Abstract
The exuberant expression of proteinases by tumor cells has long been associated with the breakdown of the extracellular matrix, tumor invasion, and metastasis to distant organs. There is both epidemiological and experimental data that support a causative role for proteinases of the matrix metalloproteinase (MMP) family in tumor progression. Optical imaging techniques provide an extraordinary opportunity for non-invasive “molecular imaging” of tumor-associated proteolytic activity. The application of optical proteolytic beacons for the detection of specific proteinase activities associated with tumors has several potential purposes: 1) Detection of small, early-stage tumors with increased sensitivity due to the catalytic nature of proteolytic activity, 2) Diagnosis and Prognosis to distinguished tumors that require particularly aggressive therapy or those that will not benefit from therapy, 3) Identification of tumors appropriate for specific anti-proteinase therapeutics and optimization of drug and dose based on determination of target modulation, and 4) as an indicator of efficacy of proteolytically-activated pro-drugs. This chapter describes the synthesis, characterization, and application of reagents that use visible and near infrared fluorescence resonance energy transfer (FRET) fluorophore pairs to detect and measure MMP-referable proteolytic activity in tumors in mouse models of cancer.
Keywords: FRET, dendrimer, optical imaging, proteolytic beacon, MMP
1. Introduction
Fluorescence or Förster Resonance Energy Transfer (FRET) is a valuable tool in the application of optical imaging to biological problems. The process is characterized by the transfer of electronic excitation energy of a donor chromophore to an acceptor molecule brought in close proximity via a coupling mechanism between the donor-acceptor pair. The efficiency of the transfer process depends on the distance between fluorophores. The advent of optical devices, especially the CCD camera and computer-based imaging technology, have afforded tools to detect the dequenching photons from this FRET mechanism, and this process underlies the emerging molecular optical imaging approaches that enable detection of proteinase activity.
There are several examples of agents that employ FRET principles for the detection of proteolytic activity in living animals and tissues (1). Weissleder and colleagues attached fluorophores to a linear copolymer by a peptide containing a proteolytic cleavage site as a means to measure protease activity and subsequent inhibition in tumor-bearing mice. Proximity of the fluorophores quenches the fluorescent signal, which is released upon cleavage of the peptide linker. Such a probe was used to detect MMP-2 activity in HT1080 human fibrosarcoma xenografts, and the signal was inhibited by treatment with a synthetic MMP inhibitor (2). The Tsien group described activatable cell-penetrating peptides consisting of a polyarginine membrane-translocating motif linked via an MMP-cleavable peptide to an appropriate masking polyanionic domain (a cleavable peptide hairpin) to deliver fluorescent labels to within tumor cells both in vitro and in vivo after cleavage by tumor-associated proteinases (3). The strategy we employed was to use a PAMAM dendrimer backbone, reference fluorophores attached directly to the dendrimer, and sensor fluorophores attached via a selective peptide linkage (4) (Figure 1). The multivalency of the dendrimer allows for adjustment in the relative amounts of sensor and reference fluorophores, provides a vehicle that is maintained in the circulation for greater than 30 minutes, and provides the opportunity to link additional agents that can alter the half-life of the reagent in circulation or provide additional functionalities. The use of two different fluorophores as the FRET pair provides the opportunity to determine the ratio of cleaved product to substrate by determining the sensor: reference ratio, thereby taking issues of substrate penetration into tumors into account.
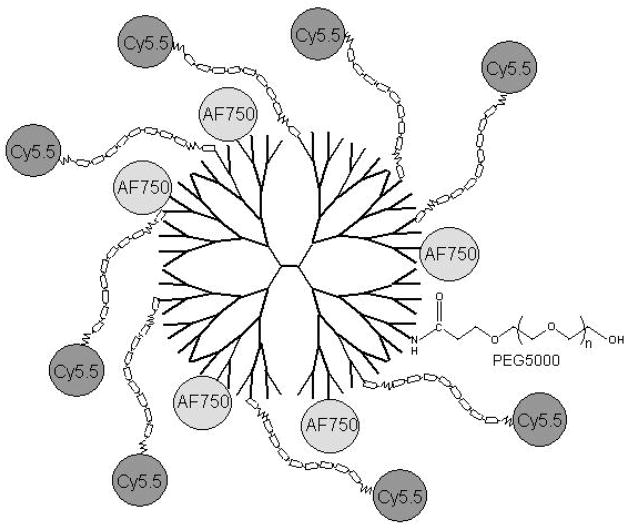
Figure 1.
Diagrammatic structure (not to scale) of PB-MXNIR constructed on a PEGylated-PAMAM generation-4 dendrimer (ethylenediamine core) with AlexafluorR750 (AF750, internal reference) linked to surface amines (not shown) and cyanine5.5 (Cy5.5)-labeled peptide proteinase sensors, Cy5.5(Ahx)XXXX*XXXX(Ahx)C-, where the octapeptide (X) linker is designed to be cleaved by various MMPs (see Table 1).
The methodology presented is for a well-characterized reagent designed for NIR imaging with Alexafluor750 (AF750, excitation 750 nm, emission 775 nm) as the reference fluorophore, and Cyanine5.5 (Cy5.5, excitation 675 nm, emission 694 nm) as the sensor fluorophore attached to a MMP7-selective peptide (5). However, this method is adaptable for peptides that have different selectivities, and thus monitor different enzymes or general proteolytic activity depending on the choice of peptide. The use of NIR fluors minimizes interference from hemoglobin and water to give enhanced visualization in biological tissues. The proteolytic beacon technology was originally developed using fluorophores in the visible region of the spectrum (450–600nm), which are particularly suited for studies at the tissue and cellular level requiring fluorescence microscopy. Table 1 gives the sequence of several peptides and FRET pairs that have been successfully employed in these probes.
Table 1.
2. Materials
2.1. Synthesis of PBs
All chemicals and biochemicals are reagent-grade and solutions are prepared in deionized filtered water (Milli-Q, Millipore Corp., www.millipore.com, Billerica, MA, USA) unless noted otherwise.
Generation 4 Starburst® PAMAM ethylenediamine core dendrimer is obtained from Sigma-Aldridge (St. Louis, MO, USA) as a 10% (w/w) solution in MeOH, equivalent to 79 mg PAMAM/ml or ~5.6 mM based on a calculated FWt of 14,215.
N-succinimidyl iodoacetate (SIA) (M. Wt: 283) (Pierce Chemical, piercenet.com, Rockford, IL, USA) is dissolved to 20mg/ml (~70 mM) in dimethylformamide (DMF).
[Ahx]-(octapeptide)-[Ahx]-C (MX), e.g., [Ahx]-RPLA*LWRS-[Ahx]-C (M7), and [Ahx]-AVRW*LLTA-[Ahx]-C (M9) where Ahx is aminohexanoic acid, are HPLC-purified peptides that include two Ahx linkers, either from Open Biosystems (www.openbiosystems.com, Huntsville, AL, USA) or from GenScript Corp. (www.genscript.com, Piscataway, NJ, USA). Peptides are dissolved at 5 mM (6–7 mg/ml) in MeOH.
The N-hydroxysuccinimidyl (NHS) ester derivatives of the near-infrared (NIR) fluorophores, Alexafluor700 (AF700), Alexafluor750 (AF750) and Cy5.5, obtained either from Molecular Probes, Invitrogen (probes.invitrogen.com, Carlsbad, CA, USA) or from GE Healthcare (www.gehealthcare.com, or GE Healthcare Bio-Sciences Corp, www6.gelifesciences.com, Piscataway, NJ) are prepared as 7 mM solutions in DMF.
0.5 M Na2CO3, pH adjusted to 9.0 (dilute 1:10 to measure) using HCl.
0.2 M cysteine in methanol.
0.2 M ethylenediaminetetraacetic acid (EDTA), pH adjusted to 8.0 with NaOH, and also diluted to 1.0 mM or 0.1 mM for use, as specified.
