Abstract
Objective
An overabundance of bacteria in the chronic wound plays a significant role in the decreased ability for primary closure. One means of decreasing the bioburden in a wound is to operatively debride the wound for wound bed optimization prior to application of other therapy, such as Negative Pressure Wound Therapy (NPWT). We undertook a prospective pilot study to assess the efficacy of wound bed preparation for a standard algorithm (sharp surgical debridement followed by NPWT) versus one employing sharp surgical debridement followed by Negative Pressure Wound Therapy with Instillation (NPWTi).
Methods
Thirteen patients, corresponding to 16 chronic lower leg and foot wounds were taken to the operating room for debridement. The patients were sequentially enrolled in 2 treatment groups: the first receiving treatment with operative debridement followed by 1 week of NPWT with the instillation of quarter strength bleach solution; the other receiving a standard algorithm consisting of operative debridement and 1 week of NPWT. Quantitative cultures were taken pre-operatively after sterile preparation and draping of the wound site (POD # 0, pre-op), post-operatively once debridement was completed (POD # 0, post-op), and on post-operative day 7 after operative debridement (POD # 7, post-op).
Results
After operative debridement (post-operative day 0) there was a mean of 3 (±1) types of bacteria per wound. The mean CFU/gram tissue culture was statistically greater – 3.7 × 106 (±4 × 106) in the NPWTi group, while in the standard group (NPWT) the mean was 1.8 × 106 (±2.36 × 106) CFU/gram tissue culture (p = 0.016); at the end of therapy there was no statistical difference between the two groups (p = 0.44). Wounds treated with NPWTi had a mean of 2.6 × 105 (±3 × 105) CFU/gram of tissue culture while wounds treated with NPWT had a mean of 2.79 × 106 (±3.18 × 106) CFU/gram of tissue culture (p = 0.43). The mean absolute reduction in bacteria for the NPWTi group was 10.6 × 106 bacteria per gram of tissue while there was a mean absolute increase in bacteria for the NPWT group of 28.7 × 106 bacteria per gram of tissue, therefore there was a statistically significant reduction in the absolute bioburden in those wounds treated with NPWTi (p = 0.016).
Conclusion
It has long been realized that NPWT does not make its greatest impact by bioburden reduction. Other work has demonstrated that debridement alone does not reduce wound bioburden by more than 1 Log. Wounds treated with NPWTi (in this case with quarter strength bleach instillation solution) had a statistically significant reduction in bioburden, while wounds treated with NPWT had an increase in bioburden over the 7 days.
Keywords: Chronic lower extremity wounds, Bioburden, Negative pressure wound therapy, Instillation
Introduction
Chronic wounds often harbor a high bacterial burden that negatively impacts tissue healing.1 Bacteria disrupt the wound healing process by elevating the metabolic requirement, stimulating a pro-inflammatory environment, and secreting detrimental cytokines that cause vasoconstriction and decreased blood flow to the wound bed.2,3 Bacteria attract polymorphonuclear neutrophils which in turn release cytotoxic enzymes responsible for degrading existing extracellular matrix, inhibiting cell migration, and preventing wound closure.4 Bacteria may even consume the nutrients and oxygen necessary for tissue repair to occur in the wound.2 To minimize the detrimental effects of a high bacterial burden on chronic wounds, surgical debridement is often necessary. Aggressive debridement of chronic wounds helps manage the devitalized tissue, decrease the wound bioburden, and may convert the wound back into the “normal” wound healing cycle of an acute wound.3
One method commonly employed to decrease wound bioburden is operative debridement followed by 1 week of negative pressure wound therapy (NPWT) for further wound bed optimization.5 The known beneficial effects of NPWT on a wound bed are the increased local blood flow, rate of granulation tissue formation, and nutrient blood flow.2,3,6–10 NPWT has also been shown to expedite wound closure rates compared to standard of care dressing.3,5,11,12 Bioburden control is not a recognized potential benefit of NPWT.12–16 Although Morykwas et al had initial success in decreasing wound bioburden in a porcine model of infected wounds, those results have not been replicated on human wounds.6 In fact, there have been reports of wound bioburden increasing with NPWT compared to standard dressings.14 Fortunately, as technology advances, new products and devices to combat wound bacterial bioburden are being introduced.
