Abstract
Children of parents with depression exhibit neural abnormalities in reward processing. Examining contributions of parenting could provide insight into the development of these abnormalities and to the etiology of depression. We evaluated whether early parenting moderates the effects of parental depression on a neural measure of reward and loss processing in mid-late childhood. Parenting was assessed when children were preschoolers. At age nine, children completed an event-related potential assessment and the feedback negativity (FN) was measured following rewards and losses (N=344). Maternal authoritative parenting moderated the effect of maternal depression; among offspring of mothers with histories of depression, low authoritative parenting predicted a blunted FN. Observed maternal positive parenting interacted with paternal depression in a comparable manner, indicating that maternal parenting may buffer the effects of paternal depression. Early parenting may be important in shaping the neural systems involved in reward processing among children at high risk for depression.
Keywords: depression, vulnerability, reward, parenting, feedback negativity, event-related potentials
The ability to respond to feedback is essential in navigating the world and adjusting behavior to achieve desired outcomes. There is evidence that the neural systems that process and modify behavior in response to feedback continue to develop across childhood and into adolescence (Peters, Braams, Raijmakers, Koolschijn, & Crone, 2014). In addition, deficits in the ability to respond effectively to feedback, particularly feedback indicating reward and loss, appear to be a key psychobiological process underlying depressive disorders in adults and youth (Eshel & Roiser, 2010; Forbes et al., 2009; Forbes et al., 2006; Henriques & Davidson, 2000; Pizzagalli et al., 2009; Pizzagalli, Jahn, & O'Shea, 2005; Smoski et al., 2009; Steele, Kumar, & Ebmeier, 2007), and may play a role in the pathogenesis of depression (e.g., Bress, Foti, Kotov, Klein, & Hajcak, 2013; Gotlib et al., 2010; Kujawa, Proudfit, & Klein, in press; McCabe, Woffindale, Harmer, & Cowen, 2012).
Parental depression is associated with heightened risk of depression in offspring (Hammen, 2009); thus, vulnerability markers and intermediate phenotypes for depression should be more evident among children of depressed parents. Consistent with this, offspring of depressed parents exhibit abnormalities in the neural processing of reward (Gotlib et al., 2010; Kujawa et al., in press; McCabe et al., 2012). In particular, we recently demonstrated that children of mothers with histories of depression, but not anxiety, exhibit a blunted feedback negativity (FN) event-related potential (ERP) component in response to monetary reward versus loss (Kujawa et al., in press).
While parental depression is associated with greater vulnerability for depressive disorders in offspring, pathways to depression are marked by both equifinality and multifinality, as not all people who develop depression have a parental history and some offspring of depressed parents never experience the disorder themselves. Thus, research evaluating moderating variables is essential for understanding the transmission of depression (Goodman, 2014; Goodman & Gotlib, 1999).
Parenting is one environmental factor that may contribute to the intergenerational transmission of depression. There is evidence that low parental warmth, as well as high parental intrusiveness and rejection, are related to depressive symptoms in youth both cross-sectionally and longitudinally (for a review, Klein, Kujawa, Black, & Pennock, 2013). On the other hand, authoritative parenting, characterized by warmth, non-punitive discipline and consistency, has been associated with positive outcomes in adolescents, including lower depression (Milevsky, Schlechter, Netter, & Keehn, 2007). In addition, problematic parenting, including low warmth and high psychological control and over-involvement, has been shown to moderate the effect of maternal depression on psychopathology and social functioning in offspring (Brennan, Le Brocque, & Hammen, 2003).
Parenting may also shape abnormalities in reward processing in offspring. For example, functional magnetic resonance imaging (fMRI) studies indicate that child maltreatment and severe early deprivation are associated with reduced basal ganglia activation during reward anticipation later in life (Dillon et al., 2009; Mehta et al., 2010; Pechtel & Pizzagalli, 2011). Work examining less extreme forms of problematic parenting is more limited; however, in one recent study, low maternal warmth was associated with enhanced striatal and medial prefrontal cortex activation during reward anticipation among a sample of adolescent girls (Casement et al., 2014). In addition, Morgan, Shaw, and Forbes (2014) examined interactions between maternal depression and early maternal warmth in predicting neural reactivity to reward among young adult males recruited for a study of the development of antisocial behaviors. Among offspring of mothers with a history of depression, low maternal warmth in early childhood was associated with an enhanced striatal response during anticipation and receipt of reward, while low maternal warmth in early adolescence was associated with reduced striatal activation during reward anticipation.
Thus, previous work provides evidence that early parenting may shape development of neural reward circuitry; however, in order to contribute to our understanding of the etiopathogenesis of depression, a number of issues must be addressed. First, examining these associations earlier in development and among never-depressed children at high and low risk for depression is an essential step toward elucidating the etiology of depression. Second, existing studies have focused either on extreme forms of problematic parenting or on maternal warmth; however, comparing multiple dimensions of parenting (e.g., intrusiveness and control, hostility, permissiveness) is needed to identify the relevant processes and aid in developing more targeted intervention strategies. Third, existing research has focused exclusively on maternal parenting (Casement et al., 2014; Morgan et al., 2014). Examination of both maternal and paternal parenting would provide insight into the complex environments that shape risk for depression. We previously failed to find effects of paternal depression on the FN in offspring (Kujawa et al., in press); however, it is possible that effects of paternal depression may be more apparent when examined in conjunction with maternal or paternal parenting.