0.5 M Hepes-NaOH, pH 7.0 (dilute 1:10 to measure) and diluted to 5 mM for use.
Glass ampoules such as 1.8 ml “Wheaton” ABC amber vial™, with Teflon/silicon lined cap (VWR International, vwr.com, Westchester, PA or Thermo Fisher Scientific Rockwood/National Scientific, nationalscientific.com, Rockwood, TN),
2.2. Analysis of PBs
NIR-glycine: add 2 μl of 7 mM NIR-NHS (in DMF) to 0.1 ml of 20 mM glycine in aqueous solution.
NIR-PAMAM: add 3 μl of 7 mM NIR-NHS (in DMF) to 5 μl (28 nMoles) of PAMAM (10 wt%)solution in MeOH, incubate one hour at ambient temperature, dilute with 1.0 ml of 0.1 mM EDTA, and concentrate by diafiltration with a Microcon™ centrifugal filter device with YM-3 membrane (Amicon, Millipore Corp.) per the manufacturers protocols and at ~8 °C.
2.3. Testing PBs in vitro
Phenylmethylsulfonylfluoride (PMSF, FWt 174) is dissolved at 0.2M in 100% ethanol and stored at 4 °C. NOTE: this reagent is highly toxic (see Note 1).
4X-Tricine assay buffer stock: 0.2 M Tricine [N-tris(hydroxymethyl)methylglycine] (>98% from Sigma-Aldrich, St. Louis, MO, USA), 0.8M NaCl, 40 mM CaCl2, 0.2 mM ZnSO4, 0.02% (w/v) Brij35, adjusted to pH 7.4 with NaOH. For convenience, some of the components of this buffer are added from aqueous stock solutions, e.g., 1.0 M CaCl2, 10 mM ZnSO4 and 1% (w/v) Brij35 (diluted from 30% w/v solution obtained from Sigma-Aldrich). This 4X-assay buffer stock solution is usually autoclaved and can be stored at ambient temperature for a number of months. Add 1 mM PMSF to the 4X-Tricine assay buffer before use, e.g., 50 μl of 0.2M PMSF to 10 ml 4X-buffer.
Various active MMPs, abbreviated generically as MMPX, e.g., MMP2, MMP3, MMP7 and MMP9, as obtained from the supplier (usually Calbiochem, San Jose, CA, USA) are stored frozen at −80 °C in appropriate aliquots (e.g., 2 μl or 3 μl) till required for use.
2.4. Quantitative Fluorescence Imaging of PBs
Matrigel™ for preparing phantoms of PB-MXVIS in the in vivo setting is from BD Biosciences (bdbiosciences.com, San Jose, CA).
2.5. In vivo Imaging of Xenograft Tumors
SW480 human colon cancer cells are obtained from the ATCC (www.atcc.org, Manassa, VA, USA).
Preparation of PB-MXNIR for administration via i.v. injection: The stock PB-MXVIS (50–100 nMoles/ml) is prepared for injection by dilution into sterile 0.9% sodium chloride solution to 1.0 nMoles/100 μl kept isotonic by addition of sterile 10X-PBS, as required. For example, for five animals, a total of 700 μl is prepared to provide 500 μl for injection and allowing for dead-volume losses in syringes, i.e., 87.5 μl of an 80 μM PB-MXNIR stock solution is added to 603 μl 0.9% saline pre-mixed with 10 μl of sterile 10X-PBS. Stock PB-MX reagents that show any propensity to precipitate are routinely filtered after dilution using 13 mm 0.2 μm filters (Product no. 4602, Supor Acrodisc, Gelman Sciences).
2.6. Ex vivo Imaging of Intestinal Adenomas
C57Bl/6-Min (Min/+) mice positive for the ApcMin allele and C57Bl/6 normal control mice (Jackson Laboratory, jax.org, Bar Harbor, ME, USA), are placed on a high fat diet 5015 (Harlan Teklad) for fifteen weeks and then on low fluorescent chow (TD-97184, Harlan Teklad) for two weeks to reduce tissue autofluorescence.
3. Methods
3.1. Synthesis of PBs
The PBs on PAMAM dendrimer scaffolds are prepared by three sequential reactions: i) using succinimidyl ester (NHS) chemistry to couple the NIRF-sensor to the amino-terminal of the protease-cleavable peptide (MX) yielding NIRF-MX; ii) linking multiple copies of NIRF-MX to the reactive terminal amines of PAMAM dendrimer; and iii) reaction of the PAMAM dendrimer core with reference fluorophore (see Note 2). For the second reaction, PAMAM is first activated with the bifunctional reagent, succinimidyl-iodoacetate (SIA) that subsequently reacts with the sulfhydryl of cysteine included usually at the C-terminal of the MX peptide.
To link the Cy5.5 sensor fluor to the N-terminal of the M7 peptide, [Ahx]RPLA*LWRS[Ahx]C (FWt: 1328), a 1 mg aliquot (886 nMoles) of the mono-reactive succinimidyl ester of Cy5.5 (Cy5.5-NHS, FWt, 1128) as obtained from the supplier (GE Healthcare Bio-Sciences Corp, www6.gelifesciences.com, Piscataway, NJ) is dissolved in 127 μl DMF (7 nMoles/μl); 122 μl of the Cy5.5-NHS solution (854 nMoles) is added to 170 μl (850 nMoles) of a methanolic solution (5mM) of the M7 peptide (see Note 3). 3 μl of triethylamine, an organic base, (see Note 4) is added to give a final concentration of ~1% (v/v) and the sensor-peptide reaction mixture (295 μl total volume) is incubated at ambient temperature overnight, in the dark, with gentle rocking.
Any residual terminal amines on the peptides in the sensor-peptide reaction mixture are blocked by addition of a 2.5-fold molar excess (with respect to peptide) of NHS-acetate (FWt: 259), prepared as a 20 mg/ml (77 mM) solution in DMSO, i.e., 28 μl of 77 mM NHS-acetate. After reaction for 1 h at ambient temperature, any residual NHS in the reaction mixture is reacted with an excess (2-fold with respect to NHS-acetate) of glycine (FWt: 75), prepared as a 2 M aqueous solution, i.e., 2 μl of 2M glycine (see Note 5). The product of this series of reactions is 850 nMoles peptide labeled with Cy5.5, i.e., Cy5.5-M7 or NIR-MX, in a total volume of 322 μl of DMF/MeOH/DMSO.
About 15–30 minutes prior to coupling the Cy5.5-M7 peptide (or other NIR-MX peptide) to PAMAM, 10 μl of a 2 mg/ml (13 mM) solution of dithiothreitol in methanol (0.15 equivalents of dithiothreitol with respect to peptide) is added to the NIR-MX reaction mixture (total volume 332 μl) to promote reduction of the C-terminal cysteine of the M7 peptide (see Note 6).
PAMAM-PEG is prepared by reacting an appropriate aliquot of the PAMAM stock solution (in methanol) with an equimolar equivalent of NHS-PEG5000 (dissolved at 22 mM in methanol). For routine synthesis, 0.2 ml of the stock methanolic solution of PAMAM, generation 4, obtained from the manufacturer as a 10 wt% methanolic solution (calculated to be 79 mg/ml, or 5.6 mM based on a theoretical FWt of 14,215), is added to 5.6 mg of PEG5000-NHS dissolved in 50 μl of methanol and incubated for >30 min at ambient temperature. The product, calculated to be 4.5 mM PAMAM-PEG, is used without purification (see Note 7).
