Abstract
Purpose of review
Acute kidney injury (AKI) is a common complication in critically ill patients and is associated with increased morbidity and mortality. Sepsis is the most common cause of AKI. Considerable evidence now suggests that the pathogenic mechanisms of sepsis-induced AKI are different from those seen in other etiologies of AKI. This review focuses on the recent advances in this area and discusses possible therapeutic interventions that might derive from these new insights into the pathogenesis of sepsis-induced AKI.
Recent findings
The traditional paradigm that sepsis-induced AKI arises from ischemia has been challenged by recent evidence that total renal blood flow (RBF) in is not universally impaired during sepsis, and AKI can develop in the presence of normal or even increased RBF. Animal and human studies suggest that adaptive responses of tubular epithelial cells to injurious signals are responsible for renal dysfunction. Simultaneously occurring renal inflammation and microcirculatory dysfunction further amplify these mechanisms.
Summary
An understanding of the pathologic mechanisms of sepsis-induced AKI emphasizes the important role of maladaptive responses to the septic insult. Preventive and therapeutic measures should be based on counteracting these maladaptive responses of tubular epithelial cells, inflammation, and microvascular dysfunction.
Keywords: Acute kidney injury, sepsis, inflammation, microvascular dysfunction, tubular epithelial cells
Introduction
Acute kidney injury (AKI) occurs in 1–35% of hospitalized patients and is associated with high mortality [1]. The incidence of AKI after general surgery has been reported to be about 1%, whereas the incidence among critically ill patients can be as high as 70%, with an in-hospital mortality of 50% when AKI is part of the multiple organ dysfunction syndrome [2, 3]. AKI is an independent risk factor for death [4], and patients who survive have an increased risk to develop chronic kidney disease. AKI is a syndrome comprising multiple clinical conditions, and outcomes are influenced by underlying disease. The most common cause of AKI in critically ill patients is sepsis. Despite considerable research during the last decades, the pathophysiology of sepsis-induced-AKI remains incompletely understood.
In the not-so-distant past, sepsis-induced AKI was considered a disease of the renal macrocirculation [5] resulting from global renal ischemia, cellular damage and acute tubular necrosis (ATN). However, an increasing body of evidence suggests that AKI can occur in the absence of hypoperfusion [6*, 7]. In a human study, Prowle et al. were able to demonstrate that decreased renal blood flow (RBF) was not a universal finding in patients with sepsis-induced AKI [8]. In addition, Murugan et al. [9] demonstrated in a prospective multicenter study of more than 1800 patients with community-acquired pneumonia that AKI was a common condition, even in patients without severe disease. Higher cytokine levels (e.g. interleukin 6) were associated with severity and worsening of AKI [9]. Moreover, most of the patients with sepsis-induced AKI were never admitted to the intensive care unit nor suffered from hemodynamic instability [9]. Complementary to the insights from clinical studies, in vitro experiments have revealed that incubating human epithelial cells with plasma from septic patients resulted in decreased cell function and shortened the survival of tubular cells and podocytes, suggesting that the plasma from septic patients can induce renal cell injury and dysfunction absent any vasculature or circulating immune effector cells. Recent postmortem studies attempted to more closely describe the pathological changes in septic kidneys [10**, 11]. Despite representing the latest stages of the disease, these kidneys were characterized by a strikingly bland histology with focal areas of tubular injury, which was also entirely discordant with the profound functional impairment seen pre-mortem. Interestingly, all these changes can occur in the presence of a normal RBF, and define the clinical phenotype characterized by a reduced glomerular filtration rate and tubular dysfunction. Although RBF does not universally decrease during sepsis, some data exist suggesting that the blood pressure can directly influence the perfusion of the kidney and GFR under some pathological conditions [12] and that a higher blood pressure in patients with previous hypertension can prevent AKI during sepsis [13]. These data support the hypothesis that mechanisms other than tissue hypoperfusion are involved in the pathogenesis of sepsis-induced AKI. A consistent finding in septic humans, independent of the severity of AKI, is the presence of three pathologic findings: microcirculatory dysfunction, inflammation, and bio-energetic adaptive response to injury. The aim of this review is to discuss the role of these mechanisms in the genesis of sepsis-induced AKI and the potential therapeutic implications.
