Abstract
Context
Thyroid associated ophthalmopathy (TAO) is a common and debilitating manifestation of Graves disease (GD). Presently little is known about factors that may increase the risk of developing TAO among patients with GD.
Objective
The purpose of this study is to identify risk factors associated with the development of TAO among individuals who are newly-diagnosed with GD.
Design, Setting, Patients
In this longitudinal cohort study, all beneficiaries age ≥ 18 years with newly-diagnosed GD who were continuously enrolled in a large nationwide U.S. managed care network and who had ≥1 visit to an eye care provider from 2001–2009 were identified. International Classification of Diseases (ICD-9-CM) billing codes were used to identify those who developed manifestations of TAO. Multivariable Cox regression was used to determine the hazard of developing TAO among persons who were newly-diagnosed with GD, with adjustment for sociodemographic factors, systemic medical conditions, thyroid stimulating hormone levels, and medical and surgical interventions for management of hyperthyroidism.
Main Outcome Measure
Hazard ratios (HR) with 95% confidence intervals (95% CIs).
Results
Of 8,404 patients with GD who met the inclusion criteria, 740 (8.8%) developed TAO (mean follow-up, 374 days since initial GD diagnosis). After adjustment for potential confounders, surgical thyroidectomy, alone or in combination with medical therapy, was associated with a 74%-decreased hazard for TAO (adjusted HR, 0.26 [95% CI, 0.12–0.51]), compared with radioactive iodine therapy alone. Statin use (for ≥60 days in the past year, vs. <60 days or nonuse) was associated with a 40%-decreased hazard (HR, 0.60 [CI, 0.37–0.93]). No significant association was found for use of non-statin cholesterol-lowering medications or COX-2 inhibitors and development of TAO.
Conclusion and Relevance
If prospective studies can confirm our finding that thyroidectomy and statin exposure are associated with substantially reduced hazards for TAO in patients with GD, deliberate preventive measures for this burdensome manifestation of GD may become a reality.
INTRODUCTION
Graves disease (GD) is among the most common autoimmune disorders in the United States, affecting nearly 1% of females.1 Some reports indicate that up to half of patients with GD develop thyroid-associated ophthalmopathy (TAO), making it the most prevalent extra-thyroidal manifestation.2,3 Debilitating components of TAO include proptosis, diplopia, and exposure keratopathy. In rare cases, vision loss may result from corneal ulceration or compressive optic neuropathy. Currently available treatments, such as corticosteroids and immune modulators, do not prevent the long term consequences of TAO. Identifying modifiable risk factors that predispose GD patients to develop TAO could dramatically alter management of these patients. Thus far, the only recognized modifiable risk factors associated with TAO are smoking, radioactive iodine (RAI) exposure, and dysthyroidemia.3
TAO is associated with inflammatory cell infiltration, accumulation of the glycosaminoglycan hyaluronan, and expansion of extraocular muscles and fat.4 Activating antibodies against the thyroid stimulating hormone (TSH) receptor drive the hyperthyroidism. However, their role in TAO has yet to be established. In fact, the proximate link between systemic, antigen-specific processes in GD and the development of TAO remains unclear.
We examined longitudinal health care claims data from 8,404 individuals with newly-diagnosed GD to identify risk factors for developing TAO. Specifically, the study sought to determine whether choice of management of hyperthyroidism in GD (anti-thyroid medications, RAI, or surgical thyroid ablation) altered the risk of developing TAO. In addition, the impact of elevated serum TSH levels, use of statins (3-hydroxy-3-methylglutaryl [HMG]-CoA reductase inhibitors) and cyclooxygenase-2 (COX-2) inhibitors were assessed. In this analysis, we find that surgical thyroidectomy and statin use significantly reduced the relative risk of developing TAO while elevations in serum TSH and exposure to RAI increased the risk.