To synthesize the thioether-bonded conjugate (Cy5.5-M7)m-PAMAM-PEG5000, the PAMAM-PEG conjugate is first activated by treatment with SIA (8 mg/ml methanol, 20 eq/PAMAM). For routine synthesis, 23 μl (104 nMoles) of PAMAM-PEG solution (4.5 nmoles/ul, prepared as in Step 4), is placed in an amber glass ampoule (e.g., 1.8 ml “Wheaton” ABC amber vial™, with Teflon/silicon lined cap) and allowed to react for 30 min. at ambient temperature with 28 μl SIA (~1.96 μMoles). The SIA/PAMAM ratio (~19) is selected to activate ~30% of the terminal primary amines calculated in PAMAM-G4 (64 surface amines/dendrimer).
325 μl of the NIR-MX solution (~2.6 mM in DMF/MeOH/DMSO) from Step 3 above (see Note 8) is added to the SIA-activated PAMAM-PEG solution (51 μl) to give a peptide/PAMAM ratio of 8 (total volume, 376 μl) (see Note 9). Minimize exposure of FL-MX to light, cover vial with foil, place upright on a rocking platform and gently rock overnight at ambient temperature.
Remove unreacted NIR-MX peptide by diafiltration after dilution with at least 8-volumes of aqueous 1 mM EDTA and ethanol to 10% with size-separation using either Centriprep™ or Microcon™ centrifugal filter devices with YM-3 membranes (Amicon, Millipore Corp.) per the manufacturers protocols and at ~8 °C (see Note 10). The product, (NIR-MX)m-PAMAM, is then concentrated to ~ 0.5 ml after at least two rounds of diafiltration following dilution (>10-fold) with aqueous 1 mM EDTA. Aliquots of the original diluted reaction mixture, the effluent, washes and retentate (product), are saved for analyses. The volume of the product, collected in a microfuge tube, is usually measured by weighing.
For labeling the PAMAM scaffold (NIR-MX)m-PAMAM with the reference NIR fluorophore such as AF750, (NIR-MX)m-PAMAM (~94 nMoles) in aqueous 1 mM EDTA is made 50 mM in Na2CO3 (pH8) by addition of 1/9 volume of 0.5 M Na2CO3 (pH 8.0) and AF750-NHS (80 μl, ~560 nMoles in DMF) is added. After gentle rocking overnight under argon, in the dark at ambient temperature, 28 μl of 0.2 M aqueous glycine (a 10-fold excess with respect to AF750-NHS) is added (see Note 11).
After 2h at ambient temperature with glycine, the reaction mixture is diluted with 8-volumes of 1 mM EDTA and 1-volume of ethanol (to 10%) and the product, (NIR-MX)m-PAMAM-(AF750)n, separated from unincorporated AF750 by diafiltration, as above, followed by at least two washes with aqueous 1 mM EDTA, a wash with dH2O (to reduce EDTA to ~0.1 mM) and a final wash with 0.1 mM EDTA prior to concentration to about 1.0 to 2.0 ml for storage under argon at 4 °C. For longer-term storage, the beacon product, referred to as PB-MXNIR (Figure 1), is adjusted to 20% ethanol (see Note 12). In addition to the product, an aliquot of the diluted reaction mixture and of the effluents from diafiltration are retained for analyses. The AF750 serves as the internal reference and provides partial quenching of the cy5.5 sensor fluorescence.
Incorporating different peptides, such as those listed in Table 1, as the cleavable substrate in the PB, yields beacons with different substrate specificities, identified generically as PB-MXNIR.
3.2. Analysis of PBs
Incorporation of Cy5.5-MX into (NIR-MX)m-PAMAM and AF750 into (Cy5.5-MX)m-PAMAM-(AF750)n is calculated from the amplitude of the absorbance spectra for FL (at 675 nm) and AF750 (at 749 nm), respectively (6). For each reaction step, the absorption spectra of the reaction mixture (after dilution into 1 mM EDTA for diafiltration), effluent, diafiltration washes and final product (usually diluted 100 or 200-fold in 1 mM EDTA) is measured and used to calculate the incorporation of each component (Cy5.5-MX and AF750, each usually >80%) into the PAMAM dendrimer.
The recovery of PAMAM is measured by ninhydrin reaction by the method of Moore and Stein as described in detail elsewhere (McIntyre et al 2004) and is routinely found to be ~90 % in each step giving a final yield of ~80% of the starting material, i.e., ~85 nMoles (NIR-MX)m-PAMAM-(AF750)n.
Fluorescence excitation and emission spectra of both the (Cy5.5-MX)m-PAMAM intermediate and the final (Cy5.5-MX)m-PAMAM-(AF750)n product are recorded after dilution (usually 500-fold) to ~0.2 μM or to an OD <0.1/cm (at both 675 nm and 749 nm) using either dH2O or 5 mM Hepes-NaOH buffer (pH 7.0). While accurate measurement of quantum yield and spectral corrections have not been implemented, the amplitude of the fluorescence spectrum of Cy5.5 in (Cy5.5-M7)8-PAMAM is ~ 60% of that for the same concentration of Cy5.5-glycine or Cy5.5-M7 in aqueous solution due to fluorescence quenching by homotransfer in the (Cy5.5-M7) 8-PAMAM dendrimer. In (Cy5.5-M7)8-PAMAM-(AF750)6 the Cy5.5 amplitude is further attenuated (to ~25%) by Förster resonance energy transfer (FRET) to AF750.
3.3. Testing PBs in vitro
For testing proteolytic cleavage of PB-MXNIR by various proteinases, the reagent is diluted, usually to ~ 0.2 μM, into buffer, dispensed in triplicate into Eppendorf snap-top conical tubes, and fluorescence of both the NIR-sensor and AF750-reference measured after incubation with or without proteinases (see Note 13). Experimental details are as follows.
Prepare a “Master Mix” working solution of PB-MXNIR in Tricine buffer: an aliquot of the PMSF-treated 4X-Tricine assay buffer is diluted with an appropriate volume of PMSF-treated H2O and PB-MXVIS is added to ~0.2 μM. For each one ml of “Master Mix”, mix together 500 μl of PMSF-treated 4X-Tricine buffer plus 2 μl of 0.1 mM PB-MXNIR (final concentration in the range of 0.1 μM in assay) and 498 μl PMSF-treated H2O. The volume of working solution required is dictated by the number of proteinases being tested; for assaying activity with a single proteinase, a minimum volume of 0.0.3 ml “Master Mix” is required, sufficient for six assays (duplicate assays of three conditions, enzyme, enzyme plus either EDTA or inhibitor and no enzyme).
To set up each the assay, 50 μl aliquots of “Master Mix” are distributed in each microfuge tube, PMSF-treated dH2O added to each tube to give a total assay volume of 100 μl (e.g., 47 μl of dH2O for the plus enzyme assays) and 15 μl 0.2M EDTA or appropriate volume of inhibitor (e.g., 10 μl of 0.1 mM aqueous GM6001).
Shortly before use, an aliquot of MMP stock solution is removed from the freezer, thawed and diluted with PMSF-treated d H2O to prepare a working solution, e.g., 2 ng/μl (~0.1 μM) MMP-7, 7 ng/μl (~0.11 μM) MMP-2, 5 ng/μl (~0.12 μM) MMP-3 or 7 ng/μl (0.11 μM) MMP-9. Working solutions of other proteinases, e.g., trypsin at 0.1 μg/μl, are prepared either fresh or by dilution from a stock (e.g., 1 μg/μl) stored in the freezer. Multiple freezing/thawing of stock proteinases solutions is to be avoided. Working proteinase solutions are kept on ice and are usually discarded after use, though the MMP7 working solution can be frozen in 50 μl aliquots for subsequent use without much loss in activity (see Note 14).
Aliquots of working solution proteinases are added to each microfuge tube, as required, e.g., 3–5 μl of MMP-7 (2 ng/μl) and tubes are closed prior to incubation at 37 °C for at least 2 h or overnight.