Pathophysiology of sepsis-induced AKI
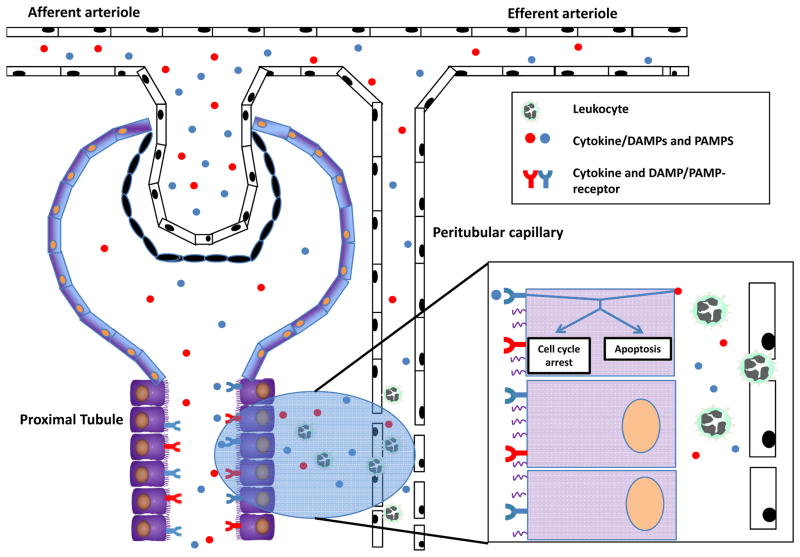
Although the functional consequences during sepsis-induced AKI are dramatic, the histological changes are moderate and do not entirely explain the clinical phenotype. Recent evidence suggests that instead of a single mechanism being responsible for its etiology, sepsis is associated with an entire orchestra of cellular mechanisms, adaptive and maladaptive, which potentiate each other and ultimately give rise to clinical AKI. The microcirculation is perhaps the more important physiological compartment where these mechanisms come together and exert their integrated and deleterious action. These mechanisms include endothelial dysfunction, inflammation, coagulation disturbance, and adaptive cell responses to injury (Figure 1) [7]. Therefore, we hypothesize that a key event in the early dysfunction of the kidney during sepsis is a bio-energetic stress of the tubular epithelial cells, in response to the amplified inflammatory signal that peritubular microvascular dysfunction generates.
Figure 1. During sepsis, DAMPs, PAMPs, and cytokines may potentially injure tubular cells from the tubular and interstitial side.
Inflammatory mediators derived from bacteria or immune cells are filtered in the glomerulus, enter the tubular space and can subsquently injure by tubular cells by binding to their respective receptors. In addition, cytokines, DAMPs, and PAMPs are released from extravasated leukocytes and can also activate tubular cells from the interstitial side. The activation of cytokine or DAMP/PAMP receptors may induce apoptosis or cell cycle arrest.
Renal microcirculation during sepsis-induced AKI
Sepsis causes a profound alteration of the macro- and microcirculation and is characterized by a decreased peripheral vascular resistance, maldistribution of tissue blood flow, and derangement of microcirculatory perfusion. These alterations cause a significant decrease in functional capillary density [14, 15] and an increment in the heterogeneity of regional blood flow distribution [16].
During the initial hyperdynamic stage of sepsis, when AKI develops, cardiac output is usually increased. RBF was markedly increased in a sheep model of sepsis [17], and yet AKI developed despite increased RBF [18]. Similarly, postmortem studies on septic patients have shown the heterogeneous distribution of tubular cellular injury with apical vacuolization, but without extensive apoptosis or necrosis [10*]. Alterations in the microcirculation in the renal cortex or renal medulla can occur despite normal or even increased global RBF [19]. Increased renal vascular resistance may represent an important hemodynamic factor that is involved in the development of sepsis-induced AKI.