METHODS
Data Source
The Clinformatics database (OptumInsight, Eden Prairie, MN) contains detailed de-identified records of all beneficiaries in a large, U.S. managed-care network. Beneficiaries receiving any form of eye care from January 1, 2001, through December 31, 2009 were identified. This subset comprises those with one or more International Classification of Diseases (ICD-9-CM)5 codes for any eye-related diagnosis (360–379.9); Current Procedural Terminology (CPT-4)6 code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499); or any other claim submitted by an ophthalmologist or optometrist during their time in the medical plan. Claims (inpatient, outpatient, skilled nursing facility) for ocular and non-ocular conditions, socio-demographic information (age, sex, race, education, financial wealth), and all records of filled outpatient medication prescriptions were analyzed. All individuals had dual enrollment in the medical and pharmacy plans. This data source has been used previously to study patients with ocular diseases.7,8
Participants and Sample Selection
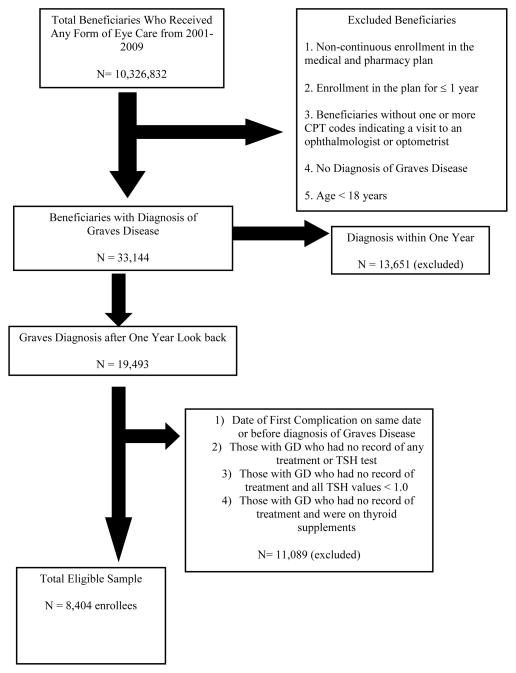
The study cohort included individuals age 18 years or older with newly-diagnosed (incident) GD who were included in the database for at least one continuous year and had visited an eye-care-provider (ophthalmologist or optometrist) at least once during their participation in the plan. Patients with GD were identified using the following ICD-9-CM diagnostic codes (242.00, 242.01). We excluded those in the plan for fewer than 365 days, those who had non-continuous enrollment, and those who had been diagnosed with GD or TAO during their first year in the plan (non-incident cases). Enrollees diagnosed with benign or malignant neoplasms of the orbit and orbital pseudotumor were also excluded. In addition, to help ensure the cohort consisted of individuals with bona fide GD, individuals with a diagnosis of GD but no recorded treatment for GD (RAI, thyroidectomy, medication, etc.) plus no record of TSH testing confirming the diagnosis were excluded. Those with no record of treatment for GD during their time in the plan but with evidence of GD based on laboratory testing were included in the analyses. After these exclusions, 8,404 patients with newly-diagnosed GD were included in the study. (Figure 1)
Figure 1.
Selection of Beneficiaries for Analysis
*Incident = the diagnosis or prescription was not present during the beneficiary’s first year of enrollment in the managed care plan. GD= Graves Disease; TSH = thyroid stimulating hormone
Identification of Individuals who Developed TAO
Diagnosis of TAO was based upon billing codes for Graves eye disease, eyelid retraction, restrictive strabismus, proptosis, exposure keratopathy, or compressive optic neuropathy. (eTable 1)
Exposure to Medications
Individuals who filled one or more prescriptions for statins, COX-2 inhibitors, or anti-thyroid medications were identified and medication use was quantified. (eTable 1) Information regarding use of non-prescription medications was not available.