The NPWT device was originally developed with the goal of providing a moist wound-healing environment while protecting the wound from outside bacteria.17–19 However, the first model did not sufficiently address wound bioburden. Wound irrigation with the goal of decreasing bioburden is a fundamental next step in the management of open wounds.2 Combining NPWT with a therapeutic solution that acts to cleanse the wound may lessen the bacterial bioburden and be a more effective therapy than NPWT alone.20 The NPWT with instillation (NPWTi) device provides continuous negative pressure therapy followed by delivery of a regulated amount of topical solution to the wound for a short, pre-determined interval, and finally, removal of the solution from the wound site as negative pressure therapy is reinstated. We believe this novel mode of therapy will provide an added benefit to standard NPWT by introducing a bactericidal topical solution that acts to decrease wound bioburden.21
While we have used the original instillation model in the past, the NPWTi device has undergone further modifications since its introduction to the market and now offers a more user-friendly console with improved dressing fluid retention.20 This therapy has been used in small, single center pilot studies for treating diverse wounds with differing instillation protocols and various outcomes evaluated.2,3,20,22
The primary objective of this study was to assess the difference in chronic wound planktonic bioburden after operative debridement and 1 week of treatment with either standard NPWT or NPWT with instillation using a mild concentration of Dakin's solution (Century Pharmaceuticals, Inc., Indianapolis, IN), a bactericidal agent. There is literature supporting that Dakin's solution, when used at concentrations below 0.025%, is efficacious in its bactericidal effect while simultaneously having a non-toxic effect on tissues.23
Methods
Study Design
A single center, prospective pilot study of contemporary case series was conducted between October 2012 and October 2013. The goal was to assess change in bacterial bioburden in chronic lower extremity wounds before and after operative debridement used in conjunction with NPWT versus NPWTi therapy. Patients were sequentially enrolled at random into 2 treatment groups: the first group received treatment with operative debridement followed by 1 week of NPWTi with quarter strength Dakin's solution; the second group received standard therapy consisting of operative debridement and 1 week of NPWT.
A total of 13 patients, corresponding to 16 chronic lower extremity wounds demonstrating significant bioburden (quantitative tissue cultures at baseline were >105 CFU per gram tissue) were included for analysis. One patient was involved in both study groups after negligent management of a split thickness skin graft (STSG) at a nursing facility, resulting in failure and a recurrent open wound. Two patients had bilateral lower extremity wounds; each wound was considered individually in the study.
Wound Treatment Protocol
Both groups underwent operative debridement under regional or general anesthesia. Quantitative planktonic tissue cultures were taken both before and after operative debridement on Day 0 and on postoperative day 7 after operative debridement, prior to wound closure with split thickness skin graft. Immediately after debridement on POD 0, either NPWT or NPWTi was initiated for a period of one week. The procedure of instillation for the NPWTi group involved regular delivery of quarter strength Dakin's solution into the wound bed. The instillation therapy setting for all patients in this group consisted of the following: instillation of a predetermined amount of Dakin's solution and a 10 min dwell time during which the wound was bathed in solution, followed by 60 min of negative pressure therapy. The volume of instillation was individualized based on wound surface area as recommended by the manufacturer (0.2 cc per cm2 wound area).24 The vacuum-assisted devices were set to a negative pressure of 125 mm Hg in both NPWT and NPWTi groups. There has been data to suggest that using variable or intermittent modes of therapy may be more beneficial than continuous negative pressure, however in order to not confound our experimental variables (namely, the effect of instillation versus no instillation), we opted to utilize a standard level of continuous negative pressure.25
Wound Instillation Agent
Dakin's solution, whose active ingredient is sodium hypochlorite, is a topical therapeutic agent that has been used for wound cleansing since World War I.26 The highest concentration tolerable on skin is 0.5% sodium hypochlorite, which is known as “full strength.” The preparation used in this study was 0.125% or “quarter strength,” which was chosen for its in vitro efficacy against MRSA, Pseudomonas and Enterococcus, among others, as well as its cost, availability and ease of use.23,27
Data Collection
This study was reviewed and approved by the Institutional Review Board. All patients provided informed consent in accordance to the Declaration of Helsinki. Patient demographics, including age, sex, comorbidities, were collected from the medical records. Wound data such as etiology, location, surface area, and chronicity were recorded at the first visit to the operating room for debridement, tissue biopsy, and NPWT or NPWTi placement.