Of course decreased RBF can cause injury to kidney and when sepsis-induced renal microvascular dysfunction is combined with an increase in intra-abdominal pressure, increased renal vascular resistance results. Measurement of renal vascular resistance using renal Doppler at the bedside has been proposed by Deruddre and colleagues [20] as a tool to titrate norepinephrine in septic shock patients based on the renal arterial resistance to determine the optimal mean arterial pressure. However, whether improvement of regional blood flow, even in this sub-population of patients will prevent tubular damage remains to be substantiated.
Platelets, fibrin, stiff red blood cells and leukocytes together with endothelial cell swelling are responsible for capillary occlusion [21]. Increased vascular permeability is a common feature in sepsis and leads to interstitial edema and fluid retention (Figure 1) [22, 23]. In addition to its association with the severity of sepsis, fluid overload and interstitial edema increase the diffusion distance for oxygen to target cells [24]. Similar findings can be observed in the renal microcirculation [25]. Furthermore, as the kidney is an encapsulated organ, fluid accumulation and tissue edema contribute to the observed deterioration of renal microcirculatory perfusion by altering transmural pressures and by aggravating venous congestion [26*, 27].
Endothelial cells are important determinants of vascular tone, leukocyte recruitment and function, and alter the responsiveness of smooth muscles [28]. Injured endothelial cells produce less vasodilators (e.g. nitric oxide) resulting in a more pronounced response to vasoconstrictors with a redistribution of blood flow. The imbalance between vasoconstrictors, vasodilators and oxidative stress at the endothelial level is receiving considerable attention as a major contributor to the development of AKI. Augmented vasoconstriction, small vessel occlusion due to the interaction of leukocytes with activated endothelial cells, and activation of the coagulation system results in local compromise of the microcirculation and regional ischemia [25, 29]. The patchy tubular cell injury [10**] probably reflects the heterogeneous distribution of RBF caused by microcirculatory dysfunction.
Nitric oxide (NO) plays a pivotal and multifaceted role in the complex pathophysiology of sepsis [30] and sepsis-induced AKI [31]. During sepsis, global NO production increases, whereas the producing enzyme, inducible NO synthase (iNOS), has a heterogeneous expression pattern, resulting in different regional concentrations of NO [30]. The uneven distribution of NO production may contribute to the heterogeneous perfusion pattern. However, elevated NO also influences renal hemodynamics and causes peroxynitrite-related tubular injury through the local generation of reactive nitrogen species during sepsis [32]. Evidence suggests that this may play an important role as up-regulation of iNOS has been associated with proximal tubular injury during systemic inflammation, and its selective inhibition, with amelioration of the functional impairment caused by cecal ligation and puncture [33]. Therefore, the selective inhibition of renal iNOS might have an implication for the treatment of sepsis-induced AKI.
Inflammation propagates renal damage during sepsis
There is a strong association between cytokine levels (interleukin (IL)-6, IL-10, and macrophage migration inhibitory factor) and the development of sepsis-induced AKI [9, 34], suggesting an important role of systemic inflammatory mediators in this process. During sepsis, infection triggers a host response, in which inflammatory mechanisms contribute to clearance of infection and tissue recovery on the one hand, and organ injury on the other [35]. Pathogens activate a variety of cells including renal epithelial and dendritic cells through an interaction with pattern-recognition receptors including toll-like receptors (TLR), C-type lectin receptors, retinoic acid inducible gene 1-like receptors, and nucleotide-binding oligomerization domain-like receptors [36]. The engagement of these receptors results in the up-regulation of inflammatory gene transcription and initiation of innate immunity. The same receptors can also detect endogenous molecules released from injured cells, so-called damage-associated molecular patterns, such as DNA, RNA, histones, HMGPB1, and S100 proteins [37].
