Abstract
BACKGROUND
The trend of increasing cervical spine multidirectional computed tomography (MDCT) imaging of pediatric trauma patients is characteristic of the overall dramatic increase in computed tomography utilization in the United States. The purpose of this study is to compare the amount of radiation a pediatric trauma patient absorbs to the thyroid from plain radiographs and MDCT of the cervical spine and to express risk by calculation of theoretical thyroid cancer induction.
METHODS
A retrospective evaluation of pediatric trauma patients admitted from October 1, 2004, to October 31, 2009, was performed at an academic, Level I trauma center. Inclusion criteria were Level I/II trauma patients, cervical spine imaging performed at our institution, and age <18 years. Absorbed thyroid radiation was calculated for patients receiving plain radiographs or MDCT. Thyroid cancer risk was calculated using the 2006 Biological Effects on Ionizing Radiation VII report.
RESULTS
Six hundred seventeen patients met inclusion criteria: 224 received cervical spine radiographs and 393 received cervical spine MDCT. The mean thyroid radiation absorbed from radiographs was 0.90 mGy for males and 0.96 mGy for females compared with 63.6 mGy (males) and 64.2 mGy (females) receiving MDCT (p < 0.001). The median excess relative risk of thyroid cancer induction from one cervical spine MDCT in males was 13.0% and females was 25.0%, compared with 0.24% (males) and 0.51% (females) for radiographs (p < 0.001).
CONCLUSIONS
The significant difference in radiation that MDCT delivers to the pediatric trauma patient when compared with plain radiographs should temper routine use of computed tomography in pediatric cervical spine clearance algorithms.
Cervical spine injury occurs in 0.98% to 2.2% of pediatric blunt trauma patients1–5 necessitating a methodical screening process to avoid the potential devastation of a missed injury.6 In patients who cannot be cleared on clinical grounds, screening with plain radiographs has historically been the recommended imaging modality. However, because of availability and improved sensitivity, computed tomography (CT) use for pediatric cervical spine screening has increased nearly fourfold.7 The comparatively higher doses of radiation from CT in pediatric trauma patients may be associated with untoward complications.8–10
Sixty-seven million CT scans are ordered annually as of 2006, accounting for 24% of the total amount of radiation the U.S. population is exposed to annually, while 11% was the contribution of all diagnostic medical imaging 20 years ago.11 Increased exposure to radiation has raised concern toward its population health effects, namely carcinogenesis.12–16 Diagnostic radiation contains high-energy ionizing radiation, X-ray, which is sufficient to displace an electron from an atom or molecule, ionize, and destabilize that molecule. At a cellular level, ionizing radiation has the ability to alter DNA and create mutations, potentially inducing cancer.17,18
One particularly radiosensitive tissue, the thyroid gland,17,19 is central to this discussion as it has become the fastest growing cancer in the United States.20 Although debate in the literature exists as to whether this is simply a function of improved detection methods,21 the incidence of thyroid cancer has increased twofold since 198520 and recent data suggest that increased diagnostic scrutiny is not the only reason.22,23 This does not directly implicate CT as a contributor to this dramatic increase in thyroid cancer, but an association should be considered given the susceptibility of the thyroid to radiation, particularly when factoring in its anatomic location with respect to cervical spine CT.17,19,24
The pediatric population raises the most concern for harmful effects of diagnostic radiation from cervical spine CT because of the age-related sensitivity of the thyroid to radiation, placing youth at higher risk.18,25–28 Their intrinsic sensitivity to the effects of ionizing radiation plus the longer life expectancy during which risk may be expressed makes reducing exposure to children particularly important.29
We hypothesize that CT examination of children to screen the cervical spine after trauma results in an important, measurable thyroid cancer risk greater than plain radiographs. The specific aims of this study are to compare the absorbed dose of radiation to the thyroid from plain radiographs with that from a cervical spine CT in pediatric trauma patients and to determine an estimated relative risk of induced thyroid cancer from the clearance process. This information will be useful to clinicians who must weigh the benefit of enhanced diagnostic imaging against the potential hazard of excess radiation exposure in a radiosensitive patient population.
PATIENTS AND METHODS
Study Groups
Institutional Review Board (M-2009-1358) exemption was obtained. All patients admitted during the 5-year window October 1, 2004, to October 31, 2009, were identified from a prospective, Institutional Review Board-registered pediatric trauma database who met the inclusion criteria: age ≤ 17 years, Level I trauma (emergent activation of the trauma team secondary to airway compromise, cardiopulmonary duress, gunshot wound to the neck, chest, or abdomen, or Glascow coma scale score <8) or Level II trauma (emergent trauma evaluation but no physiologic abnormalities),30 and had cervical spine imaging by plain radiographs or CT at our institution.
