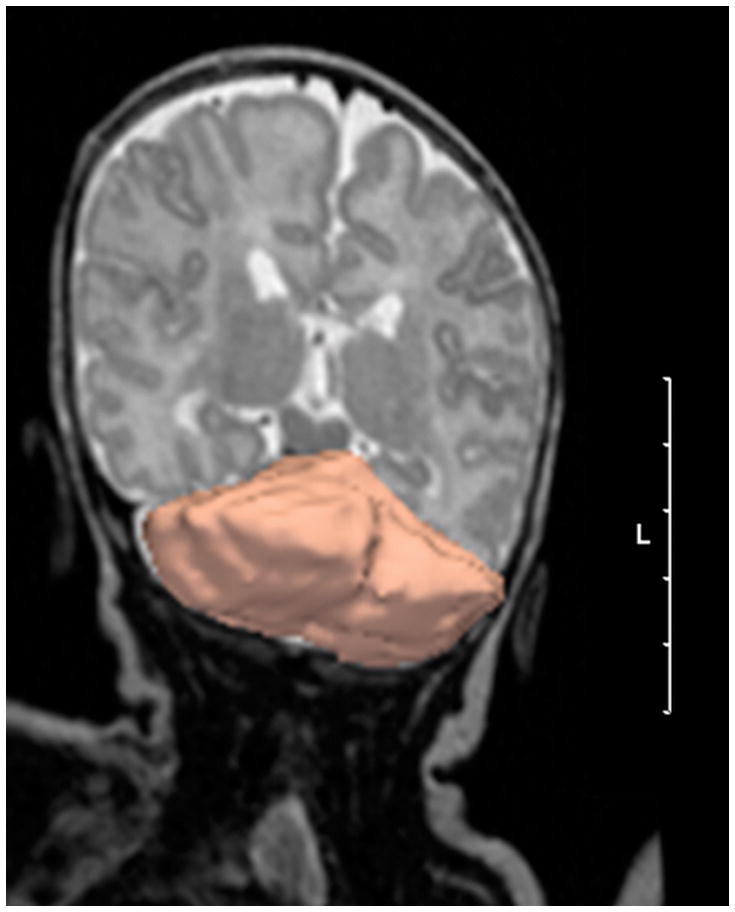
3D reconstruction of manual cerebellar outlining of reconstructed 3D image slices using 3D Slicer software. (Scale bar = 5 cm, L = left). The figure is distorted to give a 3D impression of the cerebellum.
Statistical analysis
Data are summarized as mean ± standard deviation (SD) or as median and ranges where appropriate. Differences between groups in volume of cerebral tissues and in clinical data were evaluated with one factorial ANOVA, with pair wise multiple comparison procedures, followed by a posthoc Bonferroni test if a significant difference was detected, or [chi]2 test when categorical variables were compared. We anticipated that we needed 12 infants in each group to detect the 35% difference in CGM observed following dexamethasone treatment. Furthermore, we did a bio-equivalence means power analysis with a sample size of 20, which concluded equivalence between groups. This means that it will be allowed to conclude equivalence between the groups when no statistical differences will be found with a sample size of 20 (Power 90%, 10% one side significant level). Statistical analyses were performed with SPSS for windows (SPSS version: 12.0).
RESULTS
Clinical characteristics
Table 1 summarizes the clinical data of the two groups. The infants treated with hydrocortisone had lower birth weights (826±173g vs. 1017±826g; p<0.05), without a significant difference in head circumferences at birth (24.3±2.2cm vs. 25.3±1.7cm), compared to the control group. However, at term equivalent age they had comparable weight (3016±735g vs. 3121±524g, p=ns) and head circumference (34.8±2.4cm vs. 35.3±1.5cm; p=ns). The difference between the pulmonary status of both groups was significant: The infants treated with hydrocortisone had more severe respiratory distress than the control infants, although the number of infants treated with antenatal steroids was similar for both groups (n=17 vs. n=16): hydrocortisone patients received more often surfactant (18 vs. 8 infants, p<0.001), required a longer period of ventilation than the infants in the control group (19 days vs. 2 days p<0.001 respectively), and needed CPAP and additional oxygen for a longer period (CPAP: 49 vs. 28 days, p<0.05 and oxygen: 71 vs. 33 days, p<0.001 respectively). Finally, the hydrocortisone-treated infants needed more blood pressure support (17 vs. 9, p<0.05) and developed more blood culture proven sepsis, mostly coagulase negative staphylococcus (12 vs. 6, p<0.05). The control infants did not receive hydrocortisone or dexamethasone at any time during admission.