After dilution of each reaction mixture to 1.0 ml with Tricine buffer, the sensor and reference fluorescence of each reaction mixture are measured in the fluorometer using appropriate excitation, e.g., 675 nm, 702 nm and 749 nm for Cy5.5, AF700 and AF750, respectively. As illustrated in Figure 2, proteolytic cleavage of the substrate is manifest by an increase in the sensor/reference fluorescence ratio of the beacon as compared with the uncleaved control (with EDTA). See Note 15.
With fluorescence spectra recorded over time, the enzymatic activity can be calculated from the rate of increase in sensor (Cy5.5) fluorescence by plotting fluorescence versus time and measuring the initial or maximal slope (delta fluorescence/time, ΔF.min−1); this slope can then be converted to an equivalent rate of cleavage of the substrate from the measured maximal change in fluorescence (ΔFmax, taken to represent complete cleavage of the substrate).
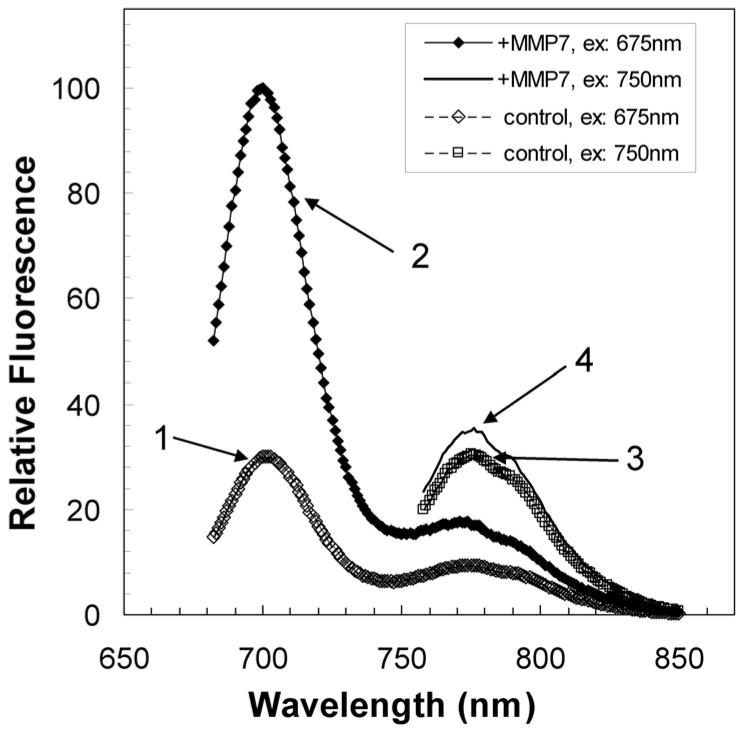
Figure 2.
Fluorescence spectra for PB-M7NIR both before and after treatment with MMP-7 [modified from (5)]. The fluorescence of the Cy5.5 sensor in PB-M7NIR (spectrum 1) increases ~5-fold after treatment with MMP-7 (spectrum 2) whereas the spectrum for the AF750 internal reference of PB-M7NIR (spectrum 3) shows a minimal increase in fluorescence post MMP-7 treatment (spectrum 4).
3.4. Quantitative Fluorescence Imaging of PBs
Quantitative fluorescence imaging is achieved by digital imaging with either a full-frame, black and white CCD camera (MicroMax 1317-K/1, Princeton Instruments, www.piacton.com, Trenton, NJ, U.S.A.) coupled to a fluorescence microscope or with a whole animal fluorescence imaging system such as the IVIS 200 (Xenogen Corp., www.xenogeny.com, Hopkinton, MA, U.S.A.). Each of these imaging systems have appropriate sensitivity for quantitative detection of fluors at nanomolar concentrations with a dynamic range of more than two orders of magnitude suitable for quantitative fluorescence imaging. Before in vivo quantitative fluorescence imaging studies can be initiated, the imaging system is calibrated both in vitro and in vivo with appropriate fluorescence standards and phantoms, as described below for the microscope and IVIS imaging systems.
3.4.1. Microscope System
Quantitative fluorescence imaging is achieved by taking digital pictures with a full-frame, black and white CCD camera (MicroMax 1317-K/1, Princeton Instruments) coupled to a fluorescence microscope with a variety of Plan-Neofluar objective lenses (Axiophot, Carl Zeiss, Inc) including a 2.5X objective and filter cubes matched to the NIR sensor and reference, e.g., Cy5.5 and AF750 (see section 3.4.2.2 below and Note 16). MetaMorph imaging software (Universal Imaging Corp., Downington, PA) is used to control the CCD camera and image acquisition as well as for data analyses. Data acquisition parameters and camera exposure conditions are adjusted to give fluorescence signal linear with exposure time and with largest dynamic range.
3.4.2. Calibration of Microscope Imaging
The fluorescence imaging system is first calibrated using a set of beacon samples prepared with the same two-color PB-MXNIR, e.g., PB-M7NIR, (Cy5.5-M7)m-PAMAM-(AF750)n, as used in vivo as well as controls prepared either with PB-MXNIR previously treated with MMPX or with each of the individual fluors, e.g., (Cy5.5-MX)m-PAMAM and PAMAM-(AF750)n. For the microscope imaging system, the reference samples are each prepared as serial dilutions, e.g., 1 μM to 10 nM and then loaded in capillary glass tubes (0.75 mm borosilicate glass, World Precision Instruments, Sarasota, FL) sealed with hematocrit putty (Critoseal®, Krackeler Scientific Inc., Albany, NY). After initial screening, capillaries with at least two of the concentrations of each of the compounds are mounted together in parallel on the microscope stage for calibration of the fluorescence of the two colors and assessing discrimination between the optical channels. In addition, the dynamic range of the fluorescence microscope for imaging PB-MXNIR cleavage by MMPX can be calibrated with a set of PB-MXNIR samples consisting of mixtures of uncleaved and MMPX - cleaved reagent, prepared at 0.2 nM in tricine buffer plus 20 mM EDTA, and loaded into capillaries.
The calibration samples are oriented and focused under low intensity white light prior to fluorescence excitation. Light is collected through one of two low magnification objectives, depending on whether the measurement was for a calibration sample (usually 10X/0.50 Plan-Neofluar) or for in vivo imaging of subcutaneous phantoms or tumors in mice (usually 2.5X/0.075 Plan-Neofluar). For each calibration sample of PB-MXNIR as well as control capillaries, triplicate images are acquired with at least two exposure times (usually 1, 3, 10 or 30 seconds) (see Note 17). The sensor (Cy5.5) fluorescence (Cy5.5 channel, 650nm with 45 nm bandpass excitation filter, HQ650/45) was discriminated using a Cy5.5 band-pass filter set (#41023, Chroma Technology Corp. Brattleboro, VT except with an HQ700/30 emission filter and Q680LP beamsplitter) and the reference (AF750) fluorescence (Cy7 channel, 740 nm with 35 nm bandpass excitation filter, HQ740/35) was discriminated using a NIR bandpass filter set (#SP-106, Chroma Technology Corp., including an HQ795/50 emission filter and Q765LP beamsplitter). The Cy5.5 channel selectively detects Cy5.5 fluorescence from PB-MXNIR with only background signal from PAMAM-(AF750)n, though only part of the total Cy5.5 emission band can be acquired. Thus, the microscope imaging system gives different Cy5.5/AF750 ratios compared with those calculated from corrected spectral amplitudes measured in a fluorometer due in part to bandpass filtering that is different in the two channels as well as possible differences in optical efficiency of the microscope and/or detector sensitivity. These factors limit the dynamic range compared to the fluorometer experiment, but it allows effective signal discrimination in the presence of both dyes. The difference in sensitivity of the two channels can be, in part, compensated by using different exposure times in the two channels.