Statistical Analysis
Wilcoxon signed rank test with Hodges–Lehmann estimates were used to determine significance of reduction of planktonic bioburden at post-operative day 7 from baseline (after post-operative debridement) and the maintenance of the reduction of bioburden after debridement to closure. All statistical analyses were carried out using MedCalc, Version 12.7.1.
Results
Patient Demographics
There were a total of 13 patients, with 7 patients in the NPWTi group, corresponding to 8 wounds, and 7 patients in the standard NPWT group, corresponding to 8 wounds (Table 1). The NPWTi group was composed of 2 female and 5 male patients. The mean age was 57. The standard NPWT group was composed of 5 female and 2 male patients. The mean age was 61. The majority of patients in both arms had significant comorbidities: diabetes mellitus (DM), chronic kidney disease (CKD), and hypertension (HTN). Two patients corresponding to 3 wounds in the NPWTi group had a history of deep vein thrombosis (DVT), while 1 patient with 1 wound in the NPWT group had a history of DVT. There were no statistical differences between groups (NS).
Table 1.
Patient Demographics
| Patient | Initials | Age | Gender | Comorbidities | Wound Etiology | Treatment Group |
|---|---|---|---|---|---|---|
| 1 | DW | 57 | F | None | Chronic venous stasis | Standard NPWT |
| 2 | CA | 65 | M | DM, HTN, CKD, COPD | Diabetic foot ulcer | NPWTi/standard NPWT |
| 3 | FA | 48 | F | HTN, h/o DVT, hypothyroidism | Unclear etiology, possibly cocaine-induced vasospasm | Standard NPWT |
| 4 | CBM | 74 | F | DM, HTN, CHF, HLD, CAD, A.fib | Diabetic foot ulcer | Standard NPWT |
| 5 | VC | 57 | F | HTN, RA | Chronic venous stasis | Standard NPWT |
| 6 | RP | 66 | M | DM, HTN, HLD, Obesity, A.fib | Chronic venous stasis | Standard NPWT |
| 7 | NS | 59 | F | DM, HTN, HLD | Chronic venous stasis | Standard NPWT |
| 8 | WC | 56 | M | DM, HTN, h/o DVT, OA, CHF | Necrotizing soft tissue infection | NPWTi |
| 9 | NA | 34 | F | DM, HTN, CKD | Diabetic foot ulcer | NPWTi |
| 10 | TD | 64 | M | HTN, BPH, seizure disorder | Trauma | NPWTi |
| 11 | TC | 54 | M | DM, HTN, h/o DVT, hepatitis | Chronic venous stasis | NPWTi |
| 12 | BL | 64 | F | HTN, h/o CVA, liver cirrhosis | Chronic venous stasis | NPWTi |
| 13 | TM | 62 | M | DM, HTN, CKD | Arterial insufficiency | NPWTi |
DM, diabetes mellitus; HTN, hypertension; CHF, congestive heart failure; DVT, deep vein thrombosis; CKD, chronic kidney disease; BPH, benign prostatic hypertrophy; CVA, cerebrovascular accident; COPD, chronic obstructive pulmonary disease; OA, osteoarthritis.