Analyses
Statistical analyses were performed using SAS, 9.3 (SAS Institute, Cary, NC) software. Participant characteristics were summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. A Cox proportional hazards model with delayed entry was used to estimate the hazard of developing TAO based on the predictors of interest. The first year of enrollment was used as a “look back” period. Individuals receiving one or more codes for TAO during this period were excluded from the analysis to exclude those with pre-existing TAO. All beneficiaries were followed from the date of first diagnosis of GD until either they developed TAO, or were censored. Censoring occurred when the individual disenrolled from the plan or at the end of study period (December 31, 2009). First, single-variable models were used to test potential predictors individually. Multivariable models were adjusted for age at first GD diagnosis, sex, race, level of education attained, household net worth, and geographic region of residence at the time of enrollment. Other variables included in the model were record of surgical thyroidectomy, RAI, hypertension, hyperlipidemia, connective tissue disease, serum TSH level, Charlson comorbidity index9 (a measure of overall health) and use of statins, other cholesterol-lowering medications, antithyroid medications, and COX-2 inhibitors. In the multivariable model, TSH levels were dichotomized into those with ≤ 5 μIU/mL at all measurements and > 5 μIU/mL at any measurement. A second multivariable model was run using a TSH concentration of 7μIU/mL as the cutoff instead of 5. In the regression model, treatment with RAI alone was the omitted reference for which other interventions were compared against. For all analyses, p-values of < 0.05 were considered statistically significant.
Medication exposure for a given individual frequently changes over time due to adherence and other factors. Assuming its constancy can lead to erroneous conclusions. To address this concern we created time-dependent covariates to quantify medication use. For each day that an individual with GD was at risk of developing TAO in the model, we determined the total number of days in the previous year that the individual was prescribed each medication class. On a particular day, if the enrollee had record of receiving a prescription for the medication of interest for at least 60 days in the past year, they were counted as a user of the medication for that day. We quantified the number of days each enrollee was prescribed each medication from the index date until the date of first diagnosis of TAO or censoring.
The University of Michigan Institutional Review Board approved this study as a non-regulated study.
RESULTS
A total of 8,404 individuals were identified with newly-diagnosed GD with an average length of medical plan enrollment of 2,050 ± 827 days. Among these, 740 enrollees (8.8%) developed TAO during the follow-up (total plan enrollment time for those who developed TAO was 2,066 ± 787 days; p=0.56 compared to all GD patients). The average length of time between first GD diagnosis and first TAO diagnosis was 374 ± 422 days. Those who developed TAO were younger (45.1 ± 11.7 years) than those without ocular manifestations (46.6 ±12.7 years) (p=0.0006). However, no predilection based on sex (p=0.63) or race (p=0.27) was identified. (Table 1)
Table 1.
Characteristics of Individuals with Graves Disease Who Develop and Do Not Develop Thyroid-Associated Ophthalmopathy