The fluorescence intensity for each of the standards and in both Cy5.5 and Cy7 channels is calculated as the average counts/pixel after subtraction of background signals from control capillaries containing only Tricine assay buffer plus 20 mM EDTA. Measurements from triplicate images of each sample are expressed as the mean (± S.E.M.). Provided signal remains below saturation, the response should be linear with exposure time and with the concentration of both Cy5.5 and AF750 (see Note 17).
3.4.3. Whole animal imaging systems
A number of commercial optical imaging systems have been developed for fluorescence imaging of small animals including steady-state fluorescence and bioluminescence imaging systems, e.g., the IVIS200 (Xenogen, www.xenogeny.com, now Califer Life Sciences, www.caliperls.com, Hopkinton, MA), the Maestro spectral imaging system (CRI Inc., www.cri-inc.com, Woburn, MA, U.S.A.), the Kodak FX Pro in vivo imaging system (Carestream Molecular Imaging, www.Kodak.com, New Haven CT, U.S.A.) and the OV100 Olympus (Leeds Precision Instruments, www.leedsmicro.com, Minneapolis, MN, U.S.A.) and time-resolved fluorescence lifetime imaging systems such as the GE Healthcare eXplore Optix (GE Healthcare, www6.gehealthcare.com). The methods described below for calibrating the IVIS200 optical imaging system should, with appropriate modification depending on instrument specifications, be useful for calibrating various whole-animal imaging systems. The IVIS200 is equipped with a cryogenically cooled CCD camera that was designed primarily for the sensitive quantitative detection of bioluminescence but that has been adapted for fluorescence imaging. The intrinsic sensitivity of the camera facilitates quantification of fluorescence images collected with either relatively narrow band-pass emission filters or at low fluor concentrations in vivo. For PB-MXNIR imaging in the IVIS200, the sensor (Cy5.5) and reference (AF750) fluorescence signals are imaged with the Cy5.5 (excitation, 615–665 nm; emission, 695–770 nm) and ICG (excitation, 710–760 nm; emission, 810–875 nm) channels, respectively, though custom filters could also be installed. With the standard Cy5.5 and ICG filters, fluorescence images are usually obtained with exposure times in the range from 1 to 10 sec. Longer exposure times, e.g., up to 30 sec, may be required for low concentrations of fluors.
3.4.4. IVIS200 Calibration
The response of the IVIS200 to the FL and TMR fluors used in PB-MXNIR is calibrated using the same strategy as for the microscope imaging system. Calibration samples, prepared as serial dilutions from both the two-color PB-MXNIR, e.g., (Cy5.5-MX)m-PAMAM-(AF750)n, reagent (with and without MMPX treatment) and from reference compounds containing only one of each of the individual fluors, i.e., (Cy5.5-MX)m-PAMAM and PAMAM-(AF750)n, are pipetted (100 μl aliquots) as an array in a black 96-well plate (#7605 Microfluor, www.thermo.com, Thermolabsystems, Franklin, MA). Fluorescence images in both sensor and reference channels are recorded with varying exposure times and for different fields of view (C, & D). For quantification using the LivingImage® software provided with the IVIS, regions of interest (ROIs) are usually placed in the center of each well so as to minimize edge-of-well imaging artifacts and fluorescence, measured as photons.sec−1.cm−2.steradian−1, in each channel plotted versus concentration of reference beacon. The fluorescence of the individual color compounds, (Cy5.5-MX)m-PAMAM and PAMAM-(AF750)n, as measured in both sensor and reference channels is used to determine the efficiency of color discrimination of the two channels. With Cy5.5 (or AF680) and AF750 in the standard Cy5.5 and ICG channels of the IVIS, there is ~10% overlap of AF750 in the Cy5.5 channel and <5% Cy5.5 signal detected in the ICG channel. While the fluorescence of adjacent duplicate wells is acceptably reproducible, there is some degree of curvature in the fluorescence field, i.e., the measured signal is dependent on position within the field-of-view. For calibrating the closest field-of-view available for fluorescence (field B), an alternative sample geometry is required as the illuminating light source casts shadows within many of the wells of the 96-well plate format.
3.4 5. Calibration for in vivo imaging
For calibration of the imaging system response to PB-MXVIS in the in vivo setting, both the uncleaved and MMPX-treated reagent are prepared as phantoms in Matrigel® (from BD Biosciences, bdbiosciences.com, San Jose, CA) injected subcutaneously in anesthetized athymic nude mice (Harlan, Harlan.com, Indianapolis, IN, U.S.A.) (see Note 18). For protease treatment, PB-MXNIR stock solution is diluted to ~5 μM in Tricine buffer (see above) and incubated overnight at 37 C with MMP7 (0.5 ng/ul). Aliquots of both uncleaved and MMPX-cleaved PB-MXNIR, prepared separately or as mixtures, e.g., 0% 25%, 50%, 75% and 100% cleaved, are then cooled on ice and diluted with nine volumes of Matrigel®, also stored on ice. For injection of duplicate 100 μl phantoms, usually 250 μl samples are prepared, drawn up into a previously chilled sterile syringe (e.g., a tuberculin syringe, 1.0 ml, 26G, 3/8″, No. 309625, Becton-Dickinson) and immediately injected subcutaneously at points along the dorsal flank of the anesthetized mouse, injecting slowly to allow the Matrigel® to gel and form a localized phantom of 3–5 mm diameter containing PB-MXNIR (see Note 19).
The in vivo phantoms can be imaged with either the microscope system, equipped with wide-field objective or with a whole animal imaging system such as the IVIS 200. For microscope imaging, the anesthetized animal is placed on the stage and each of the phantoms is imaged first with white light, and then with fluorescence in both the Cy5.5 and ICG channels. For the white-light images on the microscope stage, the surface of the skin is illuminated using a fiber-optic illuminator, e.g., Fiber-Lite High Intensity Illuminator, Series 180 (Dolan-Jenner Industries, Inc., http://www.dolan-jenner.com/, Boxborough, MA, U.S.A.). A set of three images is obtained for each field-of-view and three or more image sets are collected for each phantom, usually with at least two exposure times for each of the fluorescent images (Cy5.5 and AF750) (see Note 20). Details of data acquisition and analysis with the IVIS are outlined under tumor imaging (below).
Data analysis of microscope images is carried out using MetaMorph imaging software (Universal Imaging Corp., moleculardevices.com, Downington, PA). For each field-of-view, the white light and fluorescence images (Cy5.5 and AF750 and with different exposure times) are combined into an image “stack” for analysis as a group. Usually selected regions of interest are drawn over specific areas of the image, e.g., referable to phantom and reference or to segment the phantom in parts. For each fluorescent image, a threshold is set to exclude all saturated pixels from the analysis (including spurious pixels that are essentially unresponsive, being always saturated) and the average intensity in each ROI for each of the fluorescent images calculated. The Cy5.5 and AF750 fluorescence is determined after appropriate background subtraction and the Cy5.5/AF750 (sensor/reference) ratio than calculated for each ROI in each image set and for each of the phantoms. The fluorescence data is analyzed only for exposure times that give ROIs with fluorescence below saturation.
3.5. In vivo Imaging of Xenograft Tumors
For imaging tumor-associated proteinases activity, subcutaneous xenograft tumors that express MMP-7 (SW480mat) are established on the rear flank of nude mice with control tumors (SW480neo) on the contra-lateral flank (see Note 21). After tumors >0.5 cm diameter develop, the animals are imaged under anesthesia, either with ketamine/xylaxine (145 and 14.5mg/kg, respectively) or with isoflurane inhalation (3.5% induction and 1–2% maintenance). In the IVIS, the imaging bed is heated to maintain body temperature of the anesthetized animals; for imaging on the microscope system, a heat-lamp is used intermittently for this purpose.
3.5.1. Establishing xenograft tumors
SW480neo control and MMP7-expressing SW480mat colon cancer cells (1×106 cells) are implanted subcutaneously on the flanks of athymic nude mice (Harlan, Indianapolis, IN, U.S.A.) (see Note 22).
