Abstract
Cystic fibrosis (CF) is the most common life-limiting genetically acquired respiratory disorder. Patients with CF have thick mucus obstructing the airways leading to recurrent infections, bronchiectasis and neutrophilic airway inflammation culminating in deteriorating lung function. Current management targets airway infection and mucus clearance, but despite recent advances in care, life expectancy is still only 40 years. We investigated whether activin A is elevated in CF lung disease and whether inhibiting activin A with its natural antagonist follistatin retards lung disease progression. We measured serum activin A levels, lung function and nutritional status in CF patients. We studied the effect of activin A on CF lung pathogenesis by treating newborn CF transgenic mice (β-ENaC) intranasally with the natural activin A antagonist follistatin. Activin A levels were elevated in the serum of adult CF patients, and correlated inversely with lung function and body mass index. Follistatin treatment of newborn β-ENaC mice, noted for respiratory pathology mimicking human CF, decreased the airway activin A levels and key features of CF lung disease including mucus hypersecretion, airway neutrophilia and levels of mediators that regulate inflammation and chemotaxis. Follistatin treatment also increased body weight and survival of β-ENaC mice, with no evidence of local or systemic toxicity. Our findings demonstrate that activin A levels are elevated in CF and provide proof-of-concept for the use of the activin A antagonist, follistatin, as a therapeutic in the long-term management of lung disease in CF patients.
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator gene that cause decreased chloride secretion and increased sodium reabsorption across the airway epithelium, associated with the depletion of airway surface liquid and defective mucus rheology and reduced clearance.1 These changes contribute to a cycle of bacterial infection and inflammation leading to progressive deterioration in lung function.2 Respiratory failure is the cause of premature death in 85% of CF patients and is the major target of current therapeutic strategies.3 A drug that increases the activity of the CF transmembrane conductance regulator protein, Kalydeco (Vertex Pharmaceuticals Incorporated), is available, but offers benefit to only the 6% of patients with the uncommon G551D gene mutation.4 A new therapeutic with widespread applicability is urgently needed. Chronic infection with Pseudomonas aeruginosa (P. aeruginosa) is a prelude to bronchiectasis with a negative implication for morbidity and mortality. The mean age at which CF patients acquire chronic mucoid P. aeruginosa is 25 years,5 indicating an opportunity for preventative intervention during late adolescence or early childhood before chronic infection is established and lung function has declined.
Activin A is a member of the transforming growth factor-β superfamily of cytokines that regulates growth and differentiation,6 and has more recently been ascribed an immunoregulatory role.7, 8 Activin A, which shows 100% protein sequence conservation between human and mouse, has an important role in the regulation of lung inflammation and fibrosis9, 10, 11 and may be a final common step in the pathway to fibrosis.7 Of particular relevance to CF and other inflammatory lung disorders, activin A induces proinflammatory cytokines including interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF).8 Mice with elevated serum activin A have been shown to develop cachexia.12
The naturally produced glycoprotein follistatin binds to activin A with high affinity, blocking activin receptor binding and neutralising activin action.7 Follistatin binds to other structurally related members of the transforming growth factor-β growth factor family (GDF8 and 9, BMP2, 5, 7 and 8) but with 10-fold lower affinity than for activin A.8 The 288-amino-acid follistatin isoform (FS288) binds intrinsically to heparin sulphate-containing proteoglycans and is the main cell-associated form.13 Like activin A, the protein sequence of follistatin is highly conserved across species, with 98% conservation between human and mouse. Importantly, follistatin inhibits cachexia in inhibin-deficient mice14 and inhibits lung inflammation and fibrosis in bleomycin-induced lung injury and experimental allergic asthma.15, 16, 17 Activin A and follistatin are produced by a wide variety of cells in the lung (and other organs), including fibroblasts, dendritic cells, mast cells, macrophages, airway epithelium and T cells.7, 8, 16, 18
The research reported here supports our hypothesis that activin A is upregulated in CF and that inhibiting activin A with its natural antagonist follistatin would ameliorate CF lung immunopathology. Efficacy of follistatin was demonstrated in an established transgenic mouse model of CF (β-ENaC mice) that manifests mainly respiratory pathology, the leading cause of death in CF. Our clinical data from an adult CF patient cohort demonstrated elevated serum activin A levels with an inverse correlation with lung function and body mass index (BMI) as an index of cachexia. Collectively, our findings indicate that follistatin has the potential for a paradigm shift in management of lung disease in patients with CF.