In order to evaluate early environmental factors that shape neural processing of reward and loss in children, we examined the effects of parenting both alone and as a moderator of parental depression effects on the FN. We obtained observational and self-report measures of parenting in a large group of preschool-aged children from the community. Observational measures focused on maternal parenting behavior and included both positive (i.e., support, confidence, and instruction) and negative (i.e., hostility and intrusiveness) behaviors. Self-report measures were completed by both mothers and fathers and assessed authoritative, authoritarian and permissive parenting styles (Baumrind, 1971). Approximately six years later, when the children were nine years old, we completed a follow-up assessment in which children participated in an electroencephalogram (EEG) reward and loss feedback task to measure the FN. The FN is an ERP component that is observable approximately 250–350 ms after feedback over frontocentral recording sites as a relative negativity to monetary losses compared to rewards (Foti, Weinberg, Dien, & Hajcak, 2011; Gehring & Willoughby, 2002) and has been linked to reward-related neural activity, including ventral striatum and medial prefrontal cortex, using fMRI (Becker, Nitsch, Miltner, & Straube, 2014; Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011). At both assessments, mothers and fathers completed diagnostic interviews to assess lifetime histories of psychopathology. Because there is some evidence that blunted reward reactivity is relatively specific to depression rather than anxiety (Bress et al., 2013; Kujawa et al., in press; Shankman et al., 2012), and anxiety may be associated with heightened reward reactivity in some conditions (Guyer et al., 2012; Pérez-Edgar et al., 2013), we controlled for both parental and child anxiety in all analyses.
Our first aim was to evaluate whether early maternal parenting behavior and style predict the FN in mid-late childhood and whether maternal parenting moderates the association between maternal depression and a reduced FN. Consistent with existing evidence of a blunted FN as a marker of risk for depression (Bress et al., 2013; Kujawa et al., in press) and evidence that both maternal warmth and early childhood maltreatment may shape neural reward system activation (e.g., Morgan et al., 2014; Dillon et al., 2009), we hypothesized that low positive parenting and/or high negative parenting would be associated with a reduced FN, particularly among children at high risk for depression (e.g., with maternal histories of depression). A second, more exploratory, aim was to examine the effects of paternal parenting on the FN, alone and in conjunction with paternal history of depression. As mothers may have greater involvement than fathers in early parenting (e.g., Connell & Goodman, 2002), a third, also exploratory, aim was to examine whether maternal parenting moderates the association between paternal depression and the FN.
Method
Participants
Participants were part of a larger prospective sample (N=559) recruited through a commercial mailing list. At the initial assessment, three-year-old children with no significant medical conditions or developmental disabilities and living with at least one English-speaking biological parent were eligible to participate (for more information on the sample see Olino, Klein, Dyson, Rose, & Durbin, 2010). Since initial recruitment, the families have participated in several waves of assessments involving a range of measures, the results of which have previously been published (e.g., Kujawa, Hajcak, Torpey, Kim, & Klein, 2012; Kujawa et al., in press; Torpey et al., 2013; Shankman et al., 2011). A subset of 426 children who completed at least one of the parenting assessments at age 3 also participated in the EEG assessment approximately six years later. Data from 43 participants were excluded due to poor EEG quality. Because the aim of the study was to identify early markers of risk for unipolar depression, we also excluded 5 children with a parental history of bipolar disorder, 3 children missing data on parental mood disorders and 5 children who had already experienced a lifetime depressive episode. Lastly, data from 26 children were excluded because of parental reports of a significant learning or developmental disability at the follow up assessment. The final sample consisted of 344 children (55.8% male). At the initial assessment of parenting, the mean child age was 3.63 (SD=.29), and at the follow up assessment, the mean age was 9.19 (SD=.40). With regard to ethnicity, 7.6% of the sample was Hispanic, and with regard to race, 94.2% was Caucasian, 3.2% was African American, and 2.6% was Asian. With regard to parental education, 59.9% of mothers and 49.1% of fathers had completed college.
Procedures
This study was approved by the Stony Brook University Institutional Review Board. All parents provided written informed consent and children provided verbal assent. The initial assessment took place when the children were approximately three years old. At this time, the child and one parent completed an observational measure of parenting behavior (described below). In addition, parents completed self-report measures of parenting style and semi-structured interviews to assess their own lifetime histories of psychopathology. Participants were invited back to the laboratory as close as possible to the child’s ninth birthday, at which time the monetary reward ERP task was administered and parents completed another diagnostic interview assessing their own psychopathology. In addition, children and the primary parent participated in a semi-structured interview assessing lifetime child psychopathology.
Measures
Observational measure of maternal parenting behavior
At the age 3 assessment, the child and one biological parent participated in a parent-child interaction assessment of parenting behavior based on the Teaching Tasks (TT) battery (Egeland et al., 1995). The battery includes six standardized tasks (e.g., book reading, block building) designed to elicit parent and child behaviors. Trained coders rated videotapes of each episode for parental hostility (parent’s expression of anger, frustration, annoyance, discounting or rejection of the child), intrusiveness (parent’s failure to respect the child as an individual, or interference with the child’s needs, interests or behaviors), confidence (degree to which the parent seems to believe that she can work successfully with the child and that the child will behave appropriately), supportive presence (parent’s expression of positive regard and emotional support to the child) and quality of instruction (parent’s ability to structure the situation so that the child understands the objective and is able to attempt the task). All ratings were on a 5-point scale, with the exception of confidence, which was rated on a 3-point scale. To reduce skewness and kurtosis, hostility and confidence were dichotomized into a 2-point scale for each episode. All ratings were averaged across tasks. Interrater reliability was acceptable for all variables (ICCs=.59–.85, n = 55; Cicchetti, 1994). A negative parenting composite score was computed by combining standardized scores on hostility and intrusiveness (α = .69; Kujawa et al., 2014), and a positive parenting composite score was computed by combining standardized scores on confidence, support and instruction (α = .85). Five children were missing TT data (but parents completed self-report measures of parenting) and one participant was a significant outlier on positive parenting (p<.01) according to Grubb’s test (1969) and was excluded from analyses. Because we were interested in examining maternal and paternal parenting behavior separately and almost all TT batteries (91.9%) were completed by mothers, data from 23 participants in which the father completed the TT were also excluded from analyses of observed parenting behavior.