Table 1.
Clinical characteristics of the hydrocortisone (HC) treated and the matched non-hydrocortisone-treated infants (CTRL). Mean ± SD.
| HC (n=19) | CTRL (n=19) | P | |
|---|---|---|---|
| Gestational age (wks) | 27.0 ± 1.4 | 27.6 ± 1.1 | Ns |
| Birthweight (g) | 826 ± 173 | 1017 ± 202 | <0.05 |
| Weight at term (g) | 3016 ± 735 | 3121 ± 524 | Ns |
| Head circumference | |||
| - at birth (cm) | 24.3 ± 2.2 | 25.3 ± 1.7 | Ns |
| - at term (cm) | 34.8 ± 2.4 | 35.3 ± 1.5 | Ns |
| Gender: | |||
| - Female, n (%) | 4 (21%) | 8 (42%) | Ns |
| - Male, n (%) | 15 (79%) | 11 (58%) | Ns |
| Postnatal age at MRI (wks) | 41.1 ± 0.8 | 40.8 ± 1.0 | Ns |
| Apgar score (range) | |||
| - at 1 min | 6–8 | 2–9 | |
| - at 5 min | 8–10 | 5–10 | |
| Antenatal steroids, n (%) | 16 (84%) | 17 (89%) | Ns |
| Assisted ventilation duration (days) | 19 ± 8 | 3 ± 2 | <0.01 |
| Surfactant, n (%) | 18 (95%) | 8 (42%) | <0.01 |
| CPAP (days) | 49 ± 34 | 28 ± 15 | <0.05 |
| O2 dependence (days) | 71 ± 28 | 33 ± 25 | <0.001 |
| Postnatal age at start HC: | |||
| median (range) (days) | 14 (7–44) | ||
| 7–21days (n) | 15 | ||
| >21days (n) | 4 | ||
| Inotropic support for hypotension, n (%) | 17 (89%) | 9 (47%) | <0.05 |
| PDA, n (%) | 14 (74%) | 9 (47%) | <0.05 |
| NEC, n (%) | 3 (16%) | 1 (5%) | Ns |
| Morphine, n (%) | 18 (95%) | 6 (32%) | <0.001 |
| Postnatal proven sepsis, n (%) | 12 (63%) | 6 (32%) | <0.05 |
CPAP continuous positive airway pressure, PDA patent ductus arteriosus, NEC necrotizing enterocollitits.
MRI Volumetric measurements
All infants had their MRI between 38 and 43 weeks postmenstrual age (table 1). Age at MRI was equivalent between the groups (40.8±1.0 vs. 41.1±0.8 weeks respectively for controls vs. hydrocortisone infants).
Table 2 and figure 3 show the results of the quantitative volumetric measurements for CGM, SGM, UMWM, and MWM and CSF, and cerebellum. No significant differences were observed between the 2 groups, especially not for CGM and total cerebral and cerebellar volumes. The analyses for brain volumes were adjusted for gestational age and postmenstrual age at scan, this did not change the results.
Table 2.
Volumes in mean ± SD (confidence interval 95%) and the mean difference (confidence interval 95%) of the various cerebral tissue classes measured by 3D volumetric MRI (in ml) without statistical difference (p<0.05).
| HC (n=19) | CTRL (n=19) | Mean difference (confidence interval 95%) | |
|---|---|---|---|
| Cortical grey matter | 172 ± 32 (155–183) | 183 ± 38 (172–199) | −10 (−34–12) |
| Unmyelinated white matter | 177 ± 27 (162–191) | 194 ± 32 (180–209) | −17 (−37–3) |
| Myelinated white matter | 8.3 ± 2.2 (7.1–9.5) | 8.7 ± 2.8 (7.7–10.0) | −0.4 (−2.1–1.2) |
| Subcortical grey matter | 19 ± 3 (17–21) | 21 ± 4 (19–22) | −2 (−4–1) |
| Cerebrospinal fluid | 49 ± 18 (37–58) | 57 ± 27 (48–68) | −8 (−23–7) |
| Total cerebral tissue volume | 377 ± 52 (349–398) | 407 ± 62 (385–435) | −30 (−68–8) |
| Total intracranial volume | 425 ± 63 (393–449) | 464 ± 70 (440–496) | −38 (−82–5) |
| Cerebellum | 23 ± 4 (19–25) | 24 ± 4 (20–26) | −1 (−4 − 2) |
Figure 3.