| Overall | Persons with Graves Diseases who did not develop TAO | Persons with Graves Diseases who developed TAO | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 8404 | 7664 | 91.2 | 740 | 8.8 | ||
| Sex | 0.63 | ||||||
| Male | 1715 | 20.4 | 1569 | 20.5 | 146 | 19.7 | |
| Female | 6689 | 79.6 | 6095 | 79.5 | 594 | 80.3 | |
| Race | 0.27 | ||||||
| White | 5839 | 79.1 | 5312 | 78.9 | 527 | 81.2 | |
| Black | 524 | 7.1 | 475 | 7.1 | 49 | 7.6 | |
| Latino | 550 | 7.5 | 512 | 7.6 | 38 | 5.9 | |
| Asian American | 384 | 5.2 | 358 | 5.3 | 26 | 4.0 | |
| Other | 88 | 1.2 | 79 | 1.2 | 9 | 1.4 | |
| Household Net Worth | 0.91 | ||||||
| < $25,000 | 889 | 12.0 | 814 | 12.0 | 75 | 11.4 | |
| $25–74,999 | 626 | 8.4 | 576 | 8.5 | 50 | 7.6 | |
| $75–149,999 | 1040 | 14.0 | 947 | 14.0 | 93 | 14.2 | |
| $150– 499,999 | 3147 | 42.3 | 2868 | 42.3 | 279 | 42.5 | |
| ≥ $500,000 | 1737 | 23.4 | 1578 | 23.3 | 159 | 24.2 | |
| Education | 0.08 | ||||||
| < High school | 134 | 1.7 | 125 | 1.7 | 9 | 1.3 | |
| High School Diploma | 2533 | 32.1 | 2334 | 32.5 | 199 | 28.4 | |
| Some college | 3088 | 39.1 | 2787 | 38.8 | 301 | 42.9 | |
| College diploma | 2110 | 26.7 | 1921 | 26.7 | 189 | 26.9 | |
| Professional degree | 25 | 0.3 | 21 | 0.3 | 4 | 0.6 | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 46.5 | 12.6 | 46.6 | 12.7 | 45.1 | 11.7 | 0.0006 |
| Years in plan | 5.6 | 2.3 | 5.6 | 2.3 | 5.7 | 2.2 | 0.56 |
| Charlson Index | 2.4 | 2.9 | 2.4 | 2.9 | 1.9 | 2.4 | <0.0001 |
SD = standard deviation; TAO = thyroid associated ophthalmopathy
Individuals developing TAO were identified by the following codes: proptosis (n=602, 81.4%), Graves eye disease (n=403, 54.5%), restrictive strabismus (n=223, 30.1%), eyelid retraction (n=191, 25.8%), exposure keratopathy (n=77, 10.4%), and compressive optic neuropathy (n=29, 3.9%). Some individuals exhibited more than one of these ocular manifestations at initial diagnosis of TAO.
Treatment for thyroid dysfunction for individuals with GD included anti-thyroid medications alone (n=4,277), RAI alone (n=2,196), anti-thyroid-medications in combination with RAI (n=1,125), surgical thyroidectomy alone or anti-thyroid medications in combination with thyroidectomy (n=779). A total of 1,885 GD patients had no record of treatment for hyperthyroidism during their time in the health plan.
Multivariable Regression Results
Sociodemographic factors
In the multivariable models, we observed no significant association between age, race, geographical region of residence, household net worth, or level of education and the hazard of developing TAO. (p> 0.05 for each comparison) Females with GD demonstrated a 25% reduced hazard of developing TAO relative to males, (adjusted HR= 0.75, [CI: 0.56–1.02]), a finding that was close to but not statistically significant. (p=0.06)
Treatments of Hyperthyroidism
In the multivariable regression model, neither medical therapy with antithyroid medications alone, nor in combination with RAI, appeared to alter the risk for developing TAO compared to those receiving RAI alone. (p>0.05 for both comparisons) Individuals treated with surgical thyroidectomy alone or thyroidectomy with antithyroid medications had a 74% decreased hazard of TAO (adjusted HR = 0.26 [0.12–0.51]) when compared to those receiving RAI alone. Those patients not requiring treatment for hyperthyroidism exhibited a 73% decreased hazard of developing TAO (adjusted HR = 0.27, [0.18–0.39]) relative to those treated with RAI alone. (Table 2)
Table 2.