Self-report measures of maternal and paternal parenting style
At the age 3 assessment, the 37-item version of the Parenting Styles and Dimensions Questionnaire (PSDQ; Robinson, Mandleco, Olsen, & Hart, 2001) was administered to biological mothers (PSDQ-M) and biological fathers (PSDQ-F). The PSDQ is a self-report measure designed to assess three major parenting styles: authoritative (high control, high warmth), authoritarian (high control, low warmth), and permissive (low control, high warmth). Items are rated from 1 (never) to 5 (always). The subscales have been demonstrated to have adequate internal consistency (Olivari, 2013; Robinson et al., 2001; Robinson, Mandelco, Olsen, & Hart, 1995). Though research on the validity of scales is more limited, there is evidence for good face, concurrent, and predictive validity (Olivari, 2013). PSDQ data were missing for 24 mothers and 76 fathers.
Parental psychopathology
A detailed description of the procedures for assessing parental psychopathology is available in Kujawa et al. (in press). In summary, biological mothers and fathers completed the Structured Clinical Interview for DSM-IV non-patient version (SCID; First, Spitzer, Gibbon, & Williams, 1996) at both the age 3 and age 9 assessments. The SCID was administered by advanced doctoral students in clinical psychology and masters-level clinicians. Diagnoses from the age 3 and age 9 assessments were combined to yield lifetime diagnoses. Kappa for the interrater reliability of lifetime diagnoses of depressive disorders was .93 and .91, respectively (see Kujawa et al., in press). When one biological parent was unavailable to complete the SCID, family history information was obtained from the other parent using a semi-structured family history interview (Andreasen, Endicott, Spitzer, & Winokur, 1977). It was not possible to directly interview 13 fathers using the SCID, thus diagnoses were derived using the family history method. Data on anxiety disorders were missing for 1 of these fathers.
Monetary reward task
A detailed description of the monetary reward EEG task and EEG data acquisition/processing is provided in Kujawa et al. (in press). Participants were instructed to guess which of two doors on the computer screen had a prize behind it and were told that they would earn $0.50 or lose $0.25. The task consisted of 60 trials (30 gain, 30 loss) presented in a random order. At the start of each trial, participants were presented with two doors and pressed a mouse button to select one of the doors. Feedback was presented for 2000 ms, with a win indicated by a green “↑,” and a loss indicated by a red “↓.”
EEG data acquisition and processing
EEG was recorded using a 34-channel Biosemi system based on the 10/20 system (32 channel cap with Iz and FCz). Electrodes were placed on the left and right mastoids, and the electrooculogram was recorded from four facial electrodes. The data were digitized at 24-bit resolution with a LSB value of 31.25nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with - 3dB cutoff points at 208 Hz. Off-line analysis was performed using Brain Vision Analyzer software (Brain Products). Data were referenced to a linked mastoid reference, band-pass filtered with cutoffs of 0.1 and 30 Hz, segmented for each trial 200 ms before feedback onset and continuing for 600 ms after onset. The EEG was corrected for eye blinks (Gratton, Coles, & Donchin, 1983), and artifact rejection was completed using semi-automated procedures with the following criteria: a voltage step of more than 50 μV between data points, a voltage difference of 300 μV within a trial, and a voltage difference of less than .50 μV within 100 ms intervals. Visual inspection was used to remove additional artifacts. ERPs were averaged across win and loss trials and baseline corrected.
The FN was scored as the mean amplitude 275–375 ms following feedback at a pooling of Fz, FCz, and Cz. Analyses focused on ΔFN, which was calculated as the difference between mean amplitude on loss trials and mean amplitude on gain trials (i.e., more negative difference scores indicate greater differentiation between rewards and losses). Difference scores are typically recommended for ERP data analysis in order to isolate the cognitive process of interest (Luck, 2005). The loss minus gain FN difference score has been related to activation in reward regions using fMRI and to behavioral and self-report measures of reward sensitivity (Bress & Hajcak, 2013; Carlson et al., 2011). In addition, ΔFN has been demonstrated to relate to depressive symptoms in adults (Foti & Hajcak, 2009), risk for depression in children (Kujawa et al., in press), and the onset of MDD across adolescence (Bress et al., 2013).
Child psychopathology
At the age 9 assessment, a parent and the child completed the DSM-IV version of the Schedule of Affective Disorders and Schizophrenia for School-Age Children - Present and Lifetime (K-SADS; Axelson, Birmaher, Zelazny, Kaufman, & Gill, 2009). Advanced doctoral students in clinical psychology and a masters-level clinician administered the K-SADS first to the parent and then to the child, with further information obtained to reconcile discrepancies as needed. Lifetime symptoms were rated on a 3-point scale (0=Not present; 1=Subthreshold; 2=Threshold). Summary ratings for depression and anxiety items from the screener were summed to create dimensional scores that were used as covariates in the current analyses (depression dimensional scores could range from 0-16 and anxiety dimensional scores could range from 0-42). To assess interrater reliability, a second rater independently derived ratings from videotapes of 74 participants. Intraclass correlations (ICCs) for dimensional scores of depressive and anxiety symptoms were 0.81 and 0.82, respectively.