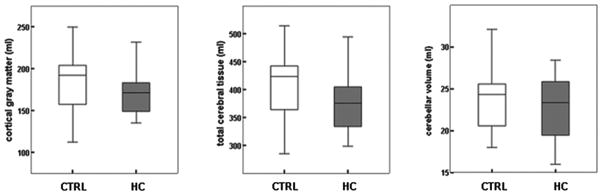
Cerebral cortical gray matter volumes, total cerebral tissue, and cerebellar volume in preterm infants at term who were never treated with postnatal hydrocortisone (CTRL) (n =19), and preterm infants at term previously treated with postnatal hydrocortisone (hydrocortisone; n =19) (expressed as means with 2SD bars).
DISCUSSION
In the present study, we compared brain development in 38 preterm infants who did or did not receive postnatal treatment with hydrocortisone for chronic lung disease. Using quantitative volumetric MRI techniques, we showed that volumes of total cerebral and cerebellar volumes, and volumes of gray and white matter were not significantly different in both groups at term equivalent age. This was even so without correction for confounding factors such as significant difference in birth weight, severity of chronic lung disease and the fact that the infants treated with hydrocortisone in the neonatal period had more complications during the first weeks of life as indicated by their pulmonary status, the higher need for inotropic support and the higher incidence of proven sepsis (17). Moreover, the volumes of all the cerebral tissue classes were comparable with previously published results, using the same advanced MRI techniques (6,12,18–21).
We were able to study a larger group of infants than Murphy et al (6), who found an impairment in brain growth, principally affecting cerebral cortical gray matter, following neonatal dexamethasone therapy in 7 preterm infants using the same advanced MRI techniques. The average CGM volumes measured in hydrocortisone infants (172 ml) were larger than average CGM volumes in Murphy’s dexamethasone infants (130 ml), which suggests that postnatal dexamethasone alters cerebral development in premature infants more than postnatal hydrocortisone.
Recent retrospective observational studies, using matched controls never treated with corticosteroids, already showed that preterm born infants who were treated with hydrocortisone during the neonatal period were not different with regard to cognitive, neurodevelopmental and motor outcome at 8–10 years of age (10,11). Moreover a volumetric MRI study at 8 years of age showed no differences in hippocampal and gray matter volumes in preterm born children who received hydrocortisone during the neonatal period as compared to a matched control group of children born preterm (9). These retrospective observational studies already suggested that the use of hydrocortisone for treatment of CLD did not adversely affect neurodevelopmental outcome, in contrast to the prospective randomized controlled studies that showed an adverse effect of dexamethasone used for this indication (2–4).
Although it is still to be shown that shorter courses of dexamethasone with lower dosages, used nowadays to treat CLD of prematurity, also have an adverse effect on neurodevelopmental outcome, there are several reasons to use hydrocortisone rather than dexamethasone. The impact of glucocorticosteroid exposure on the developing brain is dependent on the expression of mineralocorticoid receptors and glucocorticoid receptors on the neurons and microglial cells and the affinity of a particular glucosteroid for these receptors (22,23). Hydrocortisone has a mainly mineralocorticoid-mediated activity and a much shorter half-time (8-to-12 h) as compared to dexamethasone which has mainly a glucocorticosteroid-mediated action, a much longer half time (up to 48 h) triggering accumulation in the brain and an anti-inflammatory action up to 25-to-30 times higher than hydrocortisone (24). Moreover, animal studies have shown that activation of glucocorticoid receptors lead to adverse effects on neuronal cells such as promotion of apoptotic activity of granular cells in the hippocampus (1). In contrast to glucocorticoid receptors, mineralocorticoid receptors are protective against apoptosis by upregulation of anti-apoptotic proteins. Another mechanism may also play an important role here: 11βhydroxysteroid dehydrogenase type 2, which has a high expression in the fetal brain and several regions of the neonatal brain, acts as hydrogenase, inactivating cortisol (hydrocortisone is equivalent to cortisol) through conversion to dehydrocorticosterone and cortisone. Thus, it protects against abundant mineralocorticoid receptors and glucocorticoid receptors binding of exogenous corticosteroid exposure. This mechanism does not apply to exogenous dexamethasone treatment since this compound cannot be converted by 11-β-hydroxysteroid dehydrogenase type 2, which may, at least partly, explain the negative effect of dexamethasone on brain development compared to hydrocortisone. Loss of 11-β-hydroxysteroid dehydrogenase type 2 from the fetus and fetally derived tissue has been shown to alter development of the cerebellum (25,26). Although lowering the dose of dexamethasone could be one strategy to prevent abundant glucocorticoid receptors binding by dexamethasone, lower dosages used in the study of Parikh et al (7) still affected brain growth.