The cytokine storm during the initial phase of severe sepsis activates leukocytes, endothelial cells, and epithelial cells leading to leukocyte and platelet activation, microvascular dysfunction, hypoxia, and tissue damage [35]. Pro-inflammatory mediators activate endothelial cells and increase vascular permeability (Figure 1). Activated endothelial cells up-regulate the expression of adhesion molecules and release additional pro-inflammatory mediators. E-selectin, specifically induced on the endothelium upon inflammatory stimulation, has been demonstrated to play a major role in leukocyte recruitment into the kidney during the late stages of sepsis-induced AKI [38*]. Experimental data highlight the importance of leukocyte recruitment into the kidney [38*] especially in later stages of AKI. Although not seen in all models of sepsis-induced AKI [39] elimination of neutrophils or blocking adhesion molecules that are required for neutrophil recruitment into the kidney completely abolished sepsis-induced AKI in a cecal ligation and puncture (CLP)-induced sepsis model [38*]. This observation can be explained by the fact that adherent and transmigrated neutrophils release reactive oxygen species, proteases, elastases, myeloperoxidase, and other enzymes that damage the tissue. These substances, together with leukotriene B4 and platelet-activating factor, can both increase vascular permeability and up-regulate the expression of adhesion molecules that promote further inflammation [6*, 40, 41]. Leukocytes leaving peritubular capillaries have a close proximity to tubular epithelial cells and can directly activate tubular epithelial cells by releasing pro-inflammatory mediators and DAMPs (Figure 1). Based on the special location of tubular epithelial cells, these cells can also be activated from the tubular side [42]. DAMPs, PAMPs, and pro-inflammatory cytokines are filtered in the glomerulus, enter the proximal tubulus and can directly activate tubular epithelial cells resulting in a change of the metabolic and functional state of these cells (Figure 1). It has been recently shown that these molecules can bind to and activate tubular cells by binding to TLR-2 and -4 [43–44, 45*, 46]. While animal studies have linked TLR4 signaling to kidney injury, the relevance of TLR activation in human kidney was unknown until recently. In a very elegant human study, Krüger et al. [43] demonstrate that TLR4 is constitutively expressed in kidneys and that tubules in damaged kidneys also stain positively for HMGB1, a known endogenous TLR4 ligand. In vitro stimulation of human tubular epithelial cells with HMGB1 confirmed that HMGB1 can stimulate pro-inflammatory responses through TLR4 [43]. The released pro-inflammatory mediators can act in an autocrine and paracrine fashion and may contribute to further tubular cell damage. In agreement with these findings, kidneys with a TLR4 loss-of-function allele contained less TNF-α, MCP-1, and more heme oxygenase 1 [43]. During sepsis, endotoxin in the tubule binds to TLR4 on S1 proximal tubule cells, which subsequently causes oxidative stress in cells of the neighboring S2 segment [44], suggesting that targeting TLR4 signaling may have value in preventing or treating AKI.
Adaptive responses of tubular cells to changes in the local environment
Tubular cells exposed to inflammation and the consequences of microcirculatory dysfunction act as primary targets and respond by adaptation to the altered tubular environment. They may also spread this signal and shutdown other tubular cells in a paracrine fashion [43]. Microvascular dysfunction occurs in heterogeneous regions of the kidney and therefore may explain the heterogeneous histopathologic changes of tubular epithelial cells.
Oxidative stress is a hallmark of sepsis-induced AKI. Postmortem studies in humans with sepsis-induced AKI show apical epithelial tubular cell vacuolization, which has been linked to oxidative stress [47]. Cultured tubular cells and podocytes treated with components of bacteria or plasma from patients with severe burns and sepsis associated AKI produce reactive oxygen species (ROS) [48] or undergo apoptosis [48]. Oxidative stress is also linked to tubular dysfunction [50]. Recent studies demonstrate that apoptosis of tubular cells is rare during sepsis-induced AKI [10**], suggesting that tubular epithelial cells exposed to hypoxia and inflammation limit processes that can result in apoptosis or necrosis (Figure 1). This can be achieved by an adaptive response of tubular cells characterized by downregulating metabolism and undergoing cell-cycle arrest (Figure 1) [51–53]. This response may be orchestrated by mitochondria and limits further damage and provide cells with the opportunity to recover function. Swollen and injured mitochondria, which can be found in humans with sepsis-induced AKI, cause a reduced tubular cell function by prioritizing the existing energy to functions that are required for cell survival. Another important feature is mitophagy. This is process that removes damaged mitochondria through autophagy and can be induced in the kidney by several factors including inflammation and oxidative stress [54]. Decreased mitophagy is associated with a proximal tubular dysfunction, cell and organ dysfunction, and worse outcome in critically ill patients [54]. Furthermore, abnormal mitophagy has also been linked progressive renal injury [55]. However, mitophagy was significantly up-regulated in septic kidneys [10**]. As most of the patients had an already established AKI, this observation let us speculate that increased mitophagy contributes to renal recovery.