After 3 to 4 weeks of tumor growth to obtain tumors ~0.5 to 1.0 cm in diameter, mice are anesthetized using 2% isofluorane (3.5% induction, 1.5 to 2% maintenance) and imaged using either the microscope imaging system (as described above) or with a cryogenically cooled CCD camera, IVIS 200 Imaging System (Xenogen Corp., www.xenogen.com, Alameda, CA, USA, now Caliper Life Sciences, www.caliperls.com). For PB-MXNIR imaging, the filters of the IVIS are set to Cy5.5 (excitation, 615–665 nm; emission, 695–770 nm) and ICG (excitation, 710–760 nm; emission, 810–875 nm) channels to image the sensor (Cy5.5 or AF680) and reference (AF750) fluorescence signals, respectively. Background (before beacon) reference fluorescence images are obtained usually with a number of different exposure times, e.g., 1, 3 and 10 sec (see Note 23). A few minutes prior to imaging, mice are routinely given a subcutaneous 0.5 ml bolus of sterile 0.9% saline to protect against dehydration during imaging and an ophthalmic jelly (Paralube vet ointment, Pharmaderm, www.pharmaderm.com, Duluth, GA, U.S.A.) is applied to the eyes (immediately post-injection of beacon) of mice to be imaged longer than 30 min., so as to protect the cornea from dehydration.
3.5.2. Administration of Proteolytic Beacon
PB-MXNIR prepared for injection at 1.0 nMoles/100 μl in sterile 0.9% sodium chloride solution is administered by retro-orbital injection using an insulin syringe (0.3 ml, 28G, ½″, No. 309300, Becton-Dickinson) that has minimal dead-volume losses and gives reproducible i.v. administration of beacon.
3.5.3. Fluorescence Imaging of MMPX Activity In Vivo
Fluorescence imaging is achieved using one or more of the quantitative fluorescence imaging systems described above, i.e., either a fluorescence microscope with a wide-field objective and CCD camera (see Note 24) or a whole animal fluorescence imaging systems such as the IVIS 200 (Xenogen Corp. now Caliper Life Sciences, www.caliperls.com) (see Notes 25 and 26). In either imaging system, animals are first imaged before administration of PB and as soon after injection as is practical so as to provide images to validate the injection dose as measured by fluorescence in the appropriate reference channel, i.e., AF750 in PB-MXNIR.
PB-MXNIR (1.0 nmol in 100ul of sterile 0.9% saline) is injected in the retro-orbital vascular bed and animals are usually imaged as soon as practical after injection and then for either about 30 min or, for assessment of rate of clearance, up to about 60–90min post-injection. Additional image sets of animals are recorded every hour for up to usually 4–5 hrs post injection of PB-MXNIR (see Note 25). A typical image set with the Cy5.5 sensor fluorescence image overlaid on a white light photograph of the animal, as is routinely recorded in the IVIS200 imaging system, is shown in Figure 3A (shown in gray-scale but usually displayed with the fluorescence image in color). Between imaging sessions, animals are allowed to recover from anesthesia, keeping them warm and hydrated at all times (see Note 27).
Image data sets of tumors obtained with the microscope/CCD camera imaging system are analyzed using MetaMorph® software as described above for in vivo phantoms. The IVIS imaging data sets are analyzed using Living Image® software by Xenogen in the Cy5.5 and ICG channels that predominately measure Cy5.5 or AF680 (sensor) and AF750 (reference) fluorescence respectively. Regions of interest are created to measure the average radiance (photons.sec−1.cm−2.steradian−1) both pre and post injection of PB-MXNIR in the tumor bearing regions as well as an appropriate control region or regions, such as the hind leg of the mouse as illustrated in Figure 3A. In addition, a region or regions of the background usually adjacent to one or more animals is also measured over time to monitor any unanticipated changes in instrument response or detection parameters. From each of the two IVIS fluorescence imaging channels, sensor (S) and reference (R) signal is measured either as signal above pre-injection background and/or signal minus background post-injection (see Note 28). For both tumor and controls, the S/R ratio is calculated as a function of time after injection of the PB (see Notes 21, 29 and 30). Results of a typical in vivo study with PB-M7NIR, designed to be cleaved by MMP7, are illustrated in Figure 3 (5).
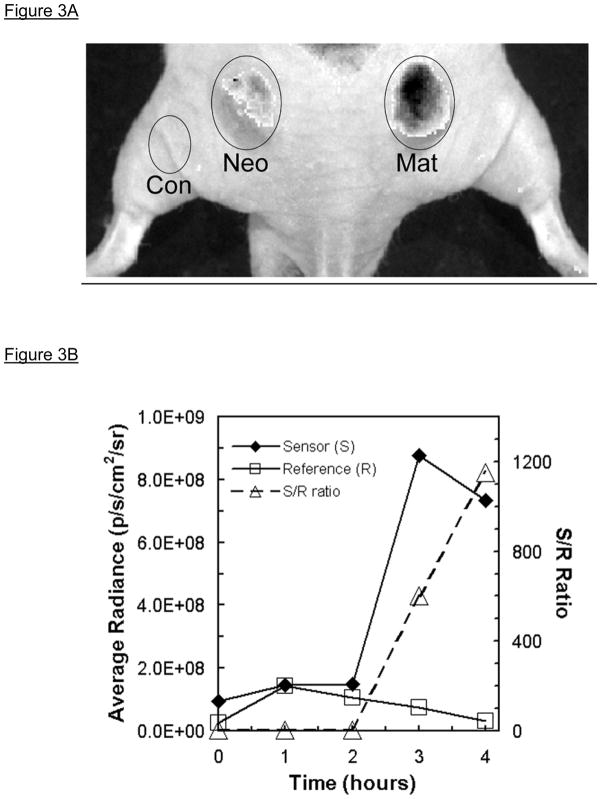
Figure 3.
Quantitative in vivo fluorescence imaging of tumor-associated MMP7 activity in mouse subcutaneous xenograft colon tumors [modified from (5)]. Panel A. Subcutaneous injection into a nude mouse of either SW480neo (Neo) or SW480mat (Mat) colon tumor cells resulted in xenograft tumors (left and right, resp.) within 3–4weeks; the mouse (dorsal, caudal view) was imaged in the Cy5.5 Sensor (S) channel 4hr post retro-orbital intravenous injection of PB-M7NIR (white light photograph overlaid with fluorescence image shown in gray scale). There is significant sensor signal associated with the SW480mat tumor indicative of cleavage of PB-M7NIR by MMP-7 with minimal signal in the SW480neo control tumor that is similar to that over a non-tumor control (Con) region. Panel B. Sensor (S) and reference (R) signals measured in an SW480mat tumor reveal increasing signal in the sensor channel over time. The S/R ratio increases markedly after 2h as the uncleaved reagent clears the circulatory system.
3.5.4. Microscopic Analysis of Tumors
The distribution of PB-MXVIS and related beacons within tumors can be assessed in frozen sections.
At an appropriate time after i.v. administration of the beacon (usually in the range from one to four hours), mice are sacrificed by carbon dioxide asphyxiation, tumors resected, immersed in OCT embedding compound (Tissue-Tek, www.emsdiasum.com, Hatfield, PA, U.S.A.) and quick-frozen under liquid nitrogen.
Frozen sections (5–10 μm) are prepared using a cryomicrotome and stored at −20°C prior to analysis. For histological analysis, OCT is removed from the samples by immersing the slides for 2–5 min in H2O followed by 70% EtOH. Slides are aqueously mounted in Gel/Mount aqueous mounting medium (Biomeda, www.biomeda.com, Foster City, CA, USA) containing 4′,6-diamidino-2-phenylindole (DAPI, usually about 2 to 5μM) for visualization of cell nuclei.