Given the results of the present study, the circumstantial evidence provided by several retrospective matched-controlled studies, the above mentioned considerations and the fact that it seems to be clinically as effective as dexamethasone (8), we would suggest that hydrocortisone is a better choice than dexamethasone for the treatment of CLD in preterm infants.
Nevertheless, the present study has several limitations, like the relatively small number of patients included and the fact that the matched controls were partly derived from a different institution (the University Hospital of Geneva) compared with the hydrocortisone patients who all came from one centre (the Wilhelmina Children’s Hospital of the University Medical Center Utrecht). At the time of this study, it was not yet routine to perform MRI at term equivalent age in high-risk preterm infants. Permission for an MRI at term equivalent age was not often obtained in the absence of severe intracranial lesions and when no hydrocortisone treatment was given. Geneva has a long-standing reputation as a center of expertise for performing neonatal MRI, also for infants with less severe neonatal problems. Since antenatal and neonatal intensive care treatment policies of very low birth weight infants and population backgrounds are comparable between these 2 hospitals and MRI acquisition protocols at term equivalent age were identical, combining data from the two centers was considered acceptable. MRI measures of tissue volume may be impacted by differences of MRI electronics noise, by gradient non-linearity, by B0 and B1 inhomogeneities, and by subject-specific physiological factors. However, the impact of these MRI phenomena is much smaller than the inter-subject variability in tissue volume, and so we did not attempt to measure or compensate for these phenomena (27).
Segmentation of the newborn brain MRI is challenging due to the small size of the developing brain. With the intensity of unmyelinated white matter being somewhere between gray matter and cerebrospinal fluid, partial volume averaging between these two tissues may result in small misclassification at border zones of these two tissues, which is a limitation of the technique. However, the algorithm utilized here has been developed and validated specifically for neonatal brain MRI. Validation experiments have demonstrated the tissue classification algorithm has high reproducibility (14), and is robust in the presence of signal intensity artifact (15). Comparison of estimates of CSF volumes from MRI to fluid removed indicates the algorithm has high absolute accuracy (28).
It is also unlikely that under powering of the study may have prevented a reliable result: in spite of the fact that the hydrocortisone group consisted of sicker prematurely born infants with lower birth weights, who had a higher need for inotropic support because of hypotension and more intense ventilatory support with significantly higher oxygen needs, and were more often affected by a septicemia, which was recently shown to adversely affect the white matter (17,29), no difference in MRI-determined brain development was detected as compared to a healthier control group. Rather than to prolong the study period by trying to collect all infants in one NICU-center, we choose for the best possible matching by collaborating with Geneva, where the volumetric analysis was performed.
In conclusion, in this small group of preterm infants we were unable to show an adverse effect of hydrocortisone on cerebral growth at term equivalent age, using volumetric 3D-MRI techniques. The preterm infants who received hydrocortisone for severe respiratory problems were compared with age-matched controls who experienced less severe respiratory problems and were less often affected by a septicemia. This study would support our previous retrospective observational long-term follow-up data after neonatal treatment with hydrocortisone in contrast to dexamethasone treatment. We nowadays tend to restrict neonatal corticosteroid therapy to exceptional clinical conditions. When corticosteroid therapy is considered, hydrocortisone may be a safer alternative than dexamethasone. A randomized controlled trial for hydrocortisone would be welcome to possibly further substantiate our findings.
Acknowledgments
Financial Support: This research was supported by Royal Netherlands Academy of Arts and Sciences, the Termeulen fund and IPRF young investigator Fellowship. And part by NIH grants R01 RR021885 and R01 GM074068.