Calculation of the Absorbed Dose to the Thyroid for Plain Radiographs
The calculation of absorbed thyroid radiation from plain radiographs is dependent on field of view (we assumed 35 × 20), the tube current and exposure time (mAs), maximum voltage (kVp), distance from source to patient (75 cm), and tissue-specific absorption value (mrad/R).31 The tissue-specific absorption value was calculated from the equation:
| 31 |
Absorbed thyroid radiation was calculated for each radiographic view, e.g., anteroposterior, lateral, and open-mouth odontoid, and summed as follows:
where 1-mrad = 0.01 mGy.31
The actual mAs and kVp for each radiograph were not recorded; therefore, we estimated these based on recommended imaging protocols that contain ranges of settings. The largest value in the range was chosen to avoid underestimating the dose and falsely expanding the difference between CT and radiographs. To confirm the validity of our calculations, a 1-month prospective survey of cervical spine radiograph image settings was performed. Data were collected on three patients, all of whom had their actual image settings fall within the range of recommended imaging protocols used to calculate the dose.
Calculation of the Absorbed Dose to the Thyroid for CT
The total thyroid radiation dose from CT was calculated via Monte Carlo technique using the ImPACT CT Patient Dosimetry Calculator (version 1.0, August 28, 2009). The Monte Carlo technique32,33 uses mathematical equations based on anthropomorphic phantoms to model photon transport and estimate the absorbed radiation doses. The calculator applies these models with data collected by the United Kingdom’s National Radiation Protection Board.32 This allows the amount of radiation absorbed by each organ to be estimated specific to the manufacturer and model of CT scanner. The variables required include CT manufacturer and model, kV, tube current (mA), rotation time (s), spiral pitch, field of view (set to the skull base through T2), and collimation (mm) and were obtained from each patient’s medical record. The absorbed thyroid dose was calculated for each patient receiving a CT.
Our institution used three CT scanners during the course of the study—GE LightSpeed VCT, GE LightSpeed Pro 16, and GE LightSpeed 16 (General Electric, Waukesha, WI). The protocols are given in Table 1 and differ based on three age groups.
TABLE 1.
CT Imaging Protocols for the Three CT Scanners Used in This Study
| CT Scan | Age (yr) | kV | mA | Time (s) | Pitch | Collimation (mm) |
|---|---|---|---|---|---|---|
| GE LightSpeed VCT | <3 | 100 | 160–620 | 0.5 | 0.53 | 20 |
| 3–6 | 120 | 210–630 | 0.6 | 0.53 | 20 | |
| >6 | 140 | 280–715 | 0.6 | 0.53 | 20 | |
| GE LightSpeed Pro 16 | <3 | 100 | 170–660 | 0.5 | 0.56 | 10 |
| 3–6 | 120 | 220–670 | 0.6 | 0.56 | 10 | |
| >6 | 140 | 300–715 | 0.6 | 0.56 | 10 | |
| GE LightSpeed 16 | <3 | 100 | 110–410 | 0.6 | 0.56 | 10 |
| 3–6 | 120 | 130–400 | 1 | 0.56 | 10 | |
| >6 | 140 | 180–380 | 1 | 0.56 | 10 |
Estimating Cancer Risk
The National Academies’ Biological Effects of Ioniz-ing Radiation 7th Report was used to estimate the thyroid cancer risk from radiation expressed as excess relative risk (ERR) according to:
The absolute risk is calculated by identifying the rate in the exposed population:
where Re was 5.2/100,000 for males and 15.2/100,000 for females.20
Statistical Methodology
The male and female groups were divided by age 0 to 6 years, 7 years to 11 years, and 12 years to 17 years. This allowed associations based on CT protocols (the 0- to 6-year-old group had different imaging protocols) and prestudy, arbitrary division of the older ages was done to allow comparison. Each group had radiation doses calculated and averaged using analysis of variance to look at differences between groups and pairwise two-tailed t test to calculate p values. The calculated age and radiation dose value for each group was used to estimate the gender-specific cancer risk. ERR data are not normally distributed, and so the nonparametric Kruskal-Wallis test was used to look at overall difference in ERR between age groups by sex. A Wilcoxon rank-sum test examined the pairwise differences between age groups. Bonferroni correction was used and p < 0.05 was considered statistically significant.
