Abstract
Vascular endothelial growth factor (VEGF) is an angiogenic cytokine that plays an important role in tumor growth and progression. Recent evidence suggests an alternate, albeit indirect, role of VEGF on host immune response to tumors. VEGF appears to diminish host immunity by altering the function of major antigen-presenting cells such as dendritic cells (DCs) [D.I. Gabrilovich, T. Ishida, S. Nadaf, J.E. Ohm, D.P. Carbone, Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function, Clin. Cancer Res. 5 (1999) 2963–2970, D. Gabrilovich, T. Ishida, T. Oyama, S. Ran, V. Kravtsov, S. Nadaf, D.P. Carbone, Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo, Blood 92 (1998) 4150–4166, T. Oyama, S. Ran, T. Ishida, S. Nadaf, L. Kerr, D.P. Carbone, D.I. Gabrilovich, Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells, J. Immunol. 160 (1998) 1224–1232.]. DCs are prime initiators of host immunity as they are known to activate both primary as well as secondary immune responses [J. Banchereau, F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.J. Liu, B. Pulendran, K. Palucka, Immunobiology of dendritic cells, Ann. Rev. Immunol. 18 (2000) 767–811.]. However, the exact nature of how VEGF suppresses DC function is not fully clear. In this report, we show that DCs cultured in the presence of VEGF are less potent in stimulating antigen-specific T-cells. Furthermore, by using DCs derived from Id1−/− mice that are defective in Flt-1 signaling, we demonstrated that the inhibitory function of VEGF on DC function is most likely mediated by Flt-1. Thus, the role of VEGF in downregulating host immunity may highlight a unique role of VEGF in the pathogenesis of cancer.
Keywords: VEGF, VPF, Cell signaling, Dendritic cells, T-cells, Id transcription factor
Dendritic cells (DCs) are regarded as the most potent antigen-presenting cells (APCs) with a unique ability to induce and sustain primary immune responses [4,5]. They originate as hematopoietic progenitors in the bone marrow and home to peripheral tissues as immature cells with high phagocytic capabilities. Upon processing foreign antigen, DCs mature and subsequently migrate to lymphoid tissues to initiate the activation of antigen-specific T-cells [6,7].
Several studies have established that DCs are crucial to anti-tumor immunity and that successful cancer immunotherapy relies on their ability to function normally [6]. Tumor escape as a result of defective DC function [1,8–14] is a major underlying cause of defective immunity against cancer. Some of these reports have been linked to the abnormal functional maturation of bone marrow progenitors into DCs in tumor-bearing hosts [13,14].
VEGF is an important cytokine required for the formation of new blood vessels. The development of tumor neovasculature and subsequent tumor angiogenesis has been mechanistically linked to the secretion of large amounts of VEGF to enhance the proliferation of endothelial cells [15]. In addition, Lissoni et al [17] have observed significant low levels of circulating immature and mature DCs in patients suffering from various forms of metastatic cancer who had very high levels of serum VEGF165. Consequently, it is argued that VEGF production in tumor-bearing hosts is directly related to a poor prognosis [16,17], not only because of VEGF-induced angiogenesis, but also due to VEGF-related immunosuppression. Recent evidences have also brought to light the underappreciated, yet important, interplay between VEGF and DCs [17–19]. In this report, we show that DCs cultured in the presence of VEGF have an impaired ability to activate antigen-specific T-cells and that this effect is mediated through the VEGF receptor, Flt-1, on DCs.