M. Tas, medical student, for collecting the clinical data.
ABBREVIATIONS
- CGM
cortical gray matter
- CLD
chronic Lung Disease
- CSF
cerebrospinal fluid
- ICV
intracranial volume
- SGM
subcortical gray matter
References
- 1.Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 2.O’Shea TM, Washburn LK, Nixon PA, Goldstein DJ. Follow-up of a randomized, placebo-controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics. 2007;120:594–602. doi: 10.1542/peds.2007-0486. [DOI] [PubMed] [Google Scholar]
- 3.Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 4.Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, Yurman S, Dolfin T, Kogan A, Dollberg S, Arbel E, Goldberg M, Gur I, Naor N, Sirota L, Mogilner S, Zaritsky A, Barak M, Gottfried E. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2000;83:F177–F181. doi: 10.1136/fn.83.3.F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrington KJ. Postnatal Steroids and Neurodevelopmental Outcomes: A Problem In the Making. Pediatrics. 2001;107:1425–1426. doi: 10.1542/peds.107.6.1425. [DOI] [PubMed] [Google Scholar]
- 6.Murphy BP, Inder TE, Hüppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 7.Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, Tyson JE. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–272. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- 8.van der Heide-Jalving M, Kamphuis PJ, van der Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, Veen S, van Bel F. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatr. 2003;92:827–835. doi: 10.1080/08035250310002425. [DOI] [PubMed] [Google Scholar]
- 9.Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Hüppi PS. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1–7. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 10.Karemaker R, Heijnen CJ, Veen S, Baerts W, Samsom J, Visser GH, Kavelaars A, van Doornen LJ, van Bel F. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res. 2006;60:745–750. doi: 10.1203/01.pdr.0000246200.76860.de. [DOI] [PubMed] [Google Scholar]
- 11.Rademaker KJ, de Vries LS, Uiterwaal CS, Groenendaal F, Grobbee DE, van Bel F. Postnatal hydrocortisone treatment for chronic lung disease in the preterm newborn and long-term neurodevelopmental follow-up. Arch Dis Child Fetal Neonatal Ed. 2008;93:F58–F63. doi: 10.1136/adc.2007.119545. [DOI] [PubMed] [Google Scholar]
- 12.Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK, Volpe JJ. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 13.Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 14.Warfield SK, Kaus M, Jolesz FA, Kikinis R. Adaptive, template moderated, spatially varying statistical classification. Med Image Anal. 2000;4:43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- 15.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limperopoulos C, Soul JS, Gauvreau K, Hüppi PS, Warfield SK, Bassan H, Robertson RL, Volpe JJ, du Plessis AJ. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115:688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 17.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, Kean MJ, Doyle LW, Egan GF, Inder TE. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 19.Mewes AU, Hüppi PS, Als H, Rybicki FJ, Inder TE, McAnulty GB, Mulkern RV, Robertson RL, Rivkin MJ, Warfield SK. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118:23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 20.Zacharia A, Zimine S, Lovblad KO, Warfield S, Thoeny H, Ozdoba C, Bossi E, Kreis R, Boesch C, Schroth G, Hüppi PS. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. AJNR Am J Neuroradiol. 2006;27:972–977. [PMC free article] [PubMed] [Google Scholar]
- 21.Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M, Hüppi PS. Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res. 2004;56:132–138. doi: 10.1203/01.PDR.0000128983.54614.7E. [DOI] [PubMed] [Google Scholar]
- 22.Almeida OF, Condé GL, Crochemore C, Demeneix BA, Fischer D, Hassan AH, Meyer M, Holsboer F, Michaelidis TM. Subtle shifts in the ratio between pro- and antiapoptotic molecules after activation of corticosteroid receptors decide neuronal fate. FASEB J. 2000;14:779–790. doi: 10.1096/fasebj.14.5.779. [DOI] [PubMed] [Google Scholar]
- 23.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57:561–570. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Taketomo CK, Hodding JH, Kraus DM. Pediatric dosage handbook. 11. Lexi-Comp; Hudson, OH: 2004. [Google Scholar]
- 25.Holmes MC, Seckl JR. The role of 11beta-hydroxysteroid dehydrogenases in the brain. Mol Cell Endocrinol. 2006;248:9–14. doi: 10.1016/j.mce.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Holmes MC, Sangra M, French KL, Whittle IR, Paterson J, Mullins JJ, Seckl JR. 11beta-Hydroxysteroid dehydrogenase type 2 protects the neonatal cerebellum from deleterious effects of glucocorticoids. Neuroscience. 2006;137:865–873. doi: 10.1016/j.neuroscience.2005.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Hunt RW, Warfield SK, Wang H, Kean M, Volpe JJ, Inder TE. Assessment of the impact of the removal of cerebrospinal fluid on cerebral tissue volumes by advanced volumetric 3D-MRI in posthaemorrhagic hydrocephalus in a premature infant. J Neurol Neurosurg Psychiatry. 2003;74:658–660. doi: 10.1136/jnnp.74.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, Ferriero DM, Miller SP. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]