RESULTS
Demographics
Six hundred seventeen patients met the inclusion criteria: 224 had plain cervical radiographs and 393 had cervical CT (Table 2).
TABLE 2.
Absorbed Radiation to the Thyroid in mGy for Males and Females From Plain Radiographs and CT Cervical Spine
| Age Category | Plain Radiograph
|
CT
|
||||||
|---|---|---|---|---|---|---|---|---|
| Male
|
Female
|
Male
|
Female
|
|||||
| Number | mGy, Mean (SD) | Number | mGy, Mean (SD) | Number | mGy, Mean (SD) | Number | mGy, Mean (SD) | |
| 0–6 | 38 | 0.36 (0.16) | 19 | 0.4 (0.25) | 46 | 51.7 (24.3) | 22 | 55.5 (21.0) |
| 7–11 | 29 | 0.81 (0.33) | 26 | 0.88 (0.28) | 34 | 51.3 (18.79) | 24 | 56.2 (16.8) |
| 12–17 | 69 | 1.51 (0.61)* | 43 | 1.59 (0.75)* | 165 | 70.2 (20.1)* | 102 | 68.3 (18.1)* |
| All | 136 | 0.9 (0.69) | 88 | 0.96 (0.73) | 245 | 63.6 (21.6) | 148 | 64.2 (19.2) |
All absorbed thyroid radiation values (mGy) for CT were significantly greater (p < 0.001) than the respective plain radiograph values.
Statistically significant difference in thyroid absorbed radiation between the 12- and 17-yr age group and the younger two age groups.
Plain Radiograph Radiation
The mean radiation absorbed to the thyroid from plain radiographs in males was 0.90 mGy and 0.96 mGy for females, which was not significantly different. Similarly, when analyzed by age there was no difference between males and females. However, increased age was associated with significantly greater thyroid radiation absorption for both genders in the oldest age group (12–17 years) compared with the two younger groups (Table 2).
CT Radiation
The mean radiation dose from CT absorbed by the thyroid in males was 63.6 mGy and 64.2 mGy for females, this difference was not statistically significant. Age significantly impacted the dose as younger patients received, on average, less radiation to the thyroid: young males (0–6 years) = 51.7 mGy, young females (0–6 years) = 55.5 mGy; males (7–11 years) = 51.3 mGy, females (7–11 years) = 56.2 mGy; and adolescent males (12–17 years) = 70.2 mGy, adolescent females (12–17 years) = 68.3 mGy. The radiation dose received by the younger two age groups was significantly lower than the adolescent age groups but not significantly different between the youngest two age groups, males and females alike (Table 2).
Excess Relative Risk
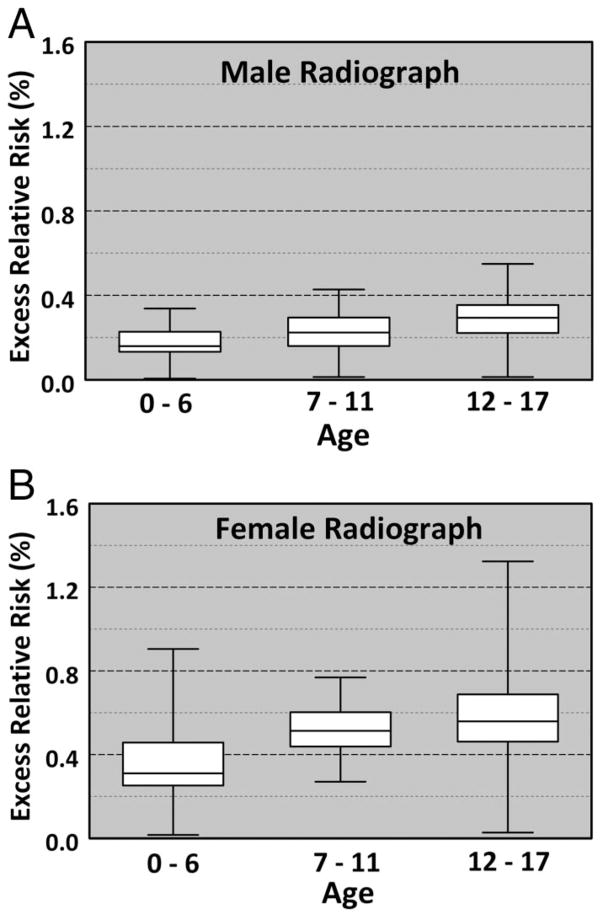
The median lifetime ERR for a plain radiograph cervical spine series in males was 0.24% and females was 0.51% (Table 3; Fig. 1, A and B).