Mitochondria are also involved in cell cycle arrest, which is a quality control process of cell division. Recent clinical studies have independently demonstrated that two markers involved in G1 cell cycle arrest, insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinase 2 (TIMP-2), predict AKI in critical ill patients and in patients undergoing cardiac surgery [56*, 57, 58**], suggesting that cell cycle arrest of tubular epithelial cells is involved in AKI. The reduction in ATP production triggers cell cycle arrest [59]. Therefore, a reduced ATP level may induce cell cycle arrest in these cells and prevent the cell from undertaking a process that could end in cell death. Interestingly, these cell cycle arrest markers can also predict renal recovery [56*, 60].
Potential for diagnostic and therapeutic targets
To date no therapeutic measures are available to prevent or treat sepsis-induced AKI. A potential reason for this may be that often therapy is started too late in the disease process. The development of new biomarkers, which also provide insights in the pathophysiology of the disease, makes it possible to detect kidneys at risk for injury and thus enable earlier initiation of interventions [56*, 57, 58**].
The knowledge that inflammation, microvascular dysfunction, and adaptive responses of tubular cells are involved in the development of sepsis-induced AKI provides new diagnostic and therapeutic avenues. As these mechanisms are closely interlinked with each other, modulating one of these components simultaneously alters other components. The recognition of inflammation has triggered the investigation of therapeutic strategies to dampen inflammation to prevent/treat AKI. As increased levels of pro-inflammatory mediators (e.g. IL-6) are associated with the development of AKI [34], it is tempting to speculate that eliminating these mediators or endotoxin can prevent sepsis-induced AKI. Indeed, elimination of cytokines and endotoxin is feasible by hemoadsorption [61, 62] and experimentally it has been shown that hemoadsorption completely protects against AKI in a CLP-model of sepsis [63]. A clinical study demonstrated that reducing endotoxin by polymyxin-B hemoperfusion reduced RIFLE scores and urine tubular enzymes [62]. Another option to interfere with cytokines and endotoxin is the application of exogenous alkaline phosphatase. Alkaline phosphatase (AP) is an endogenous enzyme that exerts detoxifying effects through dephosphorylation of endotoxins and pro-inflammatory extracellular ATP and is reduced during systemic inflammation. Heemskerk and colleagues [64] demonstrated that AP application was associated with a decreased expression of iNOS synthase in proximal tubule cells isolated from urine related to an attenuated urinary excretion of an proximal tubule injury marker. In a small, randomized trial, Pickkers et al. showed that the administration of exogenous AP in septic patients improved endogenous creatinine clearance and reduced the requirement and duration of renal replacement therapy [65]. Modulating TNF-α signaling might be another therapy option, because a polymorphism in the promoter region of the TNFA gene is associated with markers of kidney disease severity and distant organ dysfunction [66]. However, it is important to keep in mind that these pro-inflammatory mediators are required for the host response and bacterial clearance during sepsis and that they can later provide necessary signals for the resolution of injury.
Microcirculatory dysfunction during AKI initiates hypoxia and inflammation. To improve the microcirculatory perfusion, vasodilators in the setting of sepsis are currently under investigation including nitroglycerin [14, 67], NO administration, and modulation of NO production [30, 32]. Furthermore, drugs with pleiotropic effects on the vasculature, such as statins [68] and erythropoietin [69], have the potential to prevent kidney injury by enhancing eNOS expression and decreasing vascular permeability. However, on the basis of the different mechanisms involved in sepsis-induced AKI and the interrelationship among these mechanisms, it is unlikely that a single treatment modality may emerge as a magic bullet in the prevention and/or treatment of sepsis-induced AKI.