Results
Increased serum activin A levels in CF patients correlate with decreased lung function and body weight
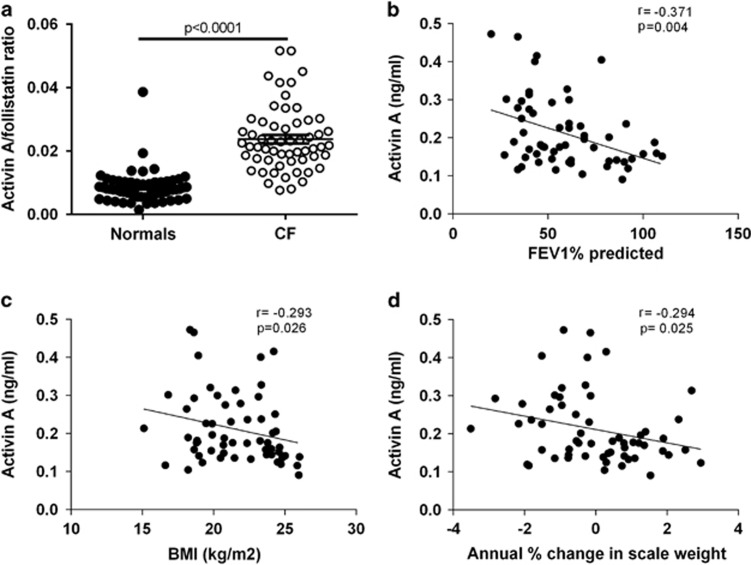
Our hypothesis that activin A is increased in CF lung disease would be reflected by high serum activin A levels and an increased activin A/follistatin ratio. Clinical sampling of a cohort of well-characterized adult CF patients19, 20, 21 confirmed elevated serum activin A levels (0.212±0.012 versus 0.082±0.003 ng ml−1 for normal; mean±s.e.m.; P<0.0001). Conversely, we observed slightly decreased follistatin levels in CF patients (9.38±0.37 versus 12.04±0.7 ng ml−1; CF versus normal, P=0.0011). Consequently, the activin A/follistatin ratio (reflecting activin A bioavailability) was elevated in CF patients (P<0.0001) compared with unaffected individuals (Figure 1a). Moreover, serum activin A levels and the activin A/follistatin ratio were inversely correlated with respiratory function (FEV1% predicted; Figure 1b, activin A P=0.004 and activin A/follistatin ratio P=0.004), BMI (Figure 1c, activin A P=0.026 and activin A/follistatin ratio P=0.019) and annual % change in body weight (Figure 1d, activin A P=0.025 and activin A/follistatin ratio P=0.07). Thus, increased serum activin A levels in CF correlate with decreased lung function and body mass.
Figure 1.
Increased serum activin A levels in CF patients correlate with the loss of respiratory function and body mass. (a) Serum activin A/follistatin ratio in adult CF patients (open circles) compared with age-matched controls (solid circles). (b–d) Correlation between serum activin A level and (b) respiratory function (FEV1% predicted), (c) BMI and (d) annual % change in scale weight (a–d; n=58–60 per group).
Follistatin treatment of newborn β-ENaC mice increases survival and inhibits activin A levels in the lung
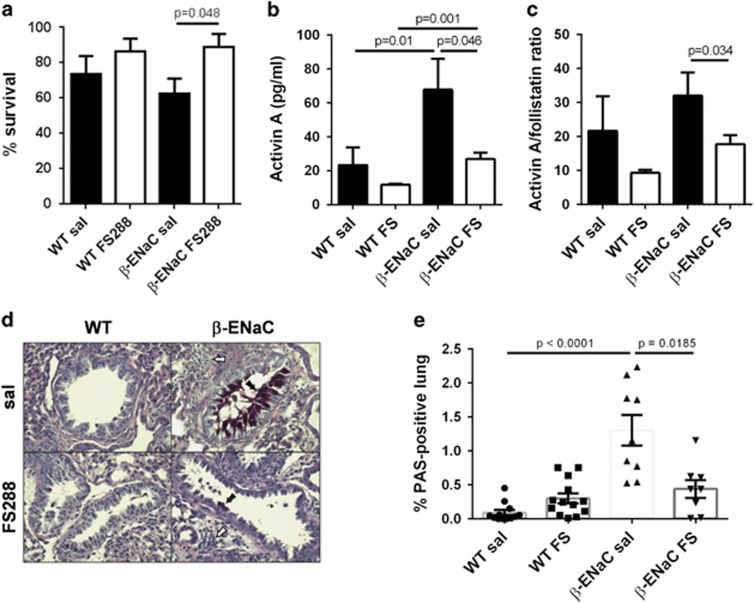
The above findings suggested a role for activin A in CF disease pathogenesis. We previously showed that blocking activin A with intranasally instilled FS288 (250 μg kg−1) inhibited pulmonary mucus production and fibrosis in mouse models of acute and chronic allergic asthma.16, 17 We therefore investigated whether intranasally delivered FS288 would ameliorate CF lung pathogenesis in an established mouse model of CF. In these β-ENaC mice, the β-subunit of the epithelial sodium channel is overexpressed in the airway epithelium.22, 23 β-ENaC mice have decreased airway surface liquid volume and severe CF-like lung pathology characterized by mucus obstruction and neutrophilic inflammation, analogous to human CF lung pathology. Approximately 40–50% of untreated β-ENaC mice die by 3 weeks of age. Our study showed that intranasal instillation of FS288 (250 μg kg−1) to β-ENaC mice every second day from day 3–21 post birth increased survival at day 22 compared with saline-treated β-ENaC mice (Figure 2a; P=0.048). We speculated that follistatin increased survival by inhibiting activin A levels and the activin A/follistatin ratio in bronchoalveolar lavage (BAL) fluid. As predicted, β-ENaC mice treated with FS288 had decreased BAL fluid activin A levels (Figure 2b; P=0.046) and activin A/follistatin ratio (Figure 2c; P=0.034) at day 22. These findings show that follistatin decreases activin A levels in the lung and increases survival of β-ENaC mice, consistent with an important pathogenic role for activin A in CF.