TABLE 3.
Excess Relative Risk of Thyroid Cancer Induction for CT Cervical Spine Versus Plain Radiographs
| Age Category | Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| Plain Radiograph | CT | P | Plain Radiograph | CT | p | |
| 0–6 | 0.16 (0.11–0.32) | 21.5 (10.0–66.0) | <0.001 | 0.31 (0.02–0.48) | 45.6 (17.0–116.0) | <0.001 |
| 7–11 | 0.22 (0.11–0.43) | 13.5 (8.0–27.0) | <0.001 | 0.51 (0.27–0.77) | 29.7 (15.0–55.0) | <0.001 |
| 12–17 | 0.29 (0.10–0.55) | 12.0 (4.0–26.0) | <0.001 | 0.56 (0.18–1.32) | 22.7 (8.0–42.0) | <0.001 |
| All | 0.24 (0.10–0.55) | 13.0 (10.0–66.0) | <0.001 | 0.51 (0.02–1.32) | 25.0 (8.0–116.0) | <0.001 |
Median values reported with the range in parentheses; p value indicates significance for age- and gender-matched groups comparing CT and plain radiographs.
Figure 1.
(A, B) Excess relative risk box plot for males and females receiving cervical spine radiographs. White boxes indicate 1 SD from the median value (single line within the box), while the bars indicate the range of values.
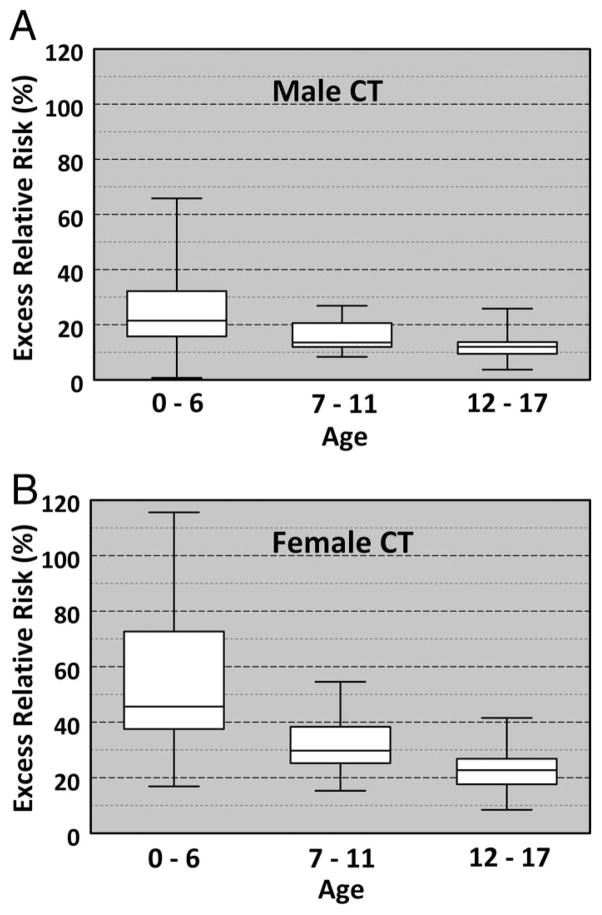
The median ERR for one cervical spine CT in males was 13.0% (range, 10–66%) and females was 25.0% (range, 8.0–116.0%), p < 0.001 when compared with plain radiographs (Table 3). Young (0–6 years) males and females have the highest ERR for one CT scan, 21.5% and 45.6%, respectively, compared with the older two groups. Males 7 to 11 years had a 13.5% ERR and females 7 to 11 years a 29.7% ERR, whereas the adolescent male group (12–17 years) had a 12.0% ERR and female adolescent group (12–17 years) a 22.7% ERR. Furthermore, younger age resulted in significantly higher ERR when compared against the older age groups (Fig. 2, A and B).
Figure 2.
(A, B) Excess relative risk box plot for males and females receiving cervical spine computed tomography. White boxes indicate 1 SD from the median value (single line within the box), while the bars indicate the range of values.
Absolute Risk
The prevalence of thyroid cancer in the general population is 5.2/100,000 males and 15.2/100,000 females.20 The risk after plain radiographs increased from 5.20 to 5.21/ 100,000 (0.0052% → 0.00521%) for males and from 15.2 to 15.3/100,000 (0.0152% →0.0153%) for females. For CT, the estimated absolute risk increased from 5.2 to 5.87/100,000 (0.0052% → 0.00587%) for males and from 15.2 to 19.0/ 100,000 (0.0152% → 0.019%) for females (Table 4).