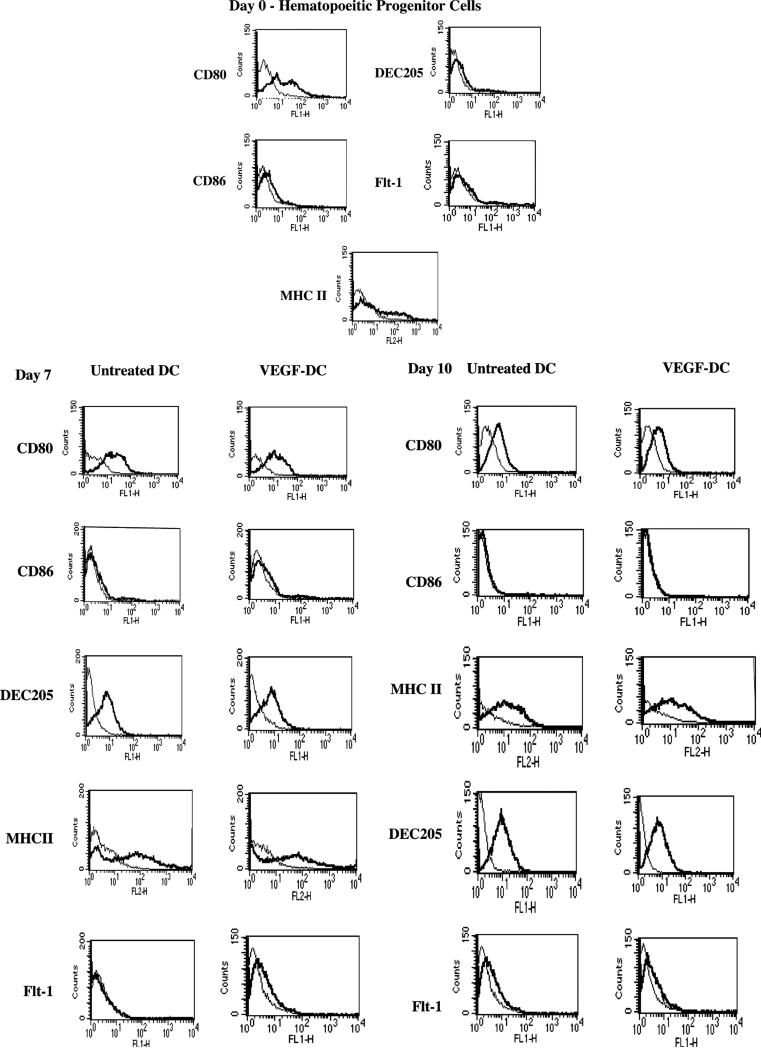
Our initial objective was to determine the phenotypic effect of VEGF on the maturation of mouse bone marrow-derived DCs. Hematopoietic progenitor cells (HPCs) were isolated from normal wild-type mouse bone marrow using the SpinSep dendritic cell enrichment kit (StemCell Technologies, BC, Canada) according to the manufacturer's protocol. The cells were cultured for the respective number of days in DC-specific medium supplemented with the cytokines Stem Cell Factor, GM-CSF, and Flt-3 ligand either in the presence or absence of 20 ng/ml VEGF, which was added every two days. FACS analysis was performed on these freshly isolated HPCs on day 0 and after culture on day 7 and day 10. As illustrated in Fig. 1A, we find there are no significant differences in the expression of the DC markers DEC 205 as well as CD80, CD86, and MHC II in the presence or absence of VEGF. Our results suggest that VEGF treatment does not affect cell-surface expression of DC markers. Since the expression of VEGF receptor, Flt-1, has been shown on CD34+ human HPCs [3,10], we also assessed for Flt-1 expression on murine HPCs, in the presence or absence of VEGF, over a 10-day period. Here again, we did not see any significant changes in Flt-1 expression on DCs cultured in the presence or absence of VEGF (Fig. 1B). Therefore, our results suggest that the effect of VEGF on DCs is at least independent of phenotype.
Fig. 1.
Assessment of phenotypes of VEGF-treated or untreated DCs. Mouse bone marrow-derived DCs were analysed by flow cytometry to ascertain changes in the expression of CD80, CD86, MHC II, DEC205, and Flt-1 on day 0, day 7, and day 10. Day 0 cells, most of which were HPCs, were subjected to FACS immediately upon isolation. For day 7 and day 10, cells were cultured in the presence of cytokines (see Methods) and 20 ng/ml murine recombinant VEGF was added every two days. CD86, MHC II, and DEC205 expression, were low on DC progenitors on day 0 as expected; no differences were observed in their expression in the VEGF-treated as well as the untreated groups. Additionally, no significant changes in Flt-1 expression were observed in the VEGF-treated group by day 7 or day 10.