Data Analysis
Hierarchical multiple regression analyses were computed to examine the main and interactive effects of parenting behavior and parental depression on ΔFN to monetary gains and losses. First, we evaluated main and interactive effects of maternal parenting behavior and maternal depression. Next, we evaluated main and interactive effects of paternal parenting behavior and paternal depression, and lastly, we evaluated interactive effects of maternal parenting behavior and paternal depression. Continuous variables were centered to examine interactions. Child characteristics (sex, anxiety symptoms, depressive symptoms)1 were entered into step 1, followed by parent variables (maternal or paternal lifetime diagnoses of depression and anxiety, observed or self-reported parenting)2 in step 2 and interactions between parental depression and parenting in step 3. As all interactions involved one continuous and one categorical variable, significant
Results
Participant Characteristics and Associations between Variables
Demographic characteristics and bivariate correlations between all variables are presented in Table 1. We previously reported a detailed description of rates of parental psychopathology and associations with the FN (Kujawa et al., in press). In the current study, 36.6% of mothers and 16.9% of fathers met criteria for a lifetime depressive disorder (MDD or dysthymia). In addition, 35.8% of mothers and 21.5% of fathers met criteria for a lifetime anxiety disorder. With regard to child diagnoses, 5.5% met criteria for lifetime separation anxiety disorder, 3.8% for social phobia, 10.2% for specific phobia, 0.3% for panic disorder, 0.6% for agoraphobia, and 3.5% for generalized anxiety disorder. In addition, 6.4% of children met criteria for lifetime attention-deficit hyperactivity disorder (ADHD) and 2.9% for oppositional defiant disorder (ODD).
Table 1.
Pearson’s correlations/phi coefficients between study variables
| Variable | Mean(SD)/ % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Sex (% male) | 55.8% | - | ||||||||||||||
| 2. Child depression | .26(.69) | .11 | - | |||||||||||||
| 3. Child anxiety | 3.24(3.41) | .03 | .18** | - | ||||||||||||
| 4. Maternal depression | 36.6% | -.02 | .05 | .21*** | - | |||||||||||
| 5. Maternal anxiety | 35.8% | -.02 | .06 | .20** | .25*** | - | ||||||||||
| 6. Paternal depression | 16.9% | .01 | .04 | .04 | .13* | .09 | - | |||||||||
| 7. Paternal anxiety | 21.6% | .05 | −.07 | −.03 | .19** | .08 | .29*** | - | ||||||||
| 8. TT Positive | 0.00(2.52) | .04 | −.12* | −.02 | −.05 | .01 | −.04 | .01 | - | |||||||
| 9. TT Negative | 0.00(1.65) | .02 | .15** | −.03 | .00 | .05 | .04 | −.09 | −.62*** | - | ||||||
| 10. PSDQ-M Authoritative | 60.97(6.41) | −.10 | −.12* | .01 | .03 | −.05 | .05 | .11* | .03 | −.03 | - | |||||
| 11. PSDQ-M Authoritarian | 20.06(4.25) | .04 | .06 | .00 | .13* | .07 | .06 | −.03 | −.16** | .09 | −.15* | - | ||||
| 12. PSDQ-M Permissive | 10.74(3.21) | .01 | .15** | .10 | .09 | .23*** | .04 | −.01 | −.19** | .20** | −.11* | .44*** | - | |||
| 13. PSDQ-F Authoritative | 56.35(8.17) | .04 | -.05 | -.04 | -.02 | -.06 | .09 | .06 | −.01 | .07 | .26*** | .03 | .04 | - | ||
| 14. PSDQ-F Authoritarian | 20.55(4.69) | .02 | .08 | −.06 | .16** | .10 | .14* | .03 | .00 | .07 | −.14* | .29*** | .18** | −.26*** | - | |
| 15. PSDQ-F Permissive | 11.30(3.39) | −.03 | .14* | .09 | .15* | .02 | .07 | .03 | −.17** | .13* | −.02 | .23*** | .36*** | .00 | .29*** | - |
| 16. "FN | -4.74(7.27) | −.17** | −.07 | −.01 | .01 | −.01 | −.04 | −.06 | −.02 | .03 | .01 | .07 | −.06 | .03 | −.13* | .00 |
p<.001;
p<.01;
p<.05
Maternal Depression and Maternal Parenting Behavior
First, a hierarchical multiple regression analysis was computed to examine effects of maternal depression and observed maternal parenting behavior from the TT on children’s ΔFN. As previously demonstrated (Kujawa et al., in press), males showed an enhanced FN compared to females, β=−.17, partial r=−.17, t(314)=−3.02, p<.01, and the effects of child anxiety and depressive symptoms on the FN did not reach significance (ps > .17). None of the main or interactive effects of TT positive parenting, TT negative parenting, maternal anxiety or maternal depression reached significance (ps > .40) in the observed parenting behavior model.