TABLE 4.
Estimated Absolute Risk of Thyroid Cancer Induction From Plain Radiographs and CT Cervical Spine
| Age Category | Male
|
Female
|
||||
|---|---|---|---|---|---|---|
| Plain Radiograph | CT | p | Plain Radiograph | CT | p | |
| 0–6 | 5.21 | 6.32 | <0.001 | 15.3 | 22.1 | <0.001 |
| 7–11 | 5.21 | 5.90 | <0.001 | 15.3 | 19.7 | <0.001 |
| 12–17 | 5.22 | 5.83 | <0.001 | 15.3 | 18.7 | <0.001 |
| All | 5.21 | 5.87 | <0.001 | 15.3 | 19.0 | <0.001 |
Values are reported in 10−5 such that they indicate the number of individuals affected per 100,000. Baseline prevalence of thyroid cancer is 5.2/100,000 males and 15.2/100,000 females.
Comparison Between Plain Radiograph and CT ERR
The overall median ERR for CT compared with plain radiographs was significantly higher, 0.24% versus 13.0% for males and 0.51% versus 25.0% for females, for inducing thyroid cancer (p < 0.001). This was true across both genders and all age groups (Table 3).
DISCUSSION
A 5-year retrospective review of our institution’s pediatric trauma database was performed to determine the relative difference in radiation dose to the thyroid between CT and plain radiographs of the cervical spine. Our protocol was to obtain CT in patients who had symptoms, were at higher risk for injury, or who were otherwise being evaluated with CT. Clearance of the cervical spine using CT at our institution resulted in a 60-fold increase in radiation absorbed to the thyroid when compared with plain radiographs, 64 mGy to 0.9 mGy, p < 0.001. This amount of radiation to the thyroid from a CT cervical spine is approximately equivalent to the absorbed dose to the lungs from 80 chest radiographs.34
The true clinical value, however, lies in gauging the potential for the CT radiation dose to be carcinogenic. On the basis of the linear nonthreshold theory (LNT), the National Research Council described a formula for calculating thyroid cancer risk.18 A single CT cervical spine in our pediatric trauma population increases the median lifetime risk 13.0% in males and 25.0% in females compared with 0.24% in males and 0.51% in females for plain radiographs. Further subdividing the patients by age demonstrates the significant influence youth has on increasing risk despite overall reductions in absorbed radiation from CT. The youngest patient group (0–6 years) received the lowest amount of radiation to the thyroid, yet had the highest median ERR. This supports the age-related sensitivity of the thyroid to radiation and provides evidence for heightened scrutiny when ordering cervical CT in the younger patient populations.
The observation that significantly lower doses were absorbed by younger patients is intuitive but also reflected differences in protocols between age groups at our institution. To decrease total radiation, CT protocols were adjusted in children younger than 6 years. Compared with older children, this demonstrated that imaging protocols that consider radiation dose can have a significant impact on lowering the radiation doses. The quality and diagnostic sensitivity of these studies was not assessed in this study, but clinically there was no issue with inferior image quality in the population that received less radiation. Further efforts to diminish radiation dose during CT are needed.
The impact of age on thyroid cancer induction is secondary to its innate sensitivity to radiation and the time-dependency of thyroid cancer expression.17,19,24 Therefore, the youngest patients have the most radiosensitive thyroids and the greatest time with which to express induction of thyroid cancer. The radiation dose calculator, ImPACT CT Patient Dosimetry Calculator (version 1.0, August 28, 2009), used in this study is based on adult phantoms and potentially underestimates the dose absorbed to pediatric patients.35 After performing another dosimetry study involving pediatric phantoms, Khursheed et al.35 concluded that effective doses for pediatric patients calculated on this dosimetry calculator ranged from 1.1 (15 years) to 2.2 (1 year) times higher than that of an adult. To avoid inaccurately inflating the calculated absorbed doses, this age-dependent factor was omitted from our calculations with the acknowledgment that the doses arrived at are potentially underestimated.