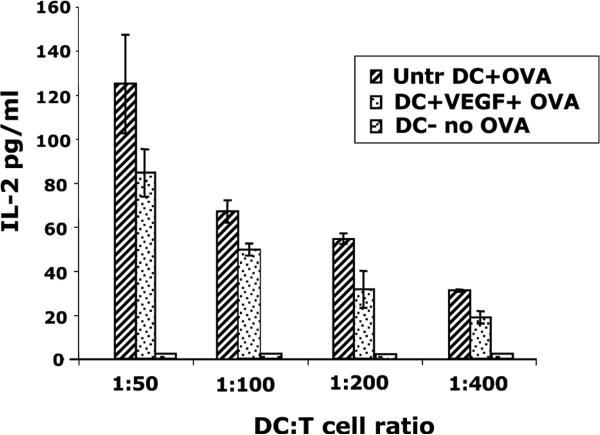
We next examined the effect of VEGF on DC function using an IL-2 secreting OVA-specific T-cell hybridoma, B097.1, which is CD4+ CD8− and is specific for Ova residues 323–339. We added 50 μM ovalbumin to day 7 DCs, cultured in the presence or absence of VEGF, and incubated them overnight. The DCs were irradiated and then mixed with B097.1 T-cell hybridoma, in an antigen-specific assay, at varying ratios and incubated at 37 °C for approximately 96 h to assess IL-2 production. As shown in Fig. 2, IL-2 production by the T-cells decreased 25–30% in the VEGF-treated DC group as compared to untreated DCs, and this was seen over a range of DC-T-cell ratios.
Fig. 2.
IL-2 assay of T-cells stimulated with VEGF-treated DCs. Untreated or VEGF-treated DCs were pulsed with 50 μM ovalbumin overnight, irradiated, and mixed at varying ratios with the Ova-specific T-cell hybridoma, B097.1. Supernatants harvested at approx. 96 h were assayed for IL-2 production. A 25–30% reduction in IL-2 levels by the T-cells stimulated with VEGF-treated DCs suggests that these cells were less able to stimulate T-cells. Untreated non-Ova-pulsed DCs were used as controls. Mean ± SEM of three experiments.
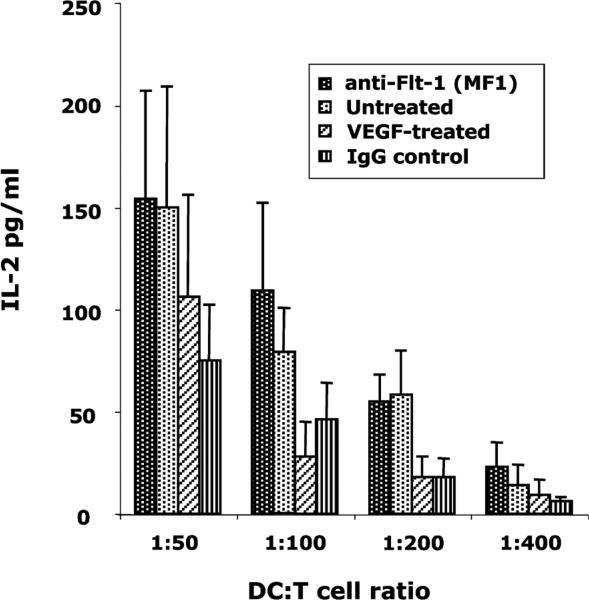
Gabrilovich et al. [10] and others [20] have detected the presence of Flt-1 mRNA in CD34+ human HPCs, and have demonstrated that it binds to VEGF [3]. We wanted to determine whether blocking Flt-1–VEGF binding with anti-Flt-1 antibodies would curtail VEGF action on DCs. Monoclonal anti-mouse Flt-1 antibody, MF1 (5 μg/ml) was added to the HPCs on day 0 as well as every 2 days 1 h prior to VEGF treatment in culture. Irradiated Ova-pulsed DCs on day 7 were mixed with B097.1 T-cells at varying ratios to stimulate IL-2 production. From Fig. 3, it is evident that MF1 successfully abrogated VEGF–DC interaction, restoring IL-2 production by the T-cells to nearly the same levels as that produced by the same cells stimulated with untreated DCs. This result clearly suggests the role of Flt-1 in VEGF–DC interaction.
Fig. 3.
Anti-Flt-1 antibody blocks the inhibitory effect of VEGF on DCs. One hour prior to the addition of VEGF, anti-Flt-1 antibody (MF1) was added to DC cultures and incubated. Isotype-matched normal IgG was used in control treatments. MF1 blocked the effect of VEGF on DCs as the T-cell hybridoma, stimulated with the anti-Flt-1 antibody-treated DCs, produced IL-2 at levels similar to T-cells that were stimulated with non-VEGF-treated DCs. Mean ± SEM of three experiments.