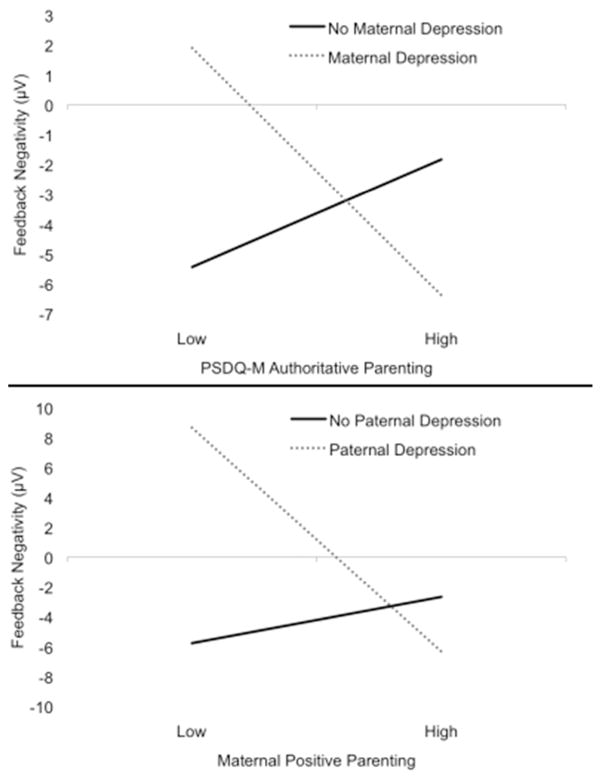
Next, the analysis was repeated using self-report measures of maternal parenting style from the PSDQ. Entry standardized regression coefficients and partial r, as well as R2 for each step and the total model, are presented in Table 2. The main effect of PSDQ-M authoritarian parenting reached significance at entry, β=.12, partial r=.11, t(319)=−1.99, p<.05, but was no longer significant after the interactions were entered, β=.08, partial r=.05, t(319)=.93, p=.35. The interaction between maternal depression and PSDQ-M authoritative parenting style was significant, β=−.17, partial r=−.14, t(319)=−2.46, p=.02. To interpret the interaction, the model was computed at each level of maternal depression. For offspring of mothers with a history of depression, lower PSDQ-M authoritative parenting was associated with a reduced FN, β=−.20, partial r=−.20, t(115)=−2.08, p=.04 (Figures 1 and 2). For offspring of mothers with no history of depression, the effect of PSDQ-M authoritative parenting was not significant, β=.09, partial r=.09, t(203)=1.32, p=.19.3 Main effects of PSDQ-M permissive parenting and interactive effects of PSDQM authoritarian and permissive parenting styles with maternal depression were not significant (ps>.09).
Table 2.
Hierarchical regressions: maternal psychopathology with maternal parenting (left) and paternal psychopathology with maternal parenting (right) predicting UFN
| Predictor | Entry β | Partial r | Predictor | Entry β | Partial r |
|---|---|---|---|---|---|
| 1. Child characteristics | R2=.04, F(3, 316)=3.90, p<.01 | 1. Child characteristics | R2=.04, F(3, 310)=3.85, p=.01 | ||
|
| |||||
| Sex | −.18** | −.18** | Sex | −.17** | −.17** |
| Child depression symptoms | −.04 | −.05 | Child depression symptoms | −.08 | −.09 |
| Child anxiety symptoms | .02 | .02 | Child anxiety symptoms | .04 | .02 |
|
| |||||
| 2. Mother variables | Change R2=.02, F(5, 311)=1.00, p=.42 | 2. Parent variables | Change R2=.00, F(4, 306)=0.30, p=.88 | ||
|
| |||||
| Maternal depression | .00 | .00 | Paternal depression | .01 | .01 |
| Maternal anxiety | .01 | .01 | Paternal anxiety | −.05 | −.04 |
| PSDQ-M authoritative | −.01 | −.01 | TT positive parenting | .01 | .01 |
| PSDQ-M authoritarian | .12* | .11* | TT negative parenting | .04 | .04 |
| PSDQ-M permissive | −.11 | −.10 | |||
|
| |||||
| 3. Interactions | Change R2=.02, F(3, 308)=2.49, p=.06 | 3. Interactions | Change R2=.02, F(2, 304)=2.71, p=.07 | ||
|
| |||||
| Maternal Depression X Authoritative | −.17* | −.14* | Paternal Depression X Positive Parenting |
−.22* | −.13* |
| Maternal Depression X Authoritarian | .08 | .05 | Paternal Depression X Negative Parenting |
−.13 | −.08 |
| Maternal Depression X Permissive | −.07 | −.05 | Total model R2=.06, F(9, 304)=2.02, p=.04 | ||
| Total model R2=.07, F(11, 308)=2.22, p=.01 | |||||
p<.01;
p<.05
Figure 1.
The interaction between maternal depression and maternal-reported authoritative parenting style (PSDQ-M) in predicting ΔFN in offspring (top), and the interaction between paternal depression and observed maternal positive parenting (TT) in predicting ΔFN in offspring (bottom). Note: Parental depression is plotted as the moderating variable because it is dichotomous; however, hypotheses focus on parenting moderating the effects of parental depression.
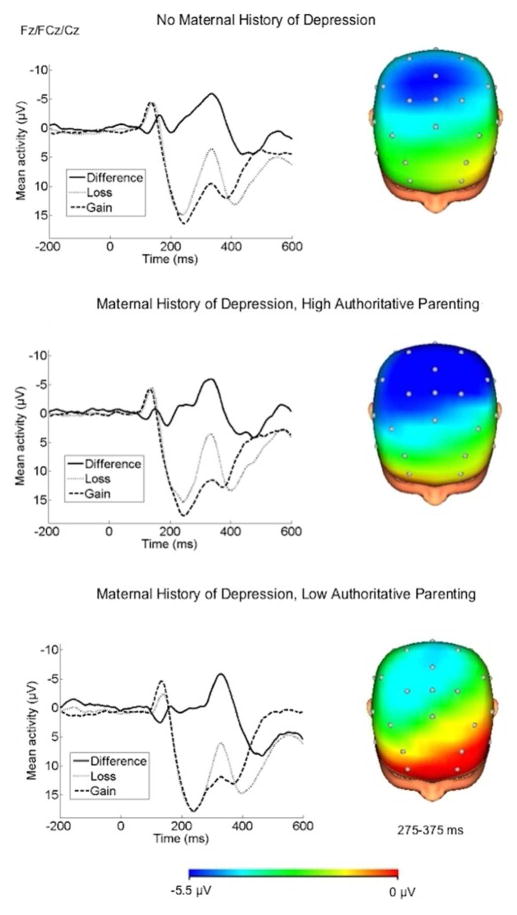
Figure 2.
ERPs (negative up) and scalp distributions depicting the loss-gain difference score for children with no maternal history of depression, children with a maternal history of depression and high PSDQ-M authoritative parenting, and children with a maternal history of depression and low PSDQ-M authoritative parenting. Note: A median split of PSDQ-M authoritative parenting was used for presentation purposes only.