Hazard of Developing Thyroid-Associated Ophthalmopathy
| Univariable model | Multivariable model 1 | Multivariable model 2 | |
|---|---|---|---|
| RAI only | Ref | Ref | Ref |
| No treatment | 0.24 (0.19–0.31) | 0.27 (0.18–0.39) | 0.28 (0.19–0.40) |
| Thyroidectomy with or without antithyroid meds | 0.34 (0.22–0.50) | 0.26 (0.12–0.51) | 0.27 (0.12–0.52) |
| Antithyroid meds only | 0.98 (0.82–1.17) | 1.14 (0.83–1.58) | 1.16 (0.85–1.62) |
| Antithyroid meds + RAI | 1.20 (0.91–1.57) | 1.48 (0.93–2.31) | 1.48 (0.93–2.31) |
| TSH > 5 μIU/mL | 1.76 (1.38–2.23) | 1.21 (0.91–1.59) | |
| TSH > 7 μIU/mL | 1.99 (1.54–2.55) | 1.31 (0.97–1.76) | |
| Statins (> 60 days) | 0.60 (0.47–0.75) | 0.60 (0.37–0.93) | 0.60 (0.37–0.93) |
| COX-2 inhibitors (>60 days) | 0.70 (0.39–1.13) | 0.79 (0.28–1.73) | 0.79 (0.28–1.73) |
| Other chol meds (>60 days) | 0.50 (0.30–0.76) | 1.00 (0.50–1.82) | 1.01 (0.50–1.83) |
Multivariable models also adjusted for age, sex, race, region of residence, household net worth, education level, Charlson comorbidity score (overall health), hyperlipidemia, hypertension, connective tissue disease
RAI = radioactive iodine; TSH = thyroid stimulating hormone level; chol = cholesterol; meds = medications
Bold indicates statistically significant at the p<0.05 level
Thyroid status
Dysthyroidemia has been associated with increased incidence and worsening of TAO.10 Therefore, we determined the impact of elevated serum TSH levels on risk of TAO. Individuals with at least one serum TSH value > 5μIU/mL were found to have a 21% increased hazard of TAO (adjusted HR = 1.21 [CI: 0.91–1.59]) relative to those with TSH levels ≤ 5 μIU/mL on all tests, though this finding did not reach statistical significance. (p=0.19) In a separate regression model adjusting for the same comorbidities, individuals with TSH >7μIU/mL manifested a 31% increased hazard (adjusted HR = 1.31 [CI: 0.97–1.76]) compared to those with TSH levels ≤ 7μIU/mL at all visits tested, a finding that was close to but not statistically significant (p=0.07). (Table 2)
Statin/Other Cholesterol Lowering Medication Use
Without adjustment for potential confounding factors, individuals with GD who were prescribed statins for at least 60 days in the year of observation had a 40% decreased hazard of developing TAO (unadjusted HR = 0.60 [CI 0.47–0.75]) compared to those not similarly treated, including exposure of fewer than 60 days (p<0.0001). After adjustment for covariates, enrollees with GD and ≥60 days of statin exposure continued to manifest a 40% decreased hazard compared to those with less exposure (adjusted HR = 0.60 [CI: 0.37–0.93]). There was no statistically significant difference in risk of developing TAO in those exposed to non-statin cholesterol lowering medications (adjusted HR = 1.00 [CI: 0.50–1.82]) relative to non-users of these medications. (Table 2)
COX-2 Inhibitor Use
In both the univariable and multivariable models, no significant difference in the hazard of TAO could be detected in individuals prescribed COX-2 inhibitors relative to others not receiving these medications (adjusted HR = 0.79 [CI:0.28–1.73]). (Table 2)
Thyroid Stimulating Immunoglobulin (TSI) and TAO
Among the 8404 subjects with GD, 536 had at least one determination of TSI. Of these, 275 (51%) had undetectable TSI values while 261 (49%) had detectable levels of > 130%. Of those with undetectable TSI levels, 19 (7%) manifested TAO while 18% of the individuals with TSI > 130% developed TAO. (p<0.0001)
COMMENT
The overarching pathogenic factors shared by GD and TAO remain unidentified. Here we followed a large cohort of patients with newly-diagnosed GD to identify risk factors associated with development of TAO. While several risk factors have been identified previously, to our knowledge, this is the first study to assess multiple potentially-modifiable risk factors in a large cohort of patients. Since both the natural history and treatment of GD can be idiosyncratic, we determined the risk factors over a several year period. Our findings indicate that surgical thyroidectomy as the treatment of hyperthyroidism and exposure to statins may lower the risk of developing TAO. Further studies will be required to determine whether a causal relationship can be identified between these factors and TAO. In addition, assessing the impact that modifying these factors might exert on the course of TAO will also require further inquiry.