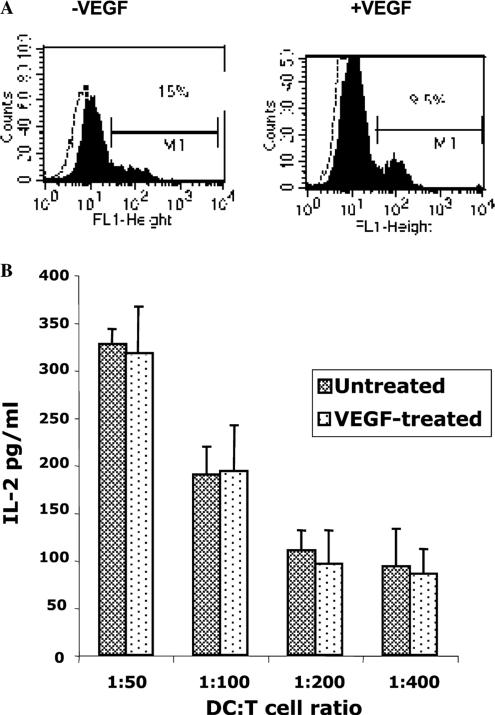
The Id protein family regulates cell cycle progression and differentiation, and Id1 and Id3 have been proven to be necessary for angiogenesis. Id mutant mice display a severe defect in VEGF-induced angio-genesis [21]. To further demonstrate Flt1-mediated VEGF–DC interaction, we used Id1−/− mutant mice that display impaired Flt-1 signaling. At first we wished to determine if DCs derived from Id1−/− mice express Flt-1. Bone marrow-derived DCs from the mutant mice, cultured either in the presence or absence of VEGF for 7 days, were subjected to FACS analyses. It is evident from Fig. 4A that the DCs from these mice do display moderate levels of Flt-1 irrespective of the presence of VEGF. We next performed the antigen-specific assay, as described above, in order to determine the functional ability of the Id1−/− DCs to stimulate T-cells. The DCs were incubated with Ova overnight and mixed with the B097.1 T-cell hybridoma. It was observed that the IL-2 levels produced by the T-cells stimulated with DCs cultured in the presence of VEGF were similar to those stimulated with untreated DCs (Fig. 4B). This suggests that Id1−/− DCs did not respond to the VEGF stimulus because of defective Flt-1 signaling in these mutant mice. Thus, Flt-1 seems to be an important player in VEGF–DC interactions.
Fig. 4.
DCs derived from Id1−/− mutant mice are non-responsive to VEGF treatment. (A) Flt-1 expression in Id1−/− DCs. Day 7 DCs from Id1−/− mice, cultured either in the presence or absence of VEGF, were subjected to FACS analyses to determine their Flt-1 expression. It was observed that these DCs express moderate levels of Flt-1on their surface suggesting that Flt-1 expression in the Id1−/− animals was normal. (B) DCs derived from Id1−/− mutant mice are non-responsive to VEGF treatment. As described under Methods, DCs isolated from Id1−/− mutant mice were treated with 20 ng/ml VEGF every two days. The DCs were then pulsed with ovalbumin, irradiated, and mixed with B097.1 T-cell hybridoma to determine IL-2 production. It was observed that IL-2 levels were almost identical in both the VEGF-treated and the untreated group. This suggests that the inhibitory effect of VEGF on DCs is largely mediated through Flt-1 as the Id1−/− mice are known to display defective Flt-1 signaling. Mean ± SEM of three experiments.
Therefore, from our brief report, we can draw two important conclusions: (1) the phenotype of DCs remains unchanged regardless of VEGF treatment, and (2) VEGF-treated DCs were less able to stimulate antigen-specific T cells. Even though we find that the effect of VEGF on DC maturation is independent of altering cell-surface expression of various molecules such as MHC II, there exists a possibility of abnormal or deficient antigen processing by the DCs under the influence of VEGF, thereby leading to some degree of non-responsiveness by the T-cells. We are not aware of any report that has addressed this issue so far, and hence warrant further investigation. The detrimental impact of VEGF, not only on DCs but also on multiple hematopoietic progenitors [2], highlights the bifunctional nature of this cytokine in pathological as well as normal physiological neoangiogenesis. The ability of the host immune system to mount a robust anti-tumor response is severely limited when DCs, rendered incapable as a result of VEGF-induced tumor angiogenesis, are less able to stimulate T-cell activity. This finding may help us better understand VEGF–DC interplays but requires improved methods to block both VEGF and Flt-1 in order to boost the function of the immune system in cancer.
Methods
Animals
The bone marrows of 5- to 6-week-old C57BL/6 (I-Ab haplotype) mice were used to generate hematopoietic progenitor cells. Id1−/− mutant mice (C57BL6/SV129, also of I-Ab haplotype), a kind gift from Dr. Robert Benezra (Memorial Sloan-Kettering Cancer Center, NY), were bred and raised at the Beth Israel Deaconess Medical Center (Boston, MA) animal facility and also used for bone marrow isolation.