Figure 2.
FS288 instillation increases survival and decreases airway activin A levels and mucus hypersecretion in β-ENaC mice. WT or β-ENaC mice were treated with saline or FS288 intranasally every second day, from 3 to 21 days of age. (a) Percentage survival of saline- or FS288-treated WT and β-ENaC mice at d22 (n=18–32 mice per group). (b and c) BAL fluid activin A (b) and activin A/follistatin ratios (c) at day 22 (n=7–12 mice per group). (d) Mucus production in representative mouse lung sections. Mucus producing cells (black arrow) and inflammation (white arrow); (PAS, original magnification 400x). (e) % Lung area occupied by mucus-secreting cells and/or mucus plugs (n=8–13 mice per group). All values mean±s.e.m. A full color version of this figure is available at Immunology and Cell Biology Journal online.
Follistatin treatment inhibits mucus production in the lungs of β-ENaC mice
Obstruction of the airways with mucus in β-ENaC mice causes respiratory failure and is the primary cause of premature death.22, 23 We therefore investigated whether the increased survival of follistatin-treated β-ENaC mice was associated with decreased mucus production. Image analysis of entire lung sections to identify mucus-producing cells and/or mucus plugs in the airways (Figure 2d) showed that the lungs of saline-treated β-ENaC mice had increased mucus production compared with wild-type (WT) mice (Figure 2e; P<0.0001), as expected.22, 23 Importantly, FS288-treated β-ENaC mice had reduced mucus production in their lungs compared with saline-treated transgenic mice (Figure 2e; P=0.0185).
Follistatin treatment inhibits airway neutrophil and macrophage numbers in β-ENaC mice
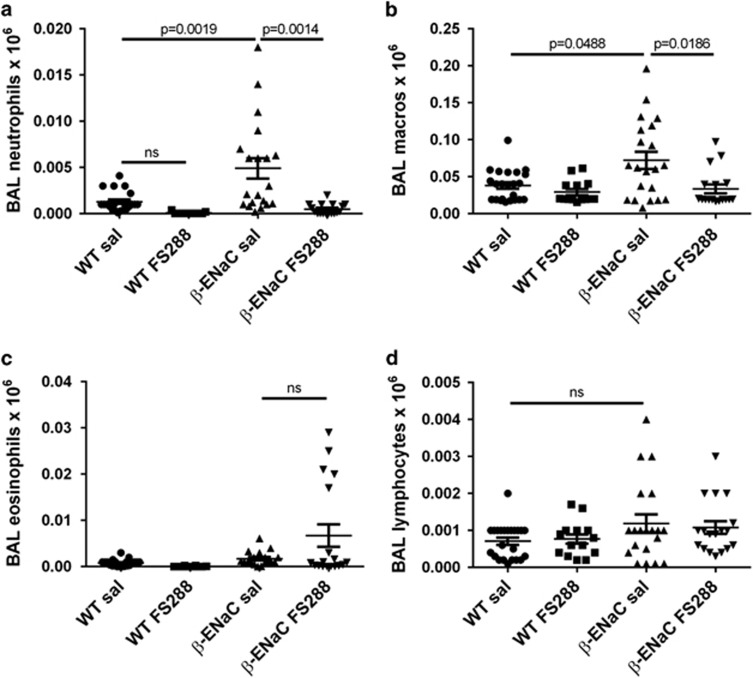
To gain insight into the effect of FS288 instillation on lung inflammation, we quantified inflammatory cells in the airways of β-ENaC mice. BAL cell differential analysis showed that FS288 treatment almost completely abrogated the neutrophilia seen in saline-treated β-ENaC mice (P<0.0014; Figure 3a). Similarly, the increase in macrophage numbers in saline-treated β-ENaC mice (P=0.0488) was significantly inhibited by FS288 treatment (P=0.0186; Figure 3b). There were no significant changes in eosinophil or lymphocyte numbers between any group (Figures 3c and d).
Figure 3.
FS288 instillation inhibits airway neutrophil and macrophage numbers in β-ENaC mice. WT or β-ENaC mice were treated with saline or FS288 intranasally as per Figure 2. (a–d) proportions of BAL neutrophils, macrophages, eosinophils and lymphocytes. Mean±s.e.m., n=14–23 mice per group.