Other limitations of the study potentially exist in both the calculation of absorbed doses to the thyroid and the determination of the risk for developing cancer. The absorbed doses from plain radiographs were estimates based on imaging protocols and not actual dose data for each patient. Rybicki et al.10 has published absorbed doses to the thyroid in adults from plain radiographs—lateral radiograph 0.09 mGy, anteroposterior 0.46 mGy, and open-mouth odontoid 1.04 for a total of 1.59 mGy. The mean radiation in the oldest patient group (that most closely resembling adults) from our data set was 1.55 mGy. Furthermore, the difference between plain radiographs and CT doses was significantly large such that any potential miscalculation would be too minimal to affect the significance. Arbitrary grouping of patients could misrepresent the true effect of age on thyroid dose and cancer induction. In addition, different-sized children within each age group creates a variability in the radiation dose calculated that may cloud comparison by age alone and necessitated data analysis using median values instead of means. Finally, three different CT scanners were used to perform the cervical spine imaging possibly introducing an uncontrolled variable.
Determining the risk for developing thyroid cancer from cervical spine CT using the linear nonthreshold theory is not uniformly accepted. Simply, this theory states that no threshold exists for radiation’s consequence and the effect is dose-dependent in a linear fashion and additive across time. Opponents of LNT refer to studies illustrating a potential protective effect of low dose radiation, so-called radiation hermesis.36–40 The science to support or negate the effect of low-dose (<100 mGy) radiation on cancer induction is weak at best and conclusions made based off the linear LNT should be regarded with caution.41 The application of LNT in this study is solely to illustrate the difference between plain radiography and CT radiation doses. It would be inaccurate to conclude that CT cervical spine causes thyroid cancer—rather, in a highly susceptible organ and sensitive patient population, the significant difference between plain radiographs and CT for potentially inducing cancer should be known so the physician can make the most educated decision to diagnose the pediatric trauma patient.
There are no national guidelines to direct clearance of the cervical spine in pediatric trauma patients and current algorithms lack support from evidence-based research.42 The Eastern Association for the Surgery of Trauma, American College of Radiology (ACR), and the American Academy of Orthopedic Surgery (AAOS) have all authored imaging guidelines for the clearance of the cervical spine, with the AAOS and ACR specifically addressing the pediatric trauma patient.43–45 The National Emergency X-Radiography Utilization Study (NEXUS) has provided practitioners with information to guide the decision to obtain imaging based on clinical findings—no midline cervical tenderness, no focal neurologic deficits, no intoxication or indication of brain injury, no painful distracting injuries, and normal alertness.1,46 However, once the decision to image a patient has been determined, the proper imaging study to use remains controversial. In the journal for the AAOS, Eubanks et al.43 state that plain radiographs are the proper imaging study in the conscious patient at risk (those that meet NEXUS criteria) with imaging by CT of the upper cervical spine if proper C1–2 visualization cannot be achieved or if head CT is indicated. The ACR agrees with applying the NEXUS criteria to older children but does not validate the criteria for younger children (<9 years). Radiographs were deemed appropriate by the ACR committee when NEXUS criteria were met while CT was not an appropriate imaging option.43 Eubanks et al.43 approach the unconscious patient differently as cervical spine CT is obtained with the head CT because of the altered mental status. Then, if unconsciousness is expected to last longer than 24 hours, a magnetic resonance imaging of the cervical spine is performed to definitively clear the cervical spine.43 A recent review of the literature by Hutchings and Willett42 agreed with the approach outlined by Eubanks et al; however, no firm statement was able to be made for the imaging choice in the unconscious/intubated patient.
The significant difference in radiation that CT delivers to the pediatric trauma patient when compared with plain radiographs should temper excessive use. Thus, CT is not recommended as the initial study in conscious patients that meet NEXUS criteria for imaging. In the unconscious patient already obtaining a CT head, we would urge practitioners to consider using plain radiographs as the initial imaging modality followed by magnetic resonance imaging if advanced imaging proved necessary—either from inadequate films or an anticipated prolonged unconscious state. The concern for missed injury is real, but the possible impact of the radiation dose on thyroid cancer induction should factor into the diagnostic algorithm. The significant difference in thyroid dose between CT and plain radiographs, along with the theoretical increase in thyroid cancer estimated relative risk, supports a more conservative cervical spine clearance protocol in the pediatric trauma patient. These results will hopefully facilitate a sensible approach to the cervical spine clearance process and more accurately allow clinicians to measure the risk of advanced imaging in the pediatric trauma patient.
Acknowledgments
We appreciate the support of Scott J. Hetzel, MS, Department of Biostatistics and Medical Informatics, University of Wisconsin–Madison.
Footnotes
Presented at Cervical Spine Research Society, December 2–4, 2010.