Wound Characteristics
Patients in the standard NPWT group had a mean wound chronicity of 30 months while in the NPWTi group had a mean wound chronicity of 23 months (p = 0.31) (Table 2). The mean wound surface area was 63 cm2 prior to debridement in the NPWTi group and 123 cm2 prior to debridement in the NPWT group (p = 0.94).
Table 2.
Wound Characteristics
| Number | Initials | Wound Etiology | Wound Site (s) | Wound Duration (months) | Wound Surface Area (cm2) | Treatment Group |
|---|---|---|---|---|---|---|
| 1 | DW | Chronic venous stasis | Right lateral lower extremity | 36 | 3.96 | Standard NPWT |
| 2a | CA | Diabetic foot ulcer | Right plantar heel | 18 | 1.5 | Standard NPWT |
| 2b | CA | Diabetic foot ulcer | Right plantar heel | 18 | 1.5 | NPWTi |
| 3 | FA | Unclear (cocaine-induced vaso-occlusive disease?) | Right transmetatarsal amputation site | 10 | 7.1 | Standard NPWT |
| 4 | CBM | Diabetic foot ulcer | Right plantar foot | 12 | 88 | Standard NPWT |
| 5 | VC | Chronic venous stasis | Right lateral ankle | 10 | 5 | Standard NPWT |
| 6a | RP | Chronic venous stasis | Right lower extremity, circumferential | 60 | 323 | Standard NPWT |
| 6b | RP | Chronic venous stasis | Left lower extremity, circumferential | 60 | 500 | Standard NPWT |
| 7 | NS | Chronic venous stasis | Right ankle | 36 | 54 | Standard NPWT |
| 8 | WC | Necrotizing soft tissue infection | Medial left lower leg | 3 | 294.5 | NPWTi |
| 9 | NA | Diabetic foot ulcer | Left plantar foot | 2 | 12.25 | NPWTi |
| 10 | TD | Trauma | Medial right lower leg | 30 | 52.5 | NPWTi |
| 11a | TC | Chronic venous stasis | Anterior left lower leg | 36 | 43.55 | NPWTi |
| 11b | TC | Chronic venous stasis | Anterior right lower leg | 36 | 5.29 | NPWTi |
| 12 | BL | Chronic venous stasis | Right ankle | 36 | 5.2 | NPWTi |
| 13 | TM | Arterial insufficiency | Left transmetatarsal amputation site | 24 | 70 | NPWTi |
Wound Bacteriology
After operative debridement (post-operative day 0) there was a mean of 3 (±1) types of bacteria per wound. The most common types of bacteria for both groups were Staphylococcus aureus, Corynebacterium, and Pseudomonas aeruginosa. The mean CFU/gram tissue culture was 3.7 × 106 (±4 × 106) in the NPWTi group, while in the NPWT group the mean was 1.8 × 106 (±2.36 × 106) CFU/gram tissue culture (Fig. 1). There was a statistically greater number of bacteria in the NPWTi cohort than the NPWT group at baseline (p = 0.016), however, at the end of therapy there was a non-statistically significant difference between the two groups (p = 0.44).
Figure 1.
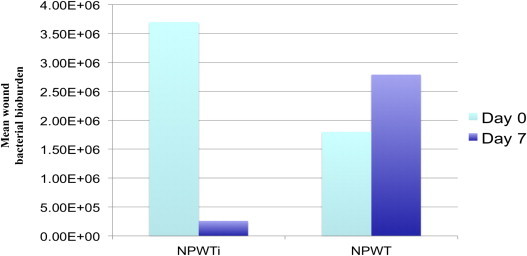
Bacterial bioburden change over time with NPWTi versus NPWT.