For quantitative fluorescence imaging, digital images are recorded with a full-frame, CCD camera (MicroMax 1317-K1; Princeton Instruments, Trenton, NJ, U.S.A.) coupled to a fluorescence microscope (see above) equipped with a variety (10X, 20X, and 40X-oil immersion) of Plan-Neofluar objective lenses (Axiophot; Carl Zeiss, www.zeiss.com, Thornwood, NY, U.S.A.). The 40X objective lens is used under oil immersion. Imaging parameters are optimized to obtain fluorescence images linear with camera exposure time and with maximum dynamic range. DAPI images are used to focus and orient the specimen field before fluorescence excitation that is usually acquired in both Cy5.5 sensor and AF750 reference channels (see above) using two or more exposure times.
The images are analyzed using MetaMorph software as described above and, for each ROI, intensity in each channel is calculated as the average counts/pixel after subtraction of background signals from control samples prepared without PB-MXVIS beacon.
3.6. Ex vivo Imaging of Intestinal Adenomas
C57Bl/6-Min (Min/+) mice positive for the ApcMin allele or C57Bl/6 normal control mice are anesthetized using 2% isofluorane and retro-orbitally injected with PB-MXNIR (1.0 nmol in 100ul sterile saline 0.9%).
Approximately 1 hr post injection (see Note 31), animals are sacrificed using CO2 asphyxiation at which point their small intestine (duodenum, jejunum, ileum), cecum, and colon are removed, rinsed with ice-cold 1X phosphate buffer saline (50 ml), and opened longitudinally to reveal adenomas on the luminal surface.
Fluorescence images are obtained in both the Cy5.5 (Cy5.5-sensor) and ICG (AF750-reference) channels using a cryogenically cooled CCD camera (IVIS 200 Imaging System, Xenogen, Alameda, CA, U.S.A.), essentially as described above for the in vivo xenograft tumors except that images are recorded both at intermediate (field C) and highest magnification (Field B).
Regions of each image corresponding to either adenomas or non-tumor (background) are analyzed using Living Image software by Xenogen to measure fluorescence intensities in each region of interest. Signal is quantified as average radiance (photons.sec−1.cm−2.steradian−1) for each of the channels and calculated as signal minus background intensity (see Note 32).
Acknowledgments
This work was supported by RO1s CA68067 and CA84360.
Footnotes
Phenylmethylsulfonylfluoride (PMSF) is a general serine proteinases inhibitor that is HIGHLY TOXIC but is rapidly hydrolyzed in aqueous solution with a reported half-life of ~2h at pH7 (http://en.wikipedia.org/wiki/PMSF).
Most of the synthetic steps are routinely carried out with minimal exposure to light (amber vials and/or foil wrapping) and under argon atmosphere to reduce exposure to oxygen.
In initial studies, reactions were usually set up with the NIRF-NHS dye dissolved in DMSO and with a slightly larger excess of peptide (~20%) to promote complete reaction of the NIRF-NHS with the terminal amine of the peptide.
For reactions with some NIRF-NHS compounds, notably AF750-NHS, the addition of triethylamine in the coupling reactions, particularly when carried out in DMSO rather than DMF, appears to affect stability of the dyes and is to be avoided.
The small volume of water, serving as the solute for glycine that is added to the sensor-peptide reaction mixture to block residual NHS in the mixture (Step 2), does not appear to markedly reduce the efficiency of subsequent peptide coupling to SIA-activated PAMAM (see reaction step 6, section 3.1.6).
The addition of dithiothreitol to the sensor-peptide solution appears to improve the efficiency of its coupling to SIA-activated PAMAM or PAMAM-PEG (dendrimer) at least with some peptide preparations. The addition of an excess of dithiothreitol is to be avoided as this reagent would react rapidly with SIA-activated dendrimer and inhibit coupling of the peptide to PAMAM.
The addition of PEG to the PAMAM improves the solubility of PB-M7NIR, a modification that was not required in the original PB-M7VIS. Note that the reaction of NHS-PEG5000 with PAMAM generates a heterogeneous product with an average of 1 PEG/PAMAM. Although the PAMAM-PEG solution in methanol precipitates upon cooling to ice temperature, it can be stored for several days in the refrigerator provided it is re-dissolved by warming to ambient temperature before use.
The NIR-MX peptide solution is usually used immediately for reaction with the dendrimer core, retaining a small sample, e.g., 5–10 μl, for measuring absorbance and fluorescence spectra of the NIR-MX solution.
Efficient coupling of ~8 peptides/PAMAM has also been obtained following addition of 10 equivalents of Cy5.5-MX peptide to SIA-activated PAMAM.
Centrifugal filter devices with YM-10 membranes may also be used.
The reaction conditions for the final step in the synthesis, labeling with the reference fluor such as AF750 have been modified from that originally described for the PB-MXVIS reagents since upon reaction with the dendrimer the AF750 dye appears to be unstable under alkaline or reducing conditions as revealed by changes in the absorption spectra and absence of appropriate NIR fluorescence.
In the original procedure (4), both the intermediate and final products are diafiltered with 0.1 M NaCl, 5 mM Hepes-NaOH (pH 7.0), 1 mM EDTA. While beacons, stored with or without 20% ethanol, are stable for several weeks at 4 °C, reagents were also stable without saline, i.e., in 1 mM EDTA. A fraction of the reagent may precipitate over time, a process usually reversed by simple vortex mixing.
With PB-MXVIS, a number of proteinases can be tested simultaneously using a 96-well plate reader measuring the visible fluors (fluorescein sensor and tetramethylrhodamine reference). With the PB-MXNIR reagents, a similar multi-well assay could be implemented using a micro-plate fluorometer fitted with appropriate NIR filters to give simultaneous kinetic data with various substrates and different enzymes. Otherwise, fluorescence spectra can be compared before and after treatment with the different proteinases using the sensor/reference ratio to compare the extent of cleavage.
When testing cleavage of a newly synthesized beacon with a variety of proteinases, it is recommended to confirm that each of the proteinases are active by setting up a number of assays with both dye-quenched(DQ)-collagen and/or DQ-gelatin, usually with these substrates at 10 μg/ml (7). Such assays are most easily accomplished using a multi-well plate-reading fluorometer with temperature-regulated stage set to 37°C, though most proteinases are also active at ambient temperature.
While the kinetics for proteinases cleavage of the NIR PBs could in principle be measured using a series of assays in a standard fluorometer, the use of a multiwell plate reading fluorometer equipped with NIR filters would be more suitable. In multi-well plate assays, collecting sets of fluorescence intensities at various points over time provides better signal-to-noise than kinetic traces of each well, where sample bleaching can occur to some extent due to prolonged exposure to the excitation light. In the absence of such multiwell fluorometer, specificity can be qualitatively assessed by comparing the relative Sensor/Reference fluorescence ratio in spectra recorded at a suitable time, e.g., 1 h and/or 4 h, after initiating cleavage of PB-MXNIR by addition of various proteases to individual reaction mixtures.
Fluorescence excitation/emission dichroic filter sets for Cy5.5 (HQ650/45 excitation filter, HQ700/30 emission filter and Q680LP beamsplitter, Chroma Technology Corp. Brattleboro, VT) and Cy7 (HQ740/35 excitation filter, HQ795/50 emission filter and Q765LP beamsplitter), provide adequate discrimination between either Cy5.5 or AF680 as sensor and AF750 as reference giving <10% overlap of sensor in the reference channel and vice versa.
For in vitro and in vivo calibration, prolonged exposure of calibration samples, particularly those prepared in capillaries, to the excitation light may result in partial bleaching of the fluorescence. It is recommended that, after initial adjustments have been made, in vitro calibration be performed using fresh samples in capillaries.
All animal experiments are carried out in accord with and after approval of the procedures and protocol by the Institutional Animal Care and Use Committeee (IACUC).