Conclusions
In conclusion, the old paradigm that sepsis-induced AKI is initiated by renal ischemia as a result of macrovascular dysfunction has been called into question, because AKI can also develop in the presence of normal or increased renal blood flow. Furthermore, in contrast to renal-ischemia reperfusion injury, which is characterized by apoptosis or necrosis of tubular epithelial cells, sepsis-induced AKI is characterized by healthy or reversible injured renal tubular epithelial cells. New evidence suggests that the inflammatory response during sepsis causes an adaptive response of the tubular epithelial cells. These alterations induce a downregulation of the cell function in order to minimize energy demand and to ensure cell survival. The result is reduced kidney function. The simultaneous occurrence of renal inflammation and microvascular dysfunction exacerbates the adaptive response of tubular epithelial cells to injurious signals. In addition, the endothelial cell injury is also of importance in the initiation and development of sepsis-induced AKI through the nitric oxide pathway, leukocyte adhesion, ROS, and inflammation. Targeting tubular epithelial cells and components of the microcirculation may be an effective strategy in preventing and/or treating sepsis-induced AKI.
Key points.
The heterogeneous distribution of renal blood flow induced by the microcirculatory dysfunction probably causes patchy tubular cell injury during sepsis-induced AKI, whereas hypoxia and hypoperfusion may amplify inflammation and contributes to an adaptive response of tubular epithelial cells.
Pro-inflammatory cytokines released during sepsis are filtered in the glomerulus, enter the proximal tubulus and can directly activate tubular epithelial cells resulting in a change of the metabolic and functional state of these cells.
Recent clinical evidence suggests that G1 cell cycle arrest of tubular epithelial cells is involved in AKI.
As different mechanisms are involved in sepsis-induced AKI, it is unlikely that a single treatment may be able to prevent or treat sepsis-induced AKI.
Acknowledgments
This work was funded by a research grant from the German research foundation (ZA428/6-1) and Else-Kröner Fresenius Stiftung awarded to A.Z. and, NIH/NHLBI grant number 1K12HL109068-02 awarded to H.G.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Critical care medicine. 2007;35:1837–43. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 3.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney international. 2012;81:819–25. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 4.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Critical care medicine. 2008;36:S146–51. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW, Wang W. Acute renal failure and sepsis. The New England journal of medicine. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 6*.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. Excellent review on the pathophysiology of sepsis-induced acute kidney injury. A comprehensive review of different mechanims causing AKI during sepsis are presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Current opinion in critical care. 2009;15:503–8. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 8.Prowle JR, Ishikawa K, May CN, Bellomo R. Renal blood flow during acute renal failure in man. Blood purification. 2009;28:216–25. doi: 10.1159/000230813. [DOI] [PubMed] [Google Scholar]
- 9.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney international. 2010;77:527–35. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. American journal of respiratory and critical care medicine. 2013;187:509–17. doi: 10.1164/rccm.201211-1983OC. This is a clinical trial to investigate the pathophysiology of cardiac and renal dysfunction in patients dying of sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerolle N, Nochy D, Guerot E, et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocyte infiltration. Inetnsive Care Med. 2010;36:471–8. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 12.Redfors B, Bragadottir G, Sellgren J, et al. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011;37:60–7. doi: 10.1007/s00134-010-2057-4. [DOI] [PubMed] [Google Scholar]
- 13.Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. The New England journal of medicine. 2014;370:1583–93. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 14.Spronk PE, Ince C, Gardien MJ, et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–6. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 15.Donati A, Damiani E, Botticelli L, et al. The aPC treatment improves microcirculation in severe sepsis/septic shock syndrome. BMC anesthesiology. 2013;13:25. doi: 10.1186/1471-2253-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Backer D, Creteur J, Preiser JC, et al. Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 17.Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest. 2003;124:1053–9. doi: 10.1378/chest.124.3.1053. [DOI] [PubMed] [Google Scholar]