At 7 days, the mean number of bacterial species per wound was 2 (±1) in the NPWTi group, with a decreased number demonstrating S. aureus as compared to the NPWT group. Wounds treated with NPWTi had a mean of 2.6 × 105 (±3 × 105) CFU/gram of tissue culture while wounds treated with NPWT had a mean of 2.79 × 106 (±3.18 × 106) CFU/gram of tissue culture (p = 0.43) (Fig. 1). The mean absolute reduction in bacteria for the NPWTi group was 10.6 × 106 bacteria per gram of tissue while there was a mean absolute increase in bacteria for the NPWT group of 28.7 × 106 bacteria per gram of tissue, therefore there was a statistically significant reduction in the absolute bioburden in those wounds treated with NPWTi (p = 0.016).
However this significance was not as great when examined as a percent in change from the wound bioburden at baseline, with each wound acting as its own control. The percentage change in the planktonic bacteria after debridement was 90% (84%–100%) (absolute bacterial count mean reduction 2.62 × 107 to 2.51 × 106) for the NPWTi group versus 88% (68%–100%) (absolute bacterial count mean reduction 2.14 × 106 to 4.76 × 105) for the NPWT group (p = 0.87). The ability to maintain the post-debridement reduced bioburden was a 16% increase in quantitative bacterial count in one week (81% increase to 2% reduction) (absolute mean increase from 4.76 × 105 to 5.74 × 106) for the NPWT group; when the wound was treated with NPWTi there was only a 4.6% increase in bacterial count from post debridement levels (53% increase to 15% further reduction) (absolute mean increase from 2.51 × 106 to 3.15 × 106) (p = 0.078). From baseline the NPWT group had a 16% increase in bacteria (8200% increase to 100% reduction) over the course of therapy while the NPWTi group had an 87% reduction (45%–100% reductions) (p = 0.078).
Discussion
The use of NPWTi in wound healing has been demonstrated to be useful by many authors. In 1998 Fleischmann proposed instillation of wounds with various solutions to enhance readiness for wound closure or spontaneous healing.22 More recently, NPWTi has been used to treat chronic wounds, infected wounds, osteomyelitis, and surgical implant infections.3,19,28,29 The majority of these studies did not investigate the role of NPWTi in reducing bacterial bioburden on quantitative tissue culture. Gabriel et al conducted a study to evaluate the efficacy of NPWTi in bioburden reduction using silver nitrate, and found promising results in clinical infection reduction compared to standard wet to moist dressings.3
The goal of this study was to determine the difference in wound bed preparation, with regard to bacterial bioburden, using surgical debridement in combination with novel NPWTi versus standard NPWT. We were able to demonstrate that surgical wound debridement followed by one week of NPWTi resulted in a significantly decreased wound bioburden as compared to wound debridement followed by one week of standard NPWT.
Our study confirmed the findings of other authors in that NPWTi reduced the quantity of bacteria, while standard NPWT resulted in an increased bacterial load.2,10,12,14,16,17 With regard to bioburden reduction, NPWTi may indeed provide superior therapy when compared to NPWT. In the past, we have found debridement alone to be insufficient in decreasing bacterial bioburden. The wound bacterial load reduction after sharp surgical debridement alone was not more than 1 Log.30 It follows that further wound treatment may be needed in order to achieve optimal bioburden reduction. On post-operative day 7 of this study, following operative debridement and one week of NPWTi, there was a statistically significant reduction of CFU per gram of tissue culture from baseline. This suggests that instillation therapy may provide further eradication of bacterial bioburden, above the ability of sharp debridement. Debridement must not be disregarded, however, as it is a fundamental step in wound care to first remove devitalized, damaged, or infected tissue.5 Following appropriate debridement we have shown that 7 days of NPWTi with Dakin's can act to better prepare wounds for healing and closure. It should be noted, that while the bioburden level increased in the wounds treated with NPWT, there were no apparent signs of active clinical infection and NPWT did not preclude split thickness skin grafting (STSG) on this group, on post-operative day 7.