Cell lines and antibodies
The Ova-specific (residues 323–339) T-cell hybridoma B097.1, a CD4+CD8− IL-2 secreting cell line, was a kind gift from Dr. Philippa Marrack (NJMC, CO). The CD80, CD86, and MHC II antibodies used for FACS were purchased from Pharmingen. The DEC205 antibody was purchased from Serotec and the Flt-1 antibody was from Santa Cruz Biotechnologies. The monoclonal blocking antibody for mouse Flt-1 (MF1) was kindly provided by ImClone Systems (NY). Murine IL-2 primary as well as biotinylated secondary antibodies for ELISA were purchased from Pharmingen and HRP-conjugated streptavidin was from R&D systems (Minneapolis, MN).
Cell culture
Bone marrow was flushed from mouse tibias and femurs with RPMI 1640 + 2% FBS and enriched for hematopoietic progenitors using the SpinSep murine dendritic cell enrichment kit (StemCell Technologies, BC, Canada). The cells were plated in Stem-Span Serum Free Expansion Medium (StemCell Technologies) and supplemented with 50 ng/ml Stem Cell Factor (Sigma), 10 ng/ml GM-CSF (Sigma), 100 ng/ml Flt-3 ligand (Sigma), and 20 μg/ml gentamicin (Gibco). For VEGF treatment, 20 ng/ml murine recombinant VEGF (R&D Systems) was added to the cell cultures every 2 days starting from day 0.
Elisa
DCs were pulsed overnight with or without 50 μM ovalbumin (Sigma) and irradiated at 3000 rads from a 137Cs source. They were then mixed with B097.1 T-cell hybridoma cells at varying ratios in 96-well round-bottom plates (Falcon) and incubated at 37 °C in 5% CO2. Supernatants were harvested between 72 and 96 h and added to anti-mIL-2 coated plates. Bound IL-2 was detected using a biotinylated secondary antibody and HRP-conjugated streptavidin.
Acknowledgments
We sincerely thank Dr. Robert Benezra for his generous gift of the Id knockout mice. This work was partly supported by NIH grants CA78383, HL072178, and HL70567 and also a grant from American Cancer Society to D.M.; PO1 AI50157 to D.M.B. S.W.R. was supported by a Fellowship award from the National Kidney Foundation of Massachusetts.
References
- 1.Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin. Cancer Res. 1999;5:2963–2970. [PubMed] [Google Scholar]
- 2.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 3.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J. Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 4.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Ann. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 5.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 6.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Ann. Rev. Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell. Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell. Immunol. 1996;170:111–119. doi: 10.1006/cimm.1996.0140. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 11.Chaux P, Favre N, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int. J. Cancer. 1997;72:619–624. doi: 10.1002/(sici)1097-0215(19970807)72:4<619::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin. Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 13.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 14.Ishida T, Oyama T, Carbone DP, Gabrilovich DI. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J. Immunol. 1998;161:4842–4851. [PubMed] [Google Scholar]
- 15.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 16.Ellis LM, Fidler IJ. Angiogenesis and metastasis. Eur. J. Cancer. 1996;32A:2451–2460. doi: 10.1016/s0959-8049(96)00389-9. [DOI] [PubMed] [Google Scholar]
- 17.Lissoni P, Malugani F, Bonfanti A, Bucovec R, Secondino S, Brivio F, Ferrari-Bravo A, Ferrante R, Vigore L, Rovelli F, Mandala M, Viviani S, Fumagalli L, Gardan GS. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J. Biol. Regul. Homeost. Agents. 2001;15:140–144. [PubMed] [Google Scholar]
- 18.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol. Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 19.Bonfanti A, Lissoni P, Bucovec R, Rovelli F, Brivio F, Fumagalli L. Changes in circulating dendritic cells and IL-12 in relation to the angiogenic factor VEGF during IL-2 immunotherapy of metastatic renal cell cancer. Int. J. Biol. Markers. 2000;15:161–164. doi: 10.1177/172460080001500206. [DOI] [PubMed] [Google Scholar]
- 20.Hoehn GT, Stokland T, Amin S, Ramirez M, Hawkins AL, Griffin CA, Small D, Civin CI. Tnk1: a novel intracellular tyrosine kinase gene isolated from human umbilical cord blood CD34+/Lin–/CD38– stem/progenitor cells. Oncogene. 1996;12:903–913. [PubMed] [Google Scholar]
- 21.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]