Paternal Depression and Paternal Parenting Behavior
Next, a hierarchical multiple regression analysis was computed to examine effects of paternal depression and self-report measures of paternal parenting style from the PSDQ on children’s ΔFN. PSDQ-F authoritarian parenting was significant in step 2, β=− .14, partial r=−.13, t(266)=−2.07, p=.04, but did not reach significance in the final model β=−.14, partial r=−.12, t(266)=−1.90, p=.06. None of the main effects of PSDQ-F authoritative or PSDQ-F permissive parenting style or the interactive effects of paternal parenting style with paternal depression reached significance (ps>.35).
Paternal Depression and Maternal Parenting Behavior
Lastly, two models were computed to evaluate whether maternal parenting behavior (TT and PSDQ) interacted with paternal depression to predict ΔFN. For the observed parenting model, entry standardized regression coefficients and partial r, as well as R2 for each step and the total model, are presented in Table 2. The interaction between TT positive parenting and paternal history of depression was significant, β=−.22, partial r=−.13, t(313)=−2.29, p=.02 (Figure 1)4. To interpret the interaction, the model was computed separately for children with and without paternal histories of depression. For offspring of fathers with a history of depression, less positive maternal parenting behavior was associated with a blunted FN, β=−.50, partial r=−.32, t(50)=−2.21, p=.03. For offspring of fathers with no history of depression, the effect of maternal positive parenting behavior on the FN was not significant, β=.07, partial r=.03, t(262)=0.96, p=.34.
In the maternal self-report model, maternal authoritarian parenting behavior was associated with a blunted FN, β=.13, partial r=.12, t(318)=2.06, p=.04, prior to controlling for the interactions. None of the main or interactive effects between paternal depression and maternal self-reported parenting style reached significance in the final model (ps>.13).
Discussion
We evaluated whether observational and self-reported measures of early parenting prospectively moderated the effects of maternal and paternal depression on neural reactivity to reward versus loss (i.e., ΔFN) in offspring. Results indicated that among offspring of mothers with histories of depression, low self-reported maternal authoritative parenting style in early childhood predicted a blunted ΔFN (i.e., reduced reactivity to reward versus loss feedback) in mid-late childhood. In addition, among offspring of fathers with histories of depression, low observed maternal positive parenting behavior (i.e., support, confidence and instruction) in early childhood predicted a reduced ΔFN in mid-late childhood. Thus, across multiple measures, children at high risk for depression appear to be particularly sensitive to the effects of low maternal positive parenting behavior and style in shaping abnormal neural processing of reward and loss feedback.
We have previously suggested that the FN may be a vulnerability marker for depression, as it has been shown to prospectively predict depression across adolescence (Bress et al., 2013) and has been observed among offspring of mothers with histories of depression but not anxiety (Kujawa et al., in press). Yet, prior to the current study, little was known about early environmental factors that contribute to a reduced FN. The current findings advance our understanding of the development and etiology of depression by evaluating a central aspect of the early environment (i.e., parenting) in shaping a core neural system (i.e., the reward system) in children at high risk for depression. While prior work indicates that environmental factors play a role in the intergenerational transmission of depression (e.g., Brennan et al., 2003), our study goes further in showing that parental depression and parenting act synergistically to shape the development of a neural index of reward processing (ΔFN), a potential intermediate phenotype for depression. Importantly, these effects are evident in mid-late childhood, long before the surges in reward seeking and rates of depression associated with adolescence (e.g., Avenevoli, Knight, Kessler, & Merikangas, 2008; van Leijenhorst et al., 2010).
Children of parents with histories of depression may have reduced reactivity to reward due to genetic predisposition and modeling of parental reactions to environmental stimuli. However, the effect of parental depression on ΔFN was most apparent among children whose mothers exhibited low support, warmth and structure. Positive and authoritative maternal parenting may be essential in teaching children to respond to environmental contingencies and shape behavior accordingly, and in the absence of positive parenting, at-risk children show deficits in the processing of reward versus loss. This, in turn, may increase the likelihood of developing depression later in life, as abnormal reward responding has previously been shown to prospectively predict depression (Bress et al., 2013; Forbes, Shaw, & Dahl, 2007; Rawal, Collishaw, Thapar, & Rice, 2012). Importantly, these results were relatively specific to positive aspects of parenting, rather than negative parenting (e.g., hostility, intrusiveness, and punitive discipline). This provides important clues to the processes that shape the development of reward processing, and highlights a potentially modifiable risk factor that can be readily targeted in prevention trials.
It is important to note that the results were significant after excluding children with a history of depression and controlling for child symptoms of depression, anxiety and disruptive behavior disorders. Thus, rather than being a consequence of prior depression or a correlate of child symptomatology, these findings further support the FN as a vulnerability marker or intermediate phenotype for depression that may play an important role in the pathway from familial risk to the later development of depressive symptoms.
While we did not find significant interactions between paternal depression and paternal parenting, we did find that maternal observed positive parenting moderated the effect of paternal depression on ΔFN. We have previously failed to find effects of paternal depression on the FN and other early markers of emotional processing biases in children (Kujawa et al., 2014; Kujawa et al., 2012; Kujawa et al., in press), which is consistent with smaller effects of paternal compared to maternal psychopathology on internalizing symptoms in young offspring (Connell & Goodman, 2002). It has been hypothesized that greater involvement by mothers in early parenting could account for these effects (Connell & Goodman, 2002), and the current results provide empirical evidence that maternal parenting may buffer the negative effects of paternal depression on risk for depression in offspring.