- 18.Chvojka J, Sykora R, Krouzecky A, et al. Renal haemodynamic, microcirculatory, metabolic and histopathological responses to peritonitis-induced septic shock in pigs. Critical care. 2008;12:R164. doi: 10.1186/cc7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezemer R, Legrand M, Klijn E, et al. Real-time assessment of renal cortical microvascular perfusion heterogeneities using near-infrared laser speckle imaging. Optics express. 2010;18:15054–61. doi: 10.1364/OE.18.015054. [DOI] [PubMed] [Google Scholar]
- 20.Deruddre S, Cheisson G, Mazoit JX, et al. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive care medicine. 2007;33:1557–62. doi: 10.1007/s00134-007-0665-4. [DOI] [PubMed] [Google Scholar]
- 21.De Backer D, Donadello K, Taccone FS, et al. Microcirculatory alterations: potential mechanisms and implications for therapy. Annals of intensive care. 2011;1:27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nature reviews Nephrology. 2010;6:107–15. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 23.Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Critical care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Critical care medicine. 2004;32:1928–48. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- 25.Holthoff JH, Wang Z, Seely KA, et al. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney international. 2012;81:370–8. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nature reviews Nephrology. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. Excellent review on the crucial role of optimal voulume management in patients with acute kidney injury. [DOI] [PubMed] [Google Scholar]
- 27.Rajendram R, Prowle JR. Venous congestion: are we adding insult to kidney injury in sepsis? Critical care. 2014;18:104. doi: 10.1186/cc13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical pharmacology. 2009;78:539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. American journal of physiology Renal physiology. 2011;301:F209–17. doi: 10.1152/ajprenal.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trzeciak S, Cinel I, Phillip Dellinger R, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2008;15:399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksu U, Demirci C, Ince C. The pathogenesis of acute kidney injury and the toxic triangle of oxygen, reactive oxygen species and nitric oxide. Contributions to nephrology. 2011;174:119–28. doi: 10.1159/000329249. [DOI] [PubMed] [Google Scholar]
- 32.Heemskerk S, Masereeuw R, Russel FG, Pickkers P. Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nature reviews Nephrology. 2009;5:629–40. doi: 10.1038/nrneph.2009.155. [DOI] [PubMed] [Google Scholar]
- 33.Tiwari MM, Brock RW, Megyesi JK, et al. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. American journal of physiology Renal physiology. 2005;289:F1324–32. doi: 10.1152/ajprenal.00124.2005. [DOI] [PubMed] [Google Scholar]
- 34.Payen D, Lukaszewicz AC, Legrand M, et al. A multicentre study of acute kidney injury in severe sepsis and septic shock: association with inflammatory phenotype and HLA genotype. PloS one. 2012;7:e35838. doi: 10.1371/journal.pone.0035838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angus DC, van der Poll T. Severe sepsis and septic shock. The New England journal of medicine. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. The Journal of clinical investigation. 2012;122:2711–9. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Herter JM, Rossaint J, Spieker T, Zarbock A. Adhesion Molecules Involved in Neutrophil Recruitment during Sepsis-Induced Acute Kidney Injury. Journal of innate immunity. 2014 doi: 10.1159/000358238. Experimental study investigating the different adhesion molecules required for neutrophil recruitment into the kidney during sepsis-induced AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singbartl K, Bishop JV, Wen X, et al. Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney international. 2011;80:633–44. doi: 10.1038/ki.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–69. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 41.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. European journal of clinical investigation. 2008;38:39–44. doi: 10.1111/j.1365-2362.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- 43.Kruger B, Krick S, Dhillon N, et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3390–5. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalakeche R, Hato T, Rhodes G, et al. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. Journal of the American Society of Nephrology: JASN. 2011;22:1505–16. doi: 10.1681/ASN.2011020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Mudaliar H, Pollock C, Komala MG, et al. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. American journal of physiology Renal physiology. 2013;305:F143–54. doi: 10.1152/ajprenal.00398.2012. Experimental study showing that the binding of damage-associated molecular pattern molecules (DAMPs) to TLR2 and 4 on tubular epithelial cells induces a dysfunction of these cells, suggesting that released DAMPs during sepsis are filtered in the glomerulus and can directly activate tubular epithelial cells. [DOI] [PubMed] [Google Scholar]