New medical technology is often associated with a period of acclimation until practitioners have identified the optimal usage to best benefit their patients. The NPWTi is a novel method of treatment currently undergoing clinical study. The manufacturer does not recommend any particular topical agent for wound instillation, but rather there are multiple approved solutions including hypochlorite-based solutions, silver nitrate (0.5%), sulfur-based solutions (sulfonadmides), biguanides (polyhexanide), cationic solutions (octenidine, benzalkonium chloride), and isotonic solutions.24
The ideal solution for topical instillation would be efficacious at reducing wound bioburden with limited cytotoxicity.2,31 This is a difficult balance to achieve as bactericidal agents commonly inhibit the cellular growth of normal, healthy tissues.1,32,33 Povidione-iodine, silver sulfadiazine, and mafenide acetate cream have shown to be cytotoxic to components of wound healing in rodent models.1,32 Dakin's solution (0.025%) was shown in vitro not to promote fibroblast toxicity, although keratinocyte monolayers were sensitive to the solution with varying response.31 Neodermal thickness of porcine wounds was significantly increased after 7 days of Dakins treatment, with an early trend toward re-epithelialization.34
In our clinical experience, Dakin's solution has demonstrated success at bacterial clearance of large lower extremity wounds with little obvious toxicity.35 We therefore elected to utilize quarter strength Dakin's solution at the upper level of concentration acceptability as provided in the NPWTi manual.24 Other clinical pilot studies have utilized various agents in infected or colonized wounds (culture directed topical antibiotics, dilute lidocaine mixed with topical antibiotics, polyhexanide antiseptic solution, PHMG, silver nitrate) with promising results.2,3,22,28,29,36 Interestingly, sterile water instillation has been successfully used to enhance complex wound closure in non-clinically infected wounds.37 This suggests that it is the action of repeated cycles of instillation and negative pressure that may enhance wound closure, rather than the specific instillation solution. To our knowledge, there have been no clinical studies evaluating the efficacy of multiple types of instillation solution on bacterial bioburden of chronic wounds.
The cost of new medical devices or technology is an increasingly growing concern for both physicians and patients. At this time, the standard protocol at our institution for wound bed preparation is surgical debridement followed by one week of NPWT. The NPWT system that we currently use is a combined NPWT and NPWTi machine (NPWT/NPWTi; V.A.C. Ulta™ Therapy; KCI USA, Inc., San Antonio, TX) and therefore the only additional cost of using the instillation mode lies in the choice of instillation solution. Dakin's solution is a cheap and cost-effective wound treatment solution in the United States. Absolute costs were not assessed in this study, though it would appear that the benefits of bioburden reduction in large chronic wounds in this patient population would provide a socioeconomic benefit as compared to conventional therapeutic modalities.
There are three main limitations that can be traced in our approach. This study was a small, non-randomized study. The type of instillation solution utilized for treatment in this study was limited to Dakin's solution; we did not compare different types of instillation solutions. Finally, as was alluded to previously, a large prospective trial would better clarify other authors' findings with other instillation solutions. Studies in the future need to examine to efficacy of utilizing various dwell times and cycles of instillation for optimal results.
Conclusion
Bioburden reduction was significantly decreased in chronic wounds using 7 days of NPWTi following operative debridement. Chronic wounds treated with 7 days of NPWT actually demonstrated an increase in bacterial load. In the future, studies should evaluate this therapy not only in larger, randomized controlled trials, but also to assess the impact of NPWTi on wound biofilm.