Follistatin treatment decreases airway levels of inflammatory mediators in β-ENaC mice
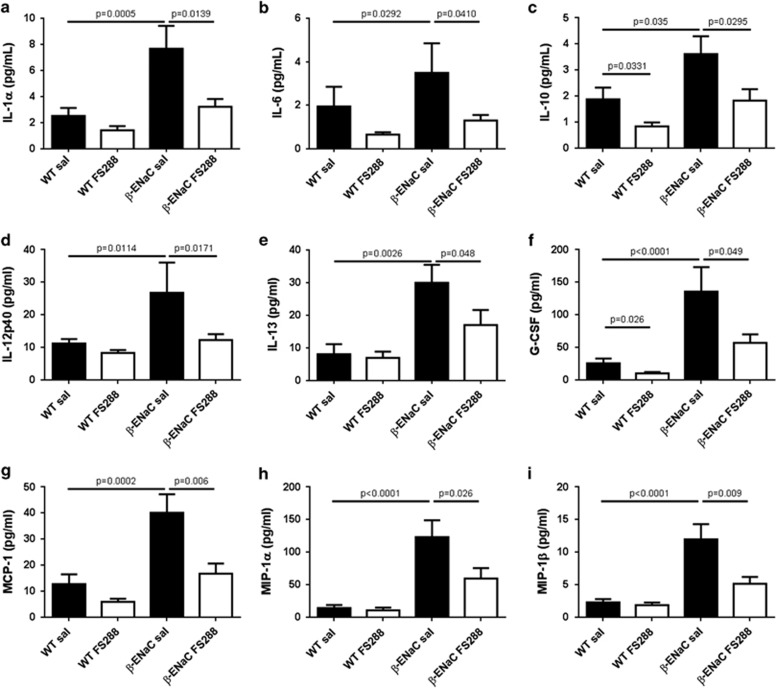
To further analyse the effects of follistatin treatment on lung inflammation, we measured the levels of BAL fluid inflammatory mediators. Multiplex analysis showed that, compared with WT mice, β-ENaC mice had increased levels of cytokines and chemokines important in (i) regulation of inflammatory responses (IL-1α, IL-6, IL-10, IL-12p40; Figures 4a–d), (ii) induction of mucus production (IL-13; Figure 4e) and (iii) maturation and/or recruitment of dendritic cells, monocyte/macrophages and neutrophils (granulocyte-colony stimulating factor [G-CSF], monocyte chemotactic protein-1 [MCP-1], monocyte inhibitory protein (MIP-1α and MIP-1β Figures 4f–i). Notably, FS288 treatment caused a two- to threefold reduction in the levels of these inflammatory mediators in the BAL fluid of β-ENaC mice such that for the majority, levels approximated those seen in saline-treated WT mice, providing a rationale for the anti-inflammatory action of FS288 in CF. No significant changes in BAL IL-17 levels were observed between WT mice and β-ENaC mice (data not shown).
Figure 4.
FS288 instillation inhibits BAL fluid cytokine and chemokine levels in β-ENaC mice. WT or β-ENaC mice were treated with saline or FS288 intranasally as per Figure 2. (a–i) BAL fluid levels of IL-1α, IL-6, IL-10, IL-12p40, IL-13, granulocyte-colony stimulating factor, monocyte chemotactic protein-1, MIP-1α and MIP-1β. Mean±s.e.m., n=15–27 mice per group.
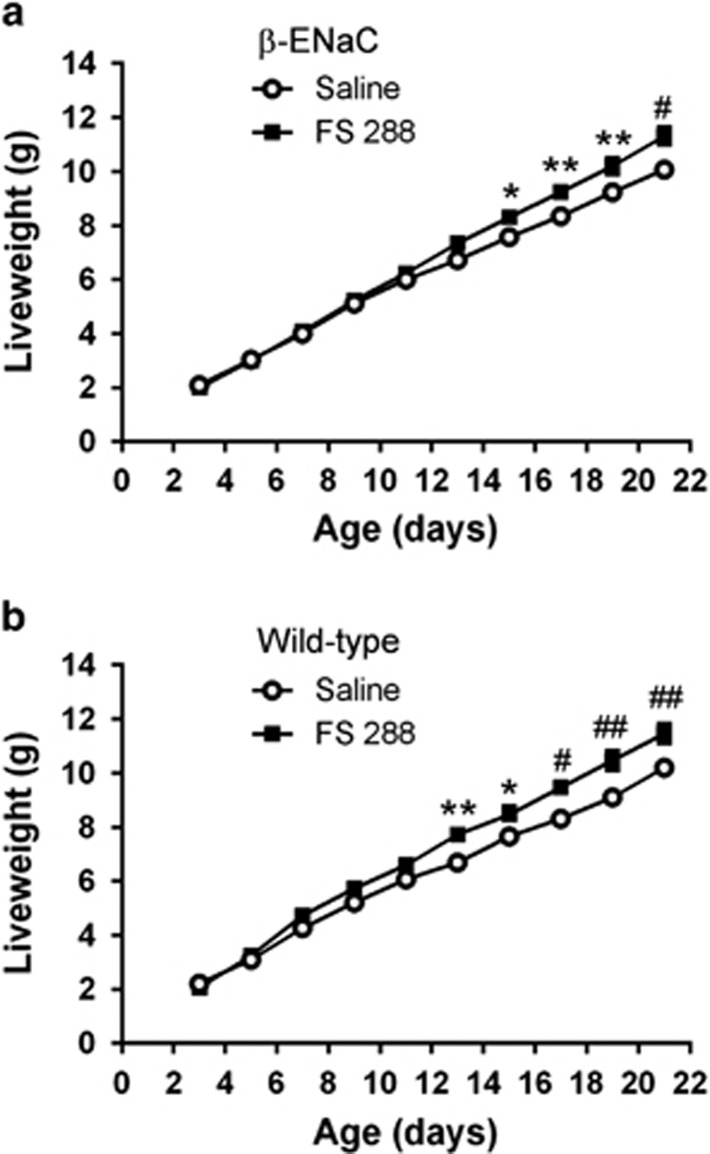
Follistatin treatment increases body weight and does not cause systemic toxicity in β-ENaC mice
Increased serum activin A levels in mice are associated with cachexia that can be inhibited by follistatin overexpression,12, 14 suggesting that follistatin could affect weight gain in β-ENaC mice. FS288 treatment of β-ENaC mice caused a significant increase in body weight from 2 weeks of age, resulting in an 11–12% increased body weight by 3 weeks of age relative to saline-treated β-ENaC mice (Figure 5a). Interestingly, FS288 treatment caused a similar small increase in body weight of WT mice (Figure 5b). Importantly, there was no histological evidence of lung pathology in FS288-treated WT mice, and histological assessment of liver, heart, brain, kidney and testes failed to identify any evidence of systemic toxicity of FS288 (data not shown). Thus, long-term follistatin treatment has no adverse developmental effects and increases weight gain.