- 46.Lin M, Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. Journal of the American Society of Nephrology: JASN. 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. Journal of the American Society of Nephrology: JASN. 2007;18:1807–15. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 48.Biancone L, Conaldi PG, Toniolo A, Camussi G. Escherichia coli porin induces proinflammatory alterations in renal tubular cells. Experimental nephrology. 1997;5:330–6. [PubMed] [Google Scholar]
- 49.Mariano F, Cantaluppi V, Stella M, et al. Circulating plasma factors induce tubular and glomerular alterations in septic burns patients. Critical care. 2008;12:R42. doi: 10.1186/cc6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrell ED, Kellum JA, Hallows KR, Pastor-Soler NM. Epithelial transport during septic acute kidney injury. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 doi: 10.1093/ndt/gft503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–8. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 52.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 53.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R491–7. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 54.Zhan M, Brooks C, Liu F, et al. Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney international. 2013;83:568–81. doi: 10.1038/ki.2012.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sansanwal P, Yen B, Gahl WA, et al. Mitochondrial autophagy promotes cellular injury in nephropathic cystinosis. Journal of the American Society of Nephrology: JASN. 2010;21:272–83. doi: 10.1681/ASN.2009040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as Early Biomarkers of Acute Kidney Injury and Renal Recovery following Cardiac Surgery. PloS one. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. This cliniacl study demonstrates that the cell-cycle arrest markers TIMP-2 and IGFBP7 serve as sensitive and specific biomarkers to predict AKI early after cardiac surgery and to predict renal recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bihorac A, Chawla LS, Shaw AD, et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. American journal of respiratory and critical care medicine. 2014 doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 58**.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Critical care. 2013;17:R25. doi: 10.1186/cc12503. This multicenter observational trial identified two new biomarkers for AKI. TIMP-2 and IGFBP7, both inducers of G1 cell cyle arrest, can predict AKI in critically ill patients. Both markers are superior to existing markers, provide additional information over clinical variables and add mechanistic insight into AKI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mandal S, Guptan P, Owusu-Ansah E, Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Developmental cell. 2005;9:843–54. doi: 10.1016/j.devcel.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Aregger F, Uehlinger DE, Witowski J, et al. Identification of IGFBP-7 by urinary proteomics as a novel prognostic marker in early acute kidney injury. Kidney international. 2013 doi: 10.1038/ki.2013.363. [DOI] [PubMed] [Google Scholar]
- 61.Kellum JA, Venkataraman R, Powner D, et al. Feasibility study of cytokine removal by hemoadsorption in brain-dead humans. Critical care medicine. 2008;36:268–72. doi: 10.1097/01.CCM.0000291646.34815.BB. [DOI] [PubMed] [Google Scholar]
- 62.Cantaluppi V, Assenzio B, Pasero D, et al. Polymyxin-B hemoperfusion inactivates circulating proapoptotic factors. Intensive care medicine. 2008;34:1638–45. doi: 10.1007/s00134-008-1124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng ZY, Wang HZ, Carter MJ, et al. Acute removal of common sepsis mediators does not explain the effects of extracorporeal blood purification in experimental sepsis. Kidney international. 2012;81:363–9. doi: 10.1038/ki.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heemskerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Critical care medicine. 2009;37:417–23. doi: 10.1097/CCM.0b013e31819598af. [DOI] [PubMed] [Google Scholar]
- 65.Pickkers P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Critical care. 2012;16:R14. doi: 10.1186/cc11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Susantitaphong P, Perianayagam MC, Tighiouart H, et al. Tumor necrosis factor alpha promoter polymorphism and severity of acute kidney injury. Nephron Clinical practice. 2013;123:67–73. doi: 10.1159/000351684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boerma EC, Koopmans M, Konijn A, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Critical care medicine. 2010;38:93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- 68.Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: a meta-analysis of over 30,000 patients. European heart journal. 2008;29:1548–59. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]
- 69.Song YR, Lee T, You SJ, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. American journal of nephrology. 2009;30:253–60. doi: 10.1159/000223229. [DOI] [PubMed] [Google Scholar]