For initial in vivo calibration, two animals are recommended, each prepared with a maximum of eight phantoms: one mouse with phantoms prepared from four different reagents, uncleaved and cleaved PB-MXVIS (each in duplicate), and the single-color (FL-MX)m-PAMAM and PAMAM-(TMR)n compounds, plus a control phantom without beacon; the second mouse is prepared with phantoms consisting of mixtures of uncleaved and cleaved PB-MXVIS.
The in vivo calibration with subcutaneous PB-MXVIS in Matrigel should be carried out by imaging the animals (see below) soon after implantation of the phantoms since the signal decreases over time (several hours). However, photobleaching of in vivo phantoms is generally negligible.
To validate in vivo targeting specificity, animal models should be designed to include a negative control such as tumor cells that have been genetically manipulated to either over-express or knock-down the target of interest. A valuable approach to demonstrate the in vivo targeting specificity is to make use of animals made null for the target of interest so that response can be compared in positive versus null animals (preferably littermates).
For subcutaneous xenograft tumors, it is important to avoid placement over the kidneys or in their immediate vicinity, as a major portion of PB-MXVIS is cleared by excretion and the kidneys retain significant fluorescence for an extended period (1–2 days) after injection of PB-MXVIS.
To reduce background autofluorescence, particularly in the Cy5.5 channel corresponding to the sensor, mice are placed on a low fluorescent diet (TD.97184, Harlan Teklad, www.teklad.com, Madison WI) about one week prior to imaging studies (5). The TD.97184 Harlan Teklad purified diet is a modification of the AIN-93G purified diet with increased vitamin levels and is suitable for sterilization by irradiation. This kind of diet has been reported by others to decrease autofluorescence in the intestinal region (8).
In the microscope system, due to the relatively small field-of view, several imaging sets must be recorded for each mouse, i.e., SW480neo and SW480mat tumors as well as a control region (usually adjacent to the spine, between the xenografts), and at multiple time points, usually limiting data acquisition to two or three animals that can be imaged alternately over a period of a few hours.
Although the IVIS is reasonably adjusted to “flat-field” for bioluminescence images, fluorescence images are somewhat dependent on location within the field and it is important when recording sequential images over time that each of the animals be positioned reproducibly within the field-of-view.
The IVIS200 whole animal imaging system is equipped with isoflurane inhalation anesthesia for simultaneous imaging of up to five mice (field of view D). However, imaging smaller numbers of animals (three or two) using one of the smaller field-of-view settings provides increased sensitivity.
For most measurements recorded over time, treatment of the skin with glycerol to enhance the detection of fluorescence from the subcutaneous xenograft tumors was not implemented so to avoid introduction of an uncontrolled variable, i.e., dehydration/rehydration of the skin over the course of the study.
Mice can be maintained on a low fluorescence diet (TD-97184, Harlan Teklad, Madison, WI, U.S.A.) for three or more days prior to imaging to reduce background fluorescence (5).
Importance of timepoint for S/R analysis: Results from studies conducted with PBs in various tumor models, e.g., SW480 colon tumor xenografts (4), Min mouse adenomas (5) and LLC lung tumors (9), have revealed the importance of carrying out exploratory studies to measure the S/R ratios as a function of time after administration of the PB. Although the half-time for whole-body clearance of the PBs studied thus far are similar (on the order of 60–90 min), maximal S/R ratios in tumors have been found at different times after injection. With the prototype PB-M7VIS, optimal S/R ratio was at ~2 h post-injection, whereas with the second generation PB-M7NIR studied in the same SW480 tumor model system, the maximal S/R ratio was at ~4 h post-injection, a temporal difference that may in part be attributed to enhanced signal-to-noise and/or signal-to-background ratios for the NIR versus VIS versions of the PB. However, for imaging PB-M7NIR in the intestinal adenomas of the Min mouse, optimal S/R ratios were found at ~1 h post-administration of the PB, i.e., much sooner than in the SW480 xenograft model, a result that may be due, at least in part, to a faster wash-out of cleaved sensor from the adenomas as compared with the xenografts.
Controls for specificity: Appropriate controls for studies with these kinds of PBs both in vitro and in vivo include the use of control PBs constructed with a scrambled peptide sequence, an uncleavable peptide of the same sequence except with D-amino acids, or with a peptide designed to target a different proteinase (see Table 1). Some validation of targeting specificity can be obtained by judicious use of pharmacological inhibitors such as the MMP inhibitors (MMPI). However, it should be noted that while the attenuation of response to a PB afforded by treatment with a pharmacological agent such as an MMPI is indicative of biological efficacy of the drug, the attenuation of PB signal may be a result of an indirect downstream response to the agent rather than direct inhibition of the proteinase target.
For the ex vivo imaging of intestinal adenomas in C57Bl/6-Min (Min/+) mice, the optimal time for sacrificing the animals after i.v. injection of the PB-MXNIR was established in a pilot study, sacrificing the animals at 30 min, 1 h, and 2 h after administration of the beacon.
The reference signal amplitude (R) is generally comparable throughout each region of the intestinal tract with a slightly higher R signal over the adenomas. With an MMP7-specific beacon, PB-M7NIR, the S/R ratio is significantly increased over the adenomas while background S/R ratio is comparable to that observed in control intestines lacking adenomas (5).
References
- 1.McIntyre JO, Matrisian LM. Molecular imaging of proteolytic activity in cancer. Journal of Cellular Biochemistry. 2003;90:1087–1097. doi: 10.1002/jcb.10713. [DOI] [PubMed] [Google Scholar]
- 2.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nature Medicine. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 3.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, et al. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochemical Journal. 2004;377:617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherer RLVMN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Molecular Imaging. 2008 [PMC free article] [PubMed] [Google Scholar]
- 6.Berlier JE, Rothe A, Buller G, Bradford J, Gray DR, Filanoski BJ, et al. Quantitative comparison of long-wavelength Alexa Fluor dyes to Cy dyes: fluorescence of the dyes and their bioconjugates. J Histochem Cytochem. 2003;51:1699–1712. doi: 10.1177/002215540305101214. [DOI] [PubMed] [Google Scholar]
- 7.Menon R, McIntyre JO, Matrisian LM, Fortunato SJ. Salivary proteinase activity: a potential biomarker for preterm premature rupture of the membranes. Am J Obstet Gynecol. 2006;194:1609–1615. doi: 10.1016/j.ajog.2006.02.052. discussion 1615. [DOI] [PubMed] [Google Scholar]
- 8.Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 2004;3:9–23. doi: 10.1162/15353500200403196. [DOI] [PubMed] [Google Scholar]
- 9.Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment 1. Cancer Research. 2006;66:259–266. doi: 10.1158/0008-5472.CAN-05-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch AR, Holman CM, Browner MF, Gehring MR, Kan CC, Vanwart HE. Purification of human matrilysin produced in escherichia coli and characterization using a new optimized fluorogenic peptide substrate. Archives of Biochemistry and Biophysics. 1995;324:59–64. doi: 10.1006/abbi.1995.9929. [DOI] [PubMed] [Google Scholar]
- 11.Chen EI, Li W, Godzik A, Howard EW, Smith JW. A residue in the S2 subsite controls substrate selectivity of matrix metalloproteinase-2 and matrix metalloproteinas-9. Journal Biological Chemistry. 2003 doi: 10.1074/jbc.M210324200. [DOI] [PubMed] [Google Scholar]
- 12.Kraft PJ, Haynes-Johnson DE, Patel L, Lenhart JA, Zivin RA, Palmer SS. Fluorescence polarization assay and SDS-PAGE confirms matrilysin degrades fibronectin and collagen IV whereas gelatinase A degrades collagen IV but not fibronectin. Connective Tissue Research. 2001;42:149–163. doi: 10.3109/03008200109014256. [DOI] [PubMed] [Google Scholar]