DISCLOSURE
Supported by University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), funded through an NIH Clinical and Translational Science Award (CSTA) grant 1 UL1 RR025011. Received stock options from TeraMedica and funding from TeraMedica and General Electric.
References
- 1.Viccellio P, Simon H, Pressman BD, et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108:E20. doi: 10.1542/peds.108.2.e20. [DOI] [PubMed] [Google Scholar]
- 2.Rana AR, Drongowski R, Breckner G, Ehrlich PF. Traumatic cervical spine injuries: characteristics of missed injuries. J Pediatr Surg. 2009;44:151, 5. doi: 10.1016/j.jpedsurg.2008.10.024. discussion 155. [DOI] [PubMed] [Google Scholar]
- 3.Cirak B, Ziegfeld S, Knight VM, Chang D, Avellino AM, Paidas CN. Spinal injuries in children. J Pediatr Surg. 2004;39:607–612. doi: 10.1016/j.jpedsurg.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Brown RL, Brunn MA, Garcia VF. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg. 2001;36:1107–1114. doi: 10.1053/jpsu.2001.25665. [DOI] [PubMed] [Google Scholar]
- 5.Kokoska ER, Keller MS, Rallo MC, Weber TR. Characteristics of pediatric cervical spine injuries. J Pediatr Surg. 2001;36:100–105. doi: 10.1053/jpsu.2001.20022. [DOI] [PubMed] [Google Scholar]
- 6.Reid DC, Henderson R, Saboe L, Miller JD. Etiology and clinical course of missed spine fractures. J Trauma. 1987;27:980–986. doi: 10.1097/00005373-198709000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Broder J, Fordham LA, Warshauer DM. Increasing utilization of computed tomography in the pediatric emergency department, 2000–2006. Emerg Radiol. 2007;14:227–232. doi: 10.1007/s10140-007-0618-9. [DOI] [PubMed] [Google Scholar]
- 8.Richards PJ, Summerfield R, George J, Hamid A, Oakley P. Major trauma & cervical clearance radiation doses & cancer induction. Injury. 2008;39:347–356. doi: 10.1016/j.injury.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Chan PN, Antonio GE, Griffith JF, Yu KW, Rainer TH, Ahuja AT. Computed tomography for cervical spine trauma. The impact of MDCT on fracture detection and dose deposition. Emerg Radiol. 2005;11:286–290. doi: 10.1007/s10140-005-0407-2. [DOI] [PubMed] [Google Scholar]
- 10.Rybicki F, Nawfel RD, Judy PF, et al. Skin and thyroid dosimetry in cervical spine screening: two methods for evaluation and a comparison between a helical CT and radiographic trauma series. AJR Am J Roentgenol. 2002;179:933–937. doi: 10.2214/ajr.179.4.1790933. [DOI] [PubMed] [Google Scholar]
- 11.National Council on Radiation Protection and Measurements. NCRP Report No. 160. Bethesda, MD: National Council on Radiation Protection and Measurement; 2009. Ionizing Radiation Exposure of the Population of the United States. [Google Scholar]
- 12.Biswas D, Bible JE, Bohan M, Simpson AK, Whang PG, Grauer JN. Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am. 2009;91:1882–1889. doi: 10.2106/JBJS.H.01199. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735–738. doi: 10.1148/radiol.2323031095. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 15.Bogdanich W. The New York Times. 2009. Radiation overdoses point up dangers of CT scans. [Google Scholar]
- 16.Associated Press. Medical radiation is a growing concern. The Wall Street Journal. 2010 [Google Scholar]
- 17.Radiation exposure and cancer. American Cancer Society; 2010. [Accessed September 4]. Available at: http://www.cancer.org/Cancer/CancerCauses/OtherCarcinogens/MedicalTreatments/radiation-exposure-and-cancer. [Google Scholar]
- 18.Monson RR, Cleaver JE, Abrams HL, et al. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington D.C: National Academy of Sciences; 2006. [Google Scholar]
- 19.Charles M. UNSCEAR report 2000: sources and effects of ionizing radiation. United Nations Scientific Committee on the Effects of Atomic Radiation. J Radiol Prot. 2001;21:83–86. doi: 10.1088/0952-4746/21/1/609. [DOI] [PubMed] [Google Scholar]
- 20.SEER Stat fact sheets: thyroid. National Cancer Institute; 2007. Available at: http://seer.cancer.gov/statfacts/html/thyro.html. [Google Scholar]
- 21.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 22.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 23.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin P, Casarett GW. Clinical radiation pathology as applied to curative radiotherapy. Cancer. 1968;22:767–778. doi: 10.1002/1097-0142(196810)22:4<767::aid-cncr2820220412>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.National Council on Radiation Protection and Measurements. NCRP Report No. 159. Bethesda, MD: National Council on Radiation Protection and Measurements; 2008. Risk to the Thyroid From Ionizing Radiation. [Google Scholar]
- 26.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 27.Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174:741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams MJ, Shore RE, Dozier A, et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann cohort. Radiat Res. 2010;174:753–762. doi: 10.1667/RR2181.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(Suppl 2):121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Accessed March 9];UWH Adminstrative Policy 8.11—Trauma Policy. Available at: https://uconnect.wisc.edu/servlet/Satellite?cid=1093039724678&pagename=B_EXTRANET_UWHC_POLICIES%2FFlexMember%2FShow_Policy&c=FlexMember.