Greater self-reported paternal authoritarian parenting style was associated with an enhanced ΔFN; however, this effect no longer reached significance when controlling for interactions with depression. As authoritarian parenting is characterized by low warmth, this finding could be consistent with recent research indicating that low maternal warmth predicted heightened striatal reactivity during reward anticipation (Casement et al., 2014). However, as the effect of authoritarian parenting did not interact with risk for depression, this finding could suggest a pathway from greater authoritarian parenting by fathers to externalizing behavior, which may also be characterized by a disrupted ΔFN (e.g., Holroyd, Baker, Kerns, & Müller, 2008; van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005). Importantly, however, the results of the current study remained the same when controlling for symptoms of disruptive behavior disorders in children. We also observed a main effect of maternal authoritarian parenting predicting a reduced ΔFN, suggesting that overcontrolling and rejecting maternal parenting may also contribute to reduced reactivity to reward and loss feedback in offspring. However, this effect was no longer significant in the final regression models after controlling for the interactions with maternal depression.
Several strengths of the current study should be noted. First, we assessed both maternal and paternal histories of depression and reports of parenting style, whereas much of the existing literature has focused only on maternal psychopathology and parenting. We also examined whether paternal depression is moderated by the effect of maternal parenting style. In addition, the interaction effects between positive parenting behavior and parental depression converged across constructs (parenting behavior vs. style), methods (observation vs. questionnaire), and parents (both maternal and paternal depression). Lastly, we used a relatively large sample and prospective design, indicating that the effects of early maternal parenting relate to reward processing in offspring across a large portion of childhood, which provides insight into trajectories from early risk to the onset of depression in later life.
Nonetheless, there are also several limitations to the current study. First, we did not find a significant effect of maternal depression and observed maternal parenting on ΔFN. This was surprising, as the interaction with maternal depression was apparent with maternal-report measures of parenting, and observed maternal parenting interacted with paternal depression. Second, our observational measure of parenting behavior was limited to mothers, and self-report measures of parenting style were missing for a large proportion of fathers (22%). Thus, we cannot rule out the possibility that methodological factors contributed to the lack of significant effects of paternal parenting. Third, the effect sizes in the current study were small in magnitude, but consistent with other work linking behavioral to ERP measures in large samples (e.g., Patrick et al., 2013; Torpey et al., 2013). In addition, as pathways from early risk to the development of depression are likely complex and marked by equifinality and multifinality, large effect sizes would not be expected.
With regards to future research, a prospective study is needed to evaluate interactions between neural reward system activation, parental risk, and parenting to predict outcomes in adolescence. Such a study could examine whether blunted reactivity to reward mediates the associations between early risk and the development of depressive symptoms in adolescence. In addition, this study could evaluate multiple components of the reward system. While the FN is a measure of reactivity to reward feedback, examining reward anticipation and learning is also essential to understanding the specific abnormalities in reward processing that contribute to the pathogenesis of depression. Lastly, this study could evaluate the whether neural measures of reward system processing predict other outcomes. That is, to what extent do reward system abnormalities mediate the relationship between early parenting and anxiety or externalizing systems? Given evidence of abnormal reward responding in some forms of anxiety and externalizing disorders (e.g., Guyer et al., 2012; Holroyd, Baker, Kerns, & Müller, 2008; van Meel, Oosterlaan, Heslenfeld, & Sergeant, 2005), it is possible that distinct disturbances in the same neural system could lead to very different clinical syndromes.
Another important question that arises from the current study is the extent to which the environment can be modified in order to shape the development of the reward system and risk for depression. A second critical study could examine whether parenting interventions are effective in modifying the FN in offspring. The current findings suggest that low positive parenting, characterized by confidence, instruction, support and warmth, is associated with reduced reward reactivity in offspring of depressed parents. Thus, children at risk for depression may benefit from interventions designed to increase positive parenting behaviors, and this could be a potentially effective early intervention or prevention approach. In addition, this study would provide a more definitive test of the causal influence of positive parenting on the development of reward processing and subsequent risk for depression.
In conclusion, the current findings are consistent with the growing focus on the core neural circuitry, rather than simply the clinical manifestations, involved in psychopathology (e.g., Sanislow et al., 2010). Moreover, they suggest a specific and potentially modifiable target for prevention/early intervention. A two-pronged approach of prospective longitudinal and mechanism-focused intervention studies are needed to test the implications of these findings, and may contribute to more biologically and contextually informed approaches to classification and treatment.
Acknowledgments
This work was supported by National Institute of Mental Health Grants RO1 MH069942 to Daniel N. Klein, R03 MH094518 to Greg Hajcak Proudfit, and F31 MH09530701 to Autumn Kujawa.
Footnotes
We also evaluated the models controlling for child symptoms of disruptive behavior disorders. No substantive changes in the results were observed.
We also evaluated the models controlling for parental completion of college and history of substance use disorders. No substantive changes in the results were observed. interactions were interpreted by evaluating the regression model separately for children with and without parental histories of depression.
We also computed the model with mean activity on loss only and gain only trials as criterion variables.Maternal depression interacted with PSDQ-M authoritative parenting to predict activation on loss trials, β=-.20, partial r=-.16, t(319)=-2.89, p<.01, but the effect did not reach significance for gain trials only, β=-.07, partial r=-.05, t(319)=-.92, p=.36.
We also computed the model with mean activity on loss only and gain only trials as criterion variables. Paternal depression interacted with TT positive parenting to predict activation on loss trials, β=-.20, partial r=-.11, t(313)=-1.99, p=.05, but the was not significant for gain trials only, β=-.02, partial r=-.01, t(313)=- .19, p=.85.
All authors contributed to study design. Data collection and processing were performed by A.K. and R.L., and A.K. performed the data analysis and interpretation under the supervision of D.N.K. and G.H.P. A.K. drafted the paper, and all other authors provided critical revisions and approved the final version of the paper for submission.