Strategies for managing GD-associated differ among practitioners. While the therapeutic options currently employed offer potentially effective control of thyroid function, each might impact the risk of developing or worsening TAO differently.11,12 Because RAI ablation can exacerbate TAO, there may be a role for prophylactic low dose oral corticosteroids, which have been found to mitigate any negative impact.13 In the present analysis, we queried the database to see whether the enrollees given RAI had record of prescriptions for corticosteroids in a 15 day window around the timing of the RAI and determined that very few patients (only 2) had been prescribed corticosteroids. Our findings indicate that the risk of developing TAO is substantially reduced if patients undergo surgical thyroidectomy as compared to RAI ablation. Elevated levels of serum TSH and TSIs may negatively impact TAO. Specifically, several groups have found an association between TSI levels and the incidence of TAO.14–16 Our study supports the notion that a greater proportion of patients with TAO exhibit high TSI levels.
Another issue related to the putative role of TSH in TAO concerns the elevations of TSH levels that sometimes occur following surgical or radioactive iodine ablation. This can result from inadequate monitoring. It has been suggested that elevated serum TSH might trigger the activity of TAO, a suspicion that has been strengthened by reports of its development of worsening in patients with hypothyroidism.18,19 In the present analysis, we observed an increased risk of TAO among individuals with elevated TSH levels, a finding that approached statistical significance using a cutoff of 7 μIU/mL.
Another goal of this study was to identify medications that might delay or prevent TAO. Since the majority of patients with GD will not develop clinically significant TAO, any preventative strategy would need to be safe and well-tolerated. This study analyzed several classes of medications with proven or suspected anti-inflammatory activity. Among them, statins represent a widely used class of drugs that reduce low-density lipoprotein cholesterol levels. The JUPITER trial assessed the potential benefit of statins in healthy participants.20 In that study, rosuvastatin reduced systemic markers of inflammation (e.g., C-reactive protein), cholesterol levels, and the number of vascular events.21–23 The study identified anti-inflammatory actions of statins that were independent of their cholesterol-lowering properties. Statins have been proposed as an adjuvant anti-inflammatory therapy.24–35
Of note, our cohort of individuals carrying the diagnosis of GD included 22% for whom no method of treatment for hyperthyroidism could be identified in the database. Some of these patients may have manifested euthyroid or subclinical GD and this may help explain why this group was found to have a reduced risk of TAO relative to the group treated with RAI only. Alternatively, exposure to RAI may increase the risk of TAO.
Our data analysis relied on diagnostic coding and is therefore subject to certain vagaries. Coding for GD is relatively straightforward. However the manifestations of TAO can be subtle and may be overlooked. Thus, the individuals identified with TAO in this database are likely those with more severe disease. While this bias is acknowledged, the goal of the study was to identify modifiable risk factors for developing TAO in patients with GD. The approach taken here appears to have identified such a population. The data analysis was also limited since environmental exposures (e.g. smoking) and over-the-counter medications could not be assessed. Only if there is differential management of GD by smoking status (i.e. smokers are more likely to receive one of these interventions relative to other interventions) could the lack of data on smoking status impact the study findings. We have no reason to believe that smokers with GD are managed differently than non-smokers. Finally, all the enrollees in the study had commercial health insurance. It is unclear whether our study findings are generalizable to persons with other forms of insurance or uninsured persons. Additional analyses using other data sources will be required to tease out the contributions of these factors.
In conclusion, this study identifies several factors that are associated with a reduction in risk of patients with GD developing TAO including surgical thyroidectomy compared to RAI alone and exposure to statins. A prospective study is needed to substantiate these findings and assess whether statins or thyroidectomy may delay or prevent TAO in patients with GD.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS:1K23EY019511); Blue Cross Blue Shield of Michigan Foundation (JDS), EY08976, EY011708, DK063121, EY016339, EY021197, EY007003, Research to Prevent Blindness, and the Bell Charitable Foundation.