Figure 5.
FS288 instillation increases body weight of WT and β-ENaC mice. WT or β-ENaC mice were treated with saline or FS288 intranasally as per Figure 2. Body weights of β-ENaC mice (a) and WT mice (b) from day 3 to 22. Mean±s.e.m., n=15–34 mice per group per time-point. *P<0.05, **P<0.01, #P<0.001, ##P<0.0001.
Discussion
Despite recent advances in the management of CF, life expectancy is still only 40 years. Pulmonary pathology and dysfunction remain the primary cause of death3 and there is a pressing need for therapeutics that can impede the aggressive cycle of inflammation and infection in the lung, which lead to progressive deterioration of lung function. Herein, we investigated the hypothesis that activin A, a key regulator of lung inflammation,9, 10, 11 is upregulated in CF, and that inhibiting activin A with its natural antagonist follistatin would ameliorate CF lung immunopathology. Our findings show that activin A levels are highly increased in CF patients, correlating with decreased lung function and body weight. Using the well-characterised β-ENaC transgenic mouse model of CF lung disease, we showed that follistatin treatment inhibited key features of CF lung immunopathology. Strengthening the association between activin A and cachexia,12, 14, 24 follistatin treatment of β-ENaC mice increased survival and body mass without any evidence of toxicity. Collectively, our findings provide impetus for the development of follistatin as a therapeutic for the amelioration of existing inflammation and the prevention of CF lung disease.
The finding that serum activin A levels correlated with CF disease severity suggests that serum activin A could be used as a marker of lung disease progression in CF, as for other lung diseases (for example, acute respiratory failure25). In this study, repeat blood samples were taken from most CF patients during a 6-month period but did not show any significant difference in activin A or follistatin levels between the three time points (P=0.77, P=0.24, respectively), consistent with their disease stability. Unfortunately, collection of BAL samples from our clinical cohort was not possible for ethical reasons as CF adults seldom have bronchoscopy/BAL due to impaired lung function leading to inability to tolerate bronchoscopy well, and it is only indicated when necessary for their specific clinical care. A prospective, longitudinal study on CF patients over a longer period, ideally starting in children, is a logical follow up to the present cross-sectional study.
Activin A levels were also markedly increased in the BAL fluid of β-ENaC mice, and this increase was inhibited by follistatin. Activin A is released into the circulation within 1 h following lipopolysaccharide injection in mice, simultaneous with TNF and before IL-1β and IL-6,26 establishing it as one of the earliest inflammatory mediators. In our study, BAL fluid TNF levels were very low (~4 pg ml−1) and not increased in β-ENaC mice, indicating that induction of activin A and TNF is not linked, and presumably depends on the type of inflammatory stimulus. In support of our hypothesis, we showed that follistatin inhibited the induction of multiple cytokines/chemokines in the lungs of β-ENaC mice, suggesting that activin A is a driver of lung inflammation in CF. A proinflammatory function of activin A in the lung is further buttressed by the finding that activin A overexpression in the lung induces severe inflammation.27 Nevertheless, activin A has also been ascribed anti-inflammatory actions,28 and the outcome may depend on the source and/or timing of expression, the tissue microenvironment and other factors.8
Follistatin treatment significantly inhibited airway neutrophil and macrophage numbers in β-ENaC mice. Since neutrophils and macrophages are producers of activin A,8, 29, 30 the decreased airway activin A levels could be partially due to the follistatin-mediated decrease in numbers of these cells. The reduction in neutrophil and macrophage numbers is likely due to the decreased chemokine production in the lung, consistent with the decreased MIP-1α and MIP-1β levels we observed in follistatin-treated mice.31 Neutrophils are widely regarded as major drivers of CF pathogenesis,32 with neutrophil elastase in BAL fluid at 3 months of age a key predictor of subsequent bronchiectasis in children.33 Although new drugs targeting neutrophil elastase show some efficacy in clinical trials,32 there are no available drugs which effectively block neutrophil recruitment to the airways in the first place. Our finding that FS288 treatment reduces airway neutrophilia in β-ENaC mice gives optimism that follistatin would be therapeutically beneficial in human CF.