References
- 1.Robson M.C., Payne W.G., Ko F. Hypochlorous acid as a potential wound care agent: Part II. Stabilized hypochlorous acid: its role in decreasing tissue bacterial bioburden and overcoming the inhibition of infection on wound healing. J Burns Wounds. 2007;6:e6. [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel A., Shores J., Bernstein B. A clinical review of infected wound treatment with Vacuum Assisted Closure (V.A.C.) therapy: experience and case series. Int Wound J. 2009;2(6 suppl):1–25. doi: 10.1111/j.1742-481X.2009.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabriel A., Shores J., Heinrich C. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5:399–413. doi: 10.1111/j.1742-481X.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin J.M., Zenilman J.M., Lazarus G.S. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol. 2010;130:38–48. doi: 10.1038/jid.2009.221. [DOI] [PubMed] [Google Scholar]
- 5.Eneroth M., van Houtum W.H. The value of debridement and Vacuum-Assisted Closure (V.A.C.) Therapy in diabetic foot ulcers. Diabetes Metab Res Rev. 2008;1(24 suppl):S76–S80. doi: 10.1002/dmrr.852. [DOI] [PubMed] [Google Scholar]
- 6.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. doi: 10.1097/00000637-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wackenfors A., Gustafsson R., Sjogren J., Algotsson L., Ingemansson R., Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 2005;79:1724–1730. doi: 10.1016/j.athoracsur.2004.10.053. discussion 30–31. [DOI] [PubMed] [Google Scholar]
- 8.Wackenfors A., Sjogren J., Algotsson L., Gustafsson R., Ingemansson R., Malmsjo M. The effect of vacuum-assisted closure therapy on the pig femoral artery vasomotor responses. Wound Repair Regen. 2004;12:244–251. doi: 10.1111/j.1067-1927.2004.012117.x. [DOI] [PubMed] [Google Scholar]
- 9.Wackenfors A., Sjogren J., Gustafsson R., Algotsson L., Ingemansson R., Malmsjo M. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen. 2004;12:600–606. doi: 10.1111/j.1067-1927.2004.12602.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S., Baharestani M., Baranoski S. Guidelines for managing pressure ulcers with negative pressure wound therapy. Adv Skin Wound Care. 2004;2(17 suppl):1–16. doi: 10.1097/00129334-200411002-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ford C.N., Reinhard E.R., Yeh D. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg. 2002;49:55–61. doi: 10.1097/00000637-200207000-00009. discussion 61. [DOI] [PubMed] [Google Scholar]
- 12.Moues C.M., van den Bemd G.J., Heule F., Hovius S.E. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg. 2007;60:672–681. doi: 10.1016/j.bjps.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Birke-Sorensen H., Malmsjo M., Rome P. Evidence-based recommendations for negative pressure wound therapy: treatment variables (pressure levels, wound filler and contact layer) – steps towards an international consensus. J Plast Reconstr Aesthet Surg. 2011;(64 suppl):S1–S16. doi: 10.1016/j.bjps.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Moues C.M., Vos M.C., van den Bemd G.J., Stijnen T., Hovius S.E. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 15.Braakenburg A., Obdeijn M.C., Feitz R., van Rooij I.A., van Griethuysen A.J., Klinkenbijl J.H. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg. 2006;118:390–397. doi: 10.1097/01.prs.0000227675.63744.af. discussion 8–400. [DOI] [PubMed] [Google Scholar]
- 16.Weed T., Ratliff C., Drake D.B. Quantifying bacterial bioburden during negative pressure wound therapy: does the wound VAC enhance bacterial clearance? Ann Plast Surg. 2004;52:276–279. doi: 10.1097/01.sap.0000111861.75927.4d. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 17.Webb L.X. New techniques in wound management: vacuum-assisted wound closure. J Am Acad Orthop Surg. 2002;10:303–311. doi: 10.5435/00124635-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Petrie N., Potter M., Banwell P. The management of lower extremity wounds using topical negative pressure. Int J Low Extrem Wounds. 2003;2:198–206. doi: 10.1177/1534734603261067. [DOI] [PubMed] [Google Scholar]
- 19.Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs. 2007;34:191–194. doi: 10.1097/01.WON.0000264834.18732.3b. [DOI] [PubMed] [Google Scholar]
- 20.Bradley B.H., Cunningham M. Biofilms in chronic wounds and the potential role of negative pressure wound therapy: an integrative review. J Wound Ostomy Continence Nurs. 2013;40:143–149. doi: 10.1097/WON.0b013e31827e8481. [DOI] [PubMed] [Google Scholar]
- 21.V.A.C. Instill® Therapy Unit. Instillation Therapy Combined With Negative Pressure Wound Therapy. Available from: http://www.kci1.com/KCI1/vacinstilltherapyunit; Cited 2013; May 13.