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Footnotes
The authors have no proprietary interest in any material discussed in this manuscript
None of the authors have any financial disclosures or conflicts of interest to disclose.
Joshua Stein, MD, MS had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions: Design and conceptualization of the study (JDS, RD, TJS);; Collection, management, analysis, and interpretation of the data (JDS, DC, NT, BN), Manuscript preparation (JDS, SG, BJL); Critical review of manuscript (RD, TJS, DC, NT, BN), Approval of manuscript (JDS, RD, TJS, SG, BJL, NT, DC, BN)
References
- 1.Vanderpump MPJ, Tunbridge WMG. The epidemiology of thyroid disease. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s the thyroid: A fundamental and clinical text. 7. Philadelphia, PA: Lippincott-Raven; 1996. pp. 474–482. [Google Scholar]
- 2.Vangheluwe O, Ducasse A, Vaudrey c, Maes B, Delisle MJ. Prevalence of ophthalmopathy in Basedow disease. Follow-up of patients one year following diagnosis of hyperthyroidism. J Fr Ophtalmol. 1994;17:331–8. [PubMed] [Google Scholar]
- 3.Bartley GB, Fatourechi V, Kadrmas EF, et al. Long-term follow-up of Graves ophthalmopathy in an incidence cohort. Ophthalmology. 1996;103:958–62. doi: 10.1016/s0161-6420(96)30579-4. [DOI] [PubMed] [Google Scholar]
- 4.Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Surv Ophthalmol. 2010;55:215–26. doi: 10.1016/j.survophthal.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Physician International Classification of Diseases (ICD-9CM) 9th Revision, Clinical Modification. 1 and 2. Chicago, IL: American Medical Association Press; 2006. [Google Scholar]
- 6.CPT 2006: Current Procedural Terminology. Chicago, IL: AMA Press; 2006. Professional ed. [Google Scholar]
- 7.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031–7. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011;118:1318–26. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, van der Gaag R. Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med. 1990;150:1098–101. [PubMed] [Google Scholar]
- 11.Traisk F, Tallstedt L, Abraham-Nordling M, et al. Thyroid-associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine-131. J Clin Endocrinol Metab. 2009;94:3700–7. doi: 10.1210/jc.2009-0747. [DOI] [PubMed] [Google Scholar]
- 12.De Bellis A, Conzo G, Cennamo G, et al. Time course of Graves’ ophthalmopathy after total thyroidectomy alone or followed by radioiodine therapy: a 2-year longitudinal study. Endocrine. 2012;41:320–6. doi: 10.1007/s12020-011-9559-x. [DOI] [PubMed] [Google Scholar]
- 13.Lai A, Sassi L, Compri E, et al. Lower dose prednisone prevents radioiodine-associated exacerbation of initially mild or absent graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010;95:1333–7. doi: 10.1210/jc.2009-2130. [DOI] [PubMed] [Google Scholar]
- 14.Dragan LR, Seiff SR, Lee DC. Longitudinal correlation of thyroid-stimulating immunoglobulin with clinical activity of disease in thyroid-associated orbitopathy. Ophthal Plast Reconstr Surg. 2006;22:13–9. doi: 10.1097/01.iop.0000192649.23508.f7. [DOI] [PubMed] [Google Scholar]
- 15.Acuna OM, Athannassaki I, Paysse EA. Association between thyroid-stimulating immunoglobulin levels and ocular findings in pediatric patients with Graves disease. Trans Am Ophthalmol Soc. 2007;105:146–50. [PMC free article] [PubMed] [Google Scholar]