A key feature of CF is the accumulation of thick neutrophil-rich mucus within the airways. β-ENaC mice have reduced airway surface liquid volume and develop goblet cell hyperplasia and mucus hypersecretion22 resulting in premature death, the primary cause of which is thought to be asphyxia due to mucus plugging.23 Importantly, we showed that follistatin treatment significantly increased survival in β-ENaC mice. Surprisingly, saline-treated WT mice had lower than expected survival, likely due to the regular saline instillations triggering low-grade mucus production and neutrophilia, and this was partially reversed by follistatin. Quantitative assessment of mucus production using a digital analysis system, which identified all Periodic acid–Schiff (PAS)-stained structures in the lung section including mucus plugs in the airways, showed that follistatin significantly inhibited mucus production in the lungs of β-ENaC mice, providing a mechanistic explanation for their increased survival. It is thought that neutrophils release proteases into the space between the mucus and airway epithelium, presenting a challenge for CF anti-protease drugs due to the difficulty in penetrating the mucus layer.3 Thus, our finding that follistatin inhibits both mucus production and neutrophilia provides a potential major breakthrough in the treatment of CF, as it circumvents two of the major stumbling blocks to successful therapy.
CF is characterised by marked loss of body weight, with the most dramatic changes occurring during adolescence.2 This loss of body mass is reflected in decreased BMI and fat-free mass, the latter associated with increased circulating IL-6 levels but not TNF,19 both of which were traditionally thought to be drivers of catabolism in CF.34 Interestingly, unrestrained activin A expression in mice (due to deletion of the inhibin α subunit) results in cachexia,12 and follistatin inhibits weight loss and increases survival of these mice.14 Furthermore, activin A overexpression in skeletal muscle causes loss of body mass (twofold loss of fat mass and 9% decreased lean mass) and muscular atrophy in mice.24 Collectively, these findings demonstrate that activin A is a key pro-cachectic cytokine, and reinforce the idea that the increased circulating activin A levels in CF patients are responsible for the association with lower BMI and weight loss that we observed. Further evidence implicating activin A as a regulator of body mass comes from our finding that follistatin treatment increased body weight of WT and β-ENaC mice, whilst decreasing lung activin A, suggesting that follistatin delivered into the lung enters the circulation and exerts its effect systemically. Of note, we did not observe any significant change in lung weights between saline and follistatin-treated mice (data not shown). Follistatin injection into skeletal muscle of mice and primates increases muscle mass35, 36 and, as mentioned above, follistatin overexpression decreases cachexia in mice that overexpress activin A,14 providing a likely explanation for the increased body mass we observed in our mice. It is possible that follistatin binding of myostatin could contribute to this change, but as the affinity of this interaction is considerably lower than for activin A,8 this pathway is likely to have a lesser role. Notably, we failed to observe any signs of toxicity in any organ examined (lung, liver, heart, brain, kidney and testes) or other signs of ill health in our follistatin-treated animals. This lack of toxicity is in agreement with others showing no detrimental effect of intramuscularly injected follistatin in any organ system in primates,36 and in our own previous long-term (5 weeks) studies delivering FS288 intranasally to the mouse lung.16
In conclusion, our findings suggest that follistatin administered preventatively before severe CF lung disease is established has the potential to safely prevent the development of excessive inflammation and mucus hypersecretion, and to decrease the infection and remodelling cascade that results in end-stage lung disease and premature death in CF. However, despite evidence of safety in this study and others, further investigation into the long-term effects of FS in CF is warranted. We propose that by blocking activin A, follistatin may interfere with the inflammatory cascade at numerous levels, collectively resulting in decreased mucus production and airway neutrophilia via impairment of cytokines that drive these responses (for example, IL-13, MIP-1α, MIP-1β). Our observation of increased body weight in follistatin-treated mice further suggests that follistatin may inhibit the cachexia seen in CF by inhibition of activin A and IL-6.19, 24 Our data including positive effects on survival and growth support efficacy of inhaled follistatin, which is a more acceptable method of administering drugs to the lung than intraperitoneally or intravenously. Thus, follistatin has the potential for a major shift in treatment of lung disease in CF patients, with the prospect of increasing quality of life and life expectancy.
Methods
Study participants and measurements
Adult CF patients (n=58; 35 males and 23 females; age range 23–63 years) attended the regional Adult Cystic Fibrosis Service at The Alfred Hospital.19, 20, 21 Pulmonary function was assessed by calculating the percentage predicted forced expiratory volume in 1 s (FEV1%).21 Height and weight measurements were used to calculate BMI (kg m−2). Annual % change in weight was determined as described.19, 20 Healthy controls (n=60; 29 males and 31 females) were mean age matched to the patient cohort.25 Serum from CF participants was sampled on one to three occasions over 6 months when clinically stable19 and once for healthy controls. The study was approved by The Alfred Hospital Institutional Ethics Committee and written informed consent was obtained. See Supplementary Methods.
Mice
β-ENaC transgenic mice were obtained from Marcus Mall22 and bred in the Alfred Medical Research and Education Precinct animal facility. Mice were genotyped using primers as described (Transnetyx Inc., Cordova, TN, USA).22 β-ENaC mice and WT controls were used for all experiments. All experimental protocols were approved by the precinct Animal Ethics Committee.