References
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Avenevoli S, Knight E, Kessler RC, Merikangas KR. Epidemiology of depression in children and adolescents. Handbook of depression in children and adolescents. 2008:6–32. [Google Scholar]
- Axelson D, Birmaher B, Zelazny J, Kaufman J, Gill MK. Advanced Centre for Intervention and Services Research, Western Psychiatric Institute and Clinic. University of Pittsburgh Department of Psychiatry; 2009. The Schedule for Affective Disorders and Schizophrenia--Present and Lifetime Version (K-SADS- PL) 2009 Working Draft. Web site. Available at: http://www.psychiatry.pitt.edu/research/tools-research/ksads-pl-2009-working-draft. [Google Scholar]
- Baumrind D. Current patterns of parental authority. Developmental Psychology. 1971;4(1 Pt 2):1–103. doi: 10.1037/h0030372. [DOI] [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. The Journal of Neuroscience. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Le Brocque R, Hammen C. Maternal depression, parent-child relationships, and resilient outcomes in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(12):1469–1477. doi: 10.1097/00004583-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bress JN, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50(7):610–616. doi: 10.1111/psyp.12053. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8(0):18–27. doi: 10.1016/j.dcn.2013.12.003. http://dx.doi.org/10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6(4):284–290. doi: 10.1037/1040-3590.6.4.284. [DOI] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children's internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128(5):746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B, Weinfield NS, Heiester M, Lawrence C, Pierce S, Chippendale K, Powell K. Teaching tasks administration and scoring manual. Institute of Child Development, University of Minnesota; 1995. Unpublished Manuscript. [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68(2):118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. The Structured Clinical Interview for DSM-IV Axis I Disorders - Non-patient editions. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May JC, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. Journal of Child Psychology and Psychiatry. 2006;47(10):1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61(5):633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81(1):1– 8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goodman S. Commentary: The multifaceted nature of maternal depression as a risk factor for child psychopathology–reflections on Sellers et al.(2014) Journal of Child Psychology and Psychiatry. 2014;55(2):121–123. doi: 10.1111/jcpp.12202. [DOI] [PubMed] [Google Scholar]
- Goodman S, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67(4):380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55(4):468– 484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grubbs FE. Procedures for detecting outlying observations in samples. Technometrics. 1969;11(1):1–21. [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. The American Journal of Psychiatry. 2012;169(2):205. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2. New York: Guilford Press; 2009. pp. 275–297. [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14(5):711–724. doi: 10.1080/02699930050117684. [DOI] [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Müller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008;46(8):2234–2242. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Klein DN, Kujawa A, Black SR, Pennock AT. Depressive disorders. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Hoboken, NJ: John Wiley & Sons; 2013. pp. 543–575. [Google Scholar]
- Kujawa A, Dougherty L, Durbin CE, Laptook R, Torpey D, Klein DN. Emotion recognition in preschool children: Associations with maternal depression and early parenting. Development and Psychopathology. 2014;26:159–170. doi: 10.1017/S0954579413000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology. doi: 10.1037/a0036285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biological Psychiatry. 2012;72(7):588–594. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Milevsky A, Schlechter M, Netter S, Keehn D. Maternal and paternal parenting styles in adolescents: Associations with self-esteem, depression and life-satisfaction. Journal of Child and Family Studies. 2007;16(1):39–47. doi: 10.1007/s10826-006-9066-5. [DOI] [Google Scholar]
- Morgan JK, Shaw DS, Forbes EE. Maternal depression and warmth during childhood predict age 20 neural response to reward. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(1):108–117. doi: 10.1016/j.jaac.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari MG, Tagliabue S, Confalonieri E. Parenting Style and Dimensions Questionnaire: A review of reliability and validity. Marriage & Family Review. 2013;49(6):465–490. doi: 10.1080/01494929.2013.770812. [DOI] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122(3):902. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Hardee J, Guyer AE, Benson B, Nelson E, Gorodetsky E, Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Braams BR, Raijmakers MEJ, Koolschijn PCMP, Crone EA. The neural coding of feedback learning across child and adolescent development. Journal of Cognitive Neuroscience. 2014 doi: 10.1162/jocn_a_00594. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal A, Collishaw S, Thapar A, Rice F. ‘The risks of playing it safe’: A prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychological Medicine. 2012;1(1):1–12. doi: 10.1017/S0033291712001158. [DOI] [PubMed] [Google Scholar]
- Robinson CC, Mandleco B, Olsen SF, Hart CH. Authoritative, authoritarian and permissive parenting practices: Development of a new measure. Psychological Reports. 1995;77(3):819–830. doi: 10.2466/pr0.1995.77.3.819. [DOI] [Google Scholar]
- Robinson C, Mandleco B, Olsen SF, Hart C. The parenting styles and dimensions questionnaire (PSDQ) Handbook of family measurement techniques. 2001;3:319–321. [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: Research domain criteria. Journal of Abnormal Psychology. 2010;119(4):631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Torpey DC, Olino TM, Dyson MW, Kim J, Tenke CE. Do positive and negative temperament traits interact in predicting risk for depression? A resting EEG study of 329 preschoolers. Development and Psychopathology. 2011;23(02):551–562. doi: 10.1017/S0954579411000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2012 doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118(1–3):69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain: A Journal of Neurology. 2007;130(9):2367– 2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Torpey DC, Hajcak G, Kim J, Kujawa AJ, Dyson MW, Olino TM, Klein DN. Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry. 2013;54(8):854–862. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Oosterlaan J, Heslenfeld DJ, Sergeant JA. Telling good from bad news: ADHD differentially affects processing of positive and negative feedback during guessing. Neuropsychologia. 2005;43(13):1946–1954. doi: 10.1016/j.neuropsychologia.2005.03.018. [DOI] [PubMed] [Google Scholar]