Abstract
Neuroregeneration is the regrowth or repair of nervous tissues, cells, or cell products involved in neurodegeneration and inflammatory diseases of the nervous system like Alzheimer’s disease and Parkinson’s disease. Nowadays, application of nanotechnology is commonly used in developing nanomedicines to advance pharmacokinetics and drug delivery exclusively for central nervous system pathologies. In addition, nanomedical advances are leading to therapies that disrupt disarranged protein aggregation in the central nervous system, deliver functional neuroprotective growth factors, and change the oxidative stress and excitotoxicity of affected neural tissues to regenerate the damaged neurons. Carbon nanotubes and graphene are allotropes of carbon that have been exploited by researchers because of their excellent physical properties and their ability to interface with neurons and neuronal circuits. This review describes the role of carbon nanotubes and graphene in neuroregeneration. In the future, it is hoped that the benefits of nanotechnologies will outweigh their risks, and that the next decade will present huge scope for developing and delivering technologies in the field of neuroscience.
Keywords: neuroregeneration, neurodegeneration, nanomedical, carbon nanotube, graphene, nanodrug delivery
Introduction
Neurodegenerative pathologies occur when the basic units of the nervous system, ie, neurons in the brain and spinal cord, start to deteriorate. Alterations in these nerve cells cause them to function abnormally, which results in the demise of the cell. Initial symptoms of neuronal deterioration may include loss of coordination or the ability to remember names. These problems progress to an advanced stage if a large number of neurons deteriorate. The patients may lose the ability to think and move on their own, and these disease are mostly incurable.1,2
Neurodegenerative diseases affect over 90,000 people every year. Among the neurodegenerative diseases, spinal cord injuries alone affect 10,000 people each year. Neurodegenerative pathologies affect millions of people worldwide. Alzheimer’s disease (AD) and Parkinson’s disease (PD) are the most common neurological diseases and occur in 5 million and 1.2 million Americans, respectively.3 If left unchecked, 30 years from now it is expected that more than 12 million Americans will suffer from neurodegenerative disease. Therefore, there is an urgent need to find therapies and a cure for these neurodegenerative pathologies.4,5