FS288 production and instillations
For follistatin production and assessment of bioactivity, see Supplementary Methods. Litters of β-ENaC mice or WT controls were allocated randomly to FS288 or saline treatment groups. FS288 (250 μg kg−1) or saline was instilled intranasally into lightly anaesthetised pups from 3 days of age, every second day for 3 weeks. Volumes were 5 μl for 3-day-old pups, increasing to 30 μl for 21-day-old pups. Pups were weighed before FS288 instillation to determine the correct dose. Mice were killed 24–48 h after the final instillation and the tissues were collected for analysis.
Blood, BAL and tissue sampling and processing
Blood was collected from the inferior vena cava. BAL was collected by cannulating the trachea and performing three lavages with 0.3 ml of phosphate-buffered saline/fetal calf serum (rather than one large volume of phosphate-buffered saline/fetal calf serum) while gently massaging the ribcage. BAL cell counts and differential analysis were performed as described previously.16 After BAL collection, the lung was perfusion fixed via the trachea with fresh 2% formalin at constant pressure (20 cm water) for 4–5 min before paraffin embedding. Extensive experience shows that this protocol does not adversely affect lung architecture, but instead results in excellent preservation of morphology while avoiding tissue fixation artefacts frequently observed in non-perfusion fixed lung. See Supplementary Methods.
Aperio quantitative image analysis
For mucus analysis, entire midline PAS-stained sections of mouse lungs were scanned, and analysed using ImageScope 11.2 (Aperio Technologies, Leica Biosystems, Mount Waverley, VIC, Australia). Colour deconvolution was used to separate PAS (pink) from haematoxylin (blue). Thresholding was performed on the acid Schiff's channel to select only mucoid staining and to minimise background selection. Data are presented as the percentage of PAS-positive pixels divided by PAS-positive pixels and hematoxylin-positive pixels (total tissue area).
Measurement of activin A, follistatin and BAL fluid cytokines and chemokines
Levels of mouse BAL fluid cytokines and chemokines were determined by Bio-Plex (BioRad, Hercules, CA, USA). Activin A and follistatin concentrations in human serum and mouse BAL fluid were measured by a specific enzyme-linked immunosorbent assay and radioimmunoassay as described.17, 37
Statistical analysis
Statistics were analysed using Graph Pad Prism v6.01 (La Jolla, CA, USA). Data were analysed for normality and log-transformed as necessary before analysis by independent samples t-test or two-way analysis of variance with Dunnett post tests. Survival data were analysed by Log-rank (Mantel–Cox) test. A Spearman correlation was used for the human correlation analysis. Differences were considered significant at P<0.05. For the PAS and BAL differential analysis, outliers were removed using a Q-value of 0.2% (meaning <1:500 outliers detected would be false). Group sizes are indicated in the figure legends. All values are mean±s.e.m.
Acknowledgments
We thank Jun Yao for assistance with the animal experimentation. We gratefully acknowledge Susan Hayward for performing the activin A and follistatin assays and Jonathan Bensley (Anatomy and Developmental Biology, Monash University) for performing the Aperio quantitative image analysis. The investigators thank Oxford Brookes University for provision of the reagents for the activin A assay. We thank NHMRC Australia; the CASS Foundation and Paranta Biosciences, Australia; the Victorian Government's Operational Infrastructure Support Program. The funding bodies had no role in the study conceptualization, design, conduct, data analysis or manuscript preparation.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Vandenbranden SL, McMullen A, Schechter MS, Pasta DJ, Michaelis RL, Konstan MW, et al. Lung function decline from adolescence to young adulthood in cystic fibrosis. Pediatr Pulmonol. 2012;47:135–143. doi: 10.1002/ppul.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmiel JF, Konstan MW.Inflammation in the Cystic Fibrosis LungIn: Allen JL, Panitch HB, Rubenstein RC (eds)Cystic Fibrosis1st ednInforma Healthcare: New York, NY; 201057–77. [Google Scholar]
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch AE, Rodgers HC. Cystic fibrosis. J R Coll Physicians Edinb. 2013;43:144–150. doi: 10.4997/JRCPE.2013.212. [DOI] [PubMed] [Google Scholar]
- Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, O'Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Mol Cell Endocrinol. 2012;359:101–106. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011;85:255–297. doi: 10.1016/B978-0-12-385961-7.00013-5. [DOI] [PubMed] [Google Scholar]
- Gregory LG, Mathie SA, Walker SA, Pegorier S, Jones CP, Lloyd CM. Overexpression of Smad2 drives house dust mite-mediated airway remodeling and airway hyperresponsiveness via activin and IL-25. Am J Respir Crit Care Med. 2010;182:143–154. doi: 10.1164/rccm.200905-0725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, et al. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Kariyawasam HH, Pegorier S, Barkans J, Xanthou G, Aizen M, Ying S, et al. Activin and transforming growth factor-beta signaling pathways are activated after allergen challenge in mild asthma. J Allergy Clin Immunol. 2009;124:454–462. doi: 10.1016/j.jaci.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci USA. 1994;91:8817–8821. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, et al. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem. 1993;268:15579–15587. [PubMed] [Google Scholar]