- 16.Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ. Clinical relevance of thyroid-stimulating immunoglobulins in graves’ ophthalmopathy. Ophthalmology. 2011;118:2279–85. doi: 10.1016/j.ophtha.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–31. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 18.Tallstedt L, Lundell G, Blomgren H, Bring J. Does early administration of thyroxine reduce the development of Graves’ ophthalmopathy after radioiodine treatment? Eur J Endocrinol. 1994;130:494–7. doi: 10.1530/eje.0.1300494. [DOI] [PubMed] [Google Scholar]
- 19.Kung AW, Yau CC, Cheng A. The incidence of ophthalmopathy after radioiodine therapy for Graves’ disease: prognostic factors and the role of methimazole. J Clin Endocrinol Metab. 1994;79:542–6. doi: 10.1210/jcem.79.2.7913934. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Group JS. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108:2292–7. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca FA, Izar MC. Primary prevention of vascular events in patients with high levels of C-reactive protein: the JUPITER study. Expert Rev Cardiovasc Ther. 2009;7:1041–56. doi: 10.1586/erc.09.93. [DOI] [PubMed] [Google Scholar]
- 22.Kones R. The Jupiter study, CRP screening, and aggressive statin therapy-implications for the primary prevention of cardiovascular disease. Ther Adv Cardiovasc Dis. 2009;3:309–15. doi: 10.1177/1753944709337056. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Lavie CJ, Milani RV. C-Reactive Protein: How Has JUPITER Impacted Clinical Practice? Ochsner J. 2009;9:204–10. [PMC free article] [PubMed] [Google Scholar]
- 24.Chang JW, Yang WS, Min WK, Lee SK, Park JS, Kim SB. Effects of simvastatin on high-sensitivity C-reactive protein and serum albumin in hemodialysis patients. Am J Kidney Dis. 2002;39:1213–7. doi: 10.1053/ajkd.2002.33393. [DOI] [PubMed] [Google Scholar]
- 25.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–8. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 26.Stuve O, Prod’homme T, Slavin A, et al. Statins and their potential targets in multiple sclerosis therapy. Expert Opin Ther Targets. 2003;7:613–22. doi: 10.1517/14728222.7.5.613. [DOI] [PubMed] [Google Scholar]
- 27.Stuve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol. 2003;16:393–401. doi: 10.1097/01.wco.0000073942.19076.d1. [DOI] [PubMed] [Google Scholar]
- 28.Walsh GM. Statins as emerging treatments for asthma and chronic obstructive pulmonary disease. Expert Rev Respir Med. 2008;2:329–35. doi: 10.1586/17476348.2.3.329. [DOI] [PubMed] [Google Scholar]
- 29.Huang CC, Chan WL, Chen YC, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest. 2011;41:507–12. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 30.Paul F, Waiczies S, Wuerfel J, et al. Oral high-dose atorvastatin treatment in relapsing-remitting multiple sclerosis. PloS one. 2008;3:e1928. doi: 10.1371/journal.pone.0001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vollmer T, Key L, Durkalski V, et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet. 2004;363:1607–8. doi: 10.1016/S0140-6736(04)16205-3. [DOI] [PubMed] [Google Scholar]
- 32.Semb AG, Holme I, Kvien TK, Pedersen TR. Intensive lipid lowering in patients with rheumatoid arthritis and previous myocardial infarction: an explorative analysis from the incremental decrease in endpoints through aggressive lipid lowering (IDEAL) trial. Rheumatology. 2011;50:324–9. doi: 10.1093/rheumatology/keq295. [DOI] [PubMed] [Google Scholar]
- 33.McCarey DW, McInnes IB, Madhok R, et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Tong G, Xu W, et al. Anti-inflammatory effects of simvastatin on adipokines in type 2 diabetic patients with carotid atherosclerosis. Diab Vasc Dis Res. 2009;6:262–8. doi: 10.1177/1479164109339966. [DOI] [PubMed] [Google Scholar]
- 35.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.