- 22.Fleischmann W., Russ M., Westhauser A., Stampehl M. Vacuum sealing as carrier system for controlled local drug administration in wound infection. Der Unfallchirurg. 1998;101:649–654. doi: 10.1007/s001130050318. [DOI] [PubMed] [Google Scholar]
- 23.Heggers J.P., Sazy J.A., Stenberg B.D. Bactericidal and wound-healing properties of sodium hypochlorite solutions: the 1991 Lindberg Award. J Burn Care Rehabil. 1991;12:420–424. doi: 10.1097/00004630-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 24.KCI Licensing Inc . 2012. V.A.C.Ulta™ Negative Pressure Wound Therapy System Monograph. March 2012 ed. [Google Scholar]
- 25.Borgquist O., Ingemansson R., Malmsjo M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage. 2010;56:60–67. [PubMed] [Google Scholar]
- 26.King M. Our historical roots: Dr Richard Drysdale Dakin, DSc, and his solution. J Wound Ostomy Continence Nurs. 2008;35:289–292. doi: 10.1097/01.WON.0000319127.98983.2c. [DOI] [PubMed] [Google Scholar]
- 27.Agostinho A.M., Hartman A., Lipp C., Parker A.E., Stewart P.S., James G.A. An invitro model for the growth and analysis of chronic wound MRA biofilms. J Appl Microbiol. 2011;111:1275–1282. doi: 10.1111/j.1365-2672.2011.05138.x. [DOI] [PubMed] [Google Scholar]
- 28.Timmers M.S., Graafland N., Bernards A.T., Nelissen R.G., van Dissel J.T., Jukema G.N. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009;17:278–286. doi: 10.1111/j.1524-475X.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehner B., Fleischmann W., Becker R., Jukema G.N. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop. 2011;35:1415–1420. doi: 10.1007/s00264-011-1274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz J., Avdagic E., Gendics C., Lantis J., II . The Symposium on Advanced Wound Care (SAWC); Denver, CO: May, 2013. Surgical Debridement Alone Does not Adequately Reduce Planktonic Bioburden in Chronic Lower Extremity Wounds. [DOI] [PubMed] [Google Scholar]
- 31.Wilson J.R., Mills J.G., Prather I.D., Dimitrijevich S.D. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care. 2005;18:373–378. doi: 10.1097/00129334-200509000-00011. [DOI] [PubMed] [Google Scholar]
- 32.McCauley R.L., Li Y.Y., Poole B. Differential inhibition of human basal keratinocyte growth to silver sulfadiazine and mafenide acetate. J Surg Res. 1992;52:276–285. doi: 10.1016/0022-4804(92)90086-f. [DOI] [PubMed] [Google Scholar]
- 33.McCauley R.L., Linares H.A., Pelligrini V., Herndon D.N., Robson M.C., Heggers J.P. In vitro toxicity of topical antimicrobial agents to human fibroblasts. J Surg Res. 1989;46:267–274. doi: 10.1016/0022-4804(89)90069-3. [DOI] [PubMed] [Google Scholar]
- 34.Bennett L.L., Rosenblum R.S., Perlov C., Davidson J.M., Barton R.M., Nanney L.B. An in vivo comparison of topical agents on wound repair. Plast Reconstr Surg. 2001;108:675–687. doi: 10.1097/00006534-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 35.Raad W., Lantis J.C., 2nd, Tyrie L., Gendics C., Todd G. Vacuum-assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81–85. doi: 10.1111/j.1742-481X.2010.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolvos T. Wound instillation–the next step in negative pressure wound therapy. Lessons learned from initial experiences. Ostomy Wound Manage. 2004;50:56–66. [PubMed] [Google Scholar]
- 37.Treot L. European Wound Management Association; Copenhagen, Denmark: May, 2013. Negative Pressure Therapy With Sterile Water Instillation for Conventional NPW Failures and Complex Wounds. [Google Scholar]