- Cipriano SC, Chen L, Kumar TR, Matzuk MM. Follistatin is a modulator of gonadal tumor progression and the activin-induced wasting syndrome in inhibin-deficient mice. Endocrinology. 2000;141:2319–2327. doi: 10.1210/endo.141.7.7535. [DOI] [PubMed] [Google Scholar]
- Aoki F, Kurabayashi M, Hasegawa Y, Kojima I. Attenuation of Bleomycin-induced Pulmonary Fibrosis by Follistatin. Am J Respir Crit Care Med. 2005;172:713–720. doi: 10.1164/rccm.200412-1620OC. [DOI] [PubMed] [Google Scholar]
- Hardy CL, Nguyen HA, Mohamud R, Yao J, Oh DY, Plebanski M, et al. The activin A antagonist follistatin inhibits asthmatic airway remodelling. Thorax. 2013;68:9–18. doi: 10.1136/thoraxjnl-2011-201128. [DOI] [PubMed] [Google Scholar]
- Hardy CL, O'Connor AE, Yao J, Sebire K, de Kretser DM, Rolland JM, et al. Follistatin is a candidate endogenous negative regulator of activin A in experimental allergic asthma. Clin Exp Allergy. 2006;36:941–950. doi: 10.1111/j.1365-2222.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- Hardy CL, Lemasurier JS, Olsson F, Dang T, Yao J, Yang M, et al. Interleukin-13 regulates secretion of the tumor growth factor-{beta} superfamily cytokine activin A in allergic airway inflammation. Am J Respir Cell Mol Biol. 2010;42:667–675. doi: 10.1165/rcmb.2008-0429OC. [DOI] [PubMed] [Google Scholar]
- King SJ, Nyulasi IB, Bailey M, Kotsimbos T, Wilson JW. Loss of fat-free mass over four years in adult cystic fibrosis is associated with high serum interleukin-6 levels but not tumour necrosis factor-alpha. Clin Nutr. 2014;33:150–155. doi: 10.1016/j.clnu.2013.04.012. [DOI] [PubMed] [Google Scholar]
- King SJ, Nyulasi IB, Strauss BJ, Kotsimbos T, Bailey M, Wilson JW. Fat-free mass depletion in cystic fibrosis: associated with lung disease severity but poorly detected by body mass index. Nutrition. 2010;26:753–759. doi: 10.1016/j.nut.2009.06.026. [DOI] [PubMed] [Google Scholar]
- King SJ, Topliss DJ, Kotsimbos T, Nyulasi IB, Bailey M, Ebeling PR, et al. Reduced bone density in cystic fibrosis: DeltaF508 mutation is an independent risk factor. Eur Respir J. 2005;25:54–61. doi: 10.1183/09031936.04.00050204. [DOI] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, et al. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med. 2008;177:730–742. doi: 10.1164/rccm.200708-1233OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JL, Walton KL, Winbanks CE, Murphy KT, Thomson RE, Makanji Y, et al. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014;28:1711–1723. doi: 10.1096/fj.13-245894. [DOI] [PubMed] [Google Scholar]
- de Kretser DM, Bensley JG, Pettila V, Linko R, Hedger MP, Hayward S, et al. Serum activin A and B levels predict outcome in patients with acute respiratory failure: a prospective cohort study. Crit Care. 2013;17:R263. doi: 10.1186/cc13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci USA. 2007;104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolou E, Stavropoulos A, Sountoulidis A, Xirakia C, Giaglis S, Protopapadakis E, et al. Activin-A overexpression in the murine lung causes pathology that simulates acute respiratory distress syndrome. Am J Respir Criti Care Med. 2012;185:382–391. doi: 10.1164/rccm.201105-0784OC. [DOI] [PubMed] [Google Scholar]
- Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, et al. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J Exp Med. 2009;206:1769–1785. doi: 10.1084/jem.20082603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Winnall WR, Loveland KL, Makanji Y, Phillips DJ, et al. Tumour necrosis factor-alpha stimulates human neutrophils to release preformed activin A. Immunol Cell Biol. 2011;89:889–896. doi: 10.1038/icb.2011.12. [DOI] [PubMed] [Google Scholar]
- Wu H, Chen Y, Winnall WR, Phillips DJ, Hedger MP. Regulation of activin A release from murine bone marrow-derived neutrophil precursors by tumour necrosis factor-alpha and insulin. Cytokine. 2013;61:199–204. doi: 10.1016/j.cyto.2012.09.018. [DOI] [PubMed] [Google Scholar]
- McDonald B, Kubes P. Chemokines: sirens of neutrophil recruitment-but is it just one song. Immunity. 2010;33:148–149. doi: 10.1016/j.immuni.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol. 2014;21:16–22. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- Ionescu AA, Nixon LS, Shale DJ. Cellular proteolysis and systemic inflammation during exacerbation in cystic fibrosis. J Cyst Fibros. 2004;3:253–258. doi: 10.1016/j.jcf.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor AE, McFarlane JR, Hayward S, Yohkaichiya T, Groome NP, de Kretser DM. Serum activin A and follistatin concentrations during human pregnancy: a cross-sectional and longitudinal study. Hum Reprod. 1999;14:827–832. doi: 10.1093/humrep/14.3.827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.