- 31.Padikal TN, Fivozinski SP. NBS Handbook 138–Medical Physics Data Book. Washington DC: United States Government Printing Office; 1982. [Google Scholar]
- 32.Shrimpton PC, Jones DG. Normalized organ doses for X ray computed tomography calculated using Monte Carlo techniques and a mathematical anthropomorphic phantom. Radiat Prot Dosim. 1993;49:241–243. [Google Scholar]
- 33.Andreo P. Monte Carlo techniques in medical radiation physics. Phys Med Biol. 1991;36:861–920. doi: 10.1088/0031-9155/36/7/001. [DOI] [PubMed] [Google Scholar]
- 34. [Accessed December 15]; Available at: http://www.bt.cdc.gov/radiation/measurement.asp.
- 35.Khursheed A, Hillier MC, Shrimpton PC, Wall BF. Influence of patient age on normalized effective doses calculated for CT examinations. Br J Radiol. 2002;75:819–830. doi: 10.1259/bjr.75.898.750819. [DOI] [PubMed] [Google Scholar]
- 36.Charles MW. LNT–an apparent rather than a real controversy? J Radiol Prot. 2006;26:325–329. doi: 10.1088/0952-4746/26/3/N02. [DOI] [PubMed] [Google Scholar]
- 37.Scott BR. Low-dose radiation risk extrapolation fallacy associated with the linear-no-threshold model. Hum Exp Toxicol. 2008;27:163–168. doi: 10.1177/0960327107083410. [DOI] [PubMed] [Google Scholar]
- 38.Jaworowski Z. Observations on the chernobyl disaster and LNT. Dose Response. 2010;8:148–171. doi: 10.2203/dose-response.09-029.Jaworowski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010;8:172–191. doi: 10.2203/dose-response.09-037.Vaiserman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tubiana M, Aurengo A, Averbeck D, Masse R. The debate on the use of linear no threshold for assessing the effects of low doses. J Radiol Prot. 2006;26:317–324. doi: 10.1088/0952-4746/26/3/N01. [DOI] [PubMed] [Google Scholar]
- 41.Wall BF, Kendall GM, Edwards AA, Bouffler S, Muirhead CR, Meara JR. What are the risks from medical X-rays and other low dose radiation? Br J Radiol. 2006;79:285–294. doi: 10.1259/bjr/55733882. [DOI] [PubMed] [Google Scholar]
- 42.Hutchings L, Willett K. Cervical spine clearance in pediatric trauma: a review of current literature. J Trauma. 2009;67:687–691. doi: 10.1097/TA.0b013e3181b5ecae. [DOI] [PubMed] [Google Scholar]
- 43.Eubanks JD, Gilmore A, Bess S, Cooperman DR. Clearing the pediatric cervical spine following injury. J Am Acad Orthop Surg. 2006;14:552–564. doi: 10.5435/00124635-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Daffner RH, Hackney DB. ACR appropriateness criteria on suspected spine trauma. J Am Coll Radiol. 2007;4:762–775. doi: 10.1016/j.jacr.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Como JL, Diaz JJ, Dunham CM, et al. Practice management guidelines for identification of cervical spine injuries following trauma—update from the eastern association for the surgery of trauma practice management guidelines committee. J Trauma. 2009;67:651– 659. doi: 10.1097/TA.0b013e3181ae583b. Available at: http://www.east.org/Portal/guidelines/tabid/57/Default.aspx. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman JR, Mower WR, Wolfson AB, Todd KH, Zucker MI. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. National Emergency X-Radiography Utilization Study Group. N Engl J Med. 2000;343:94–99. doi: 10.1056/NEJM200007133430203. [DOI] [PubMed] [Google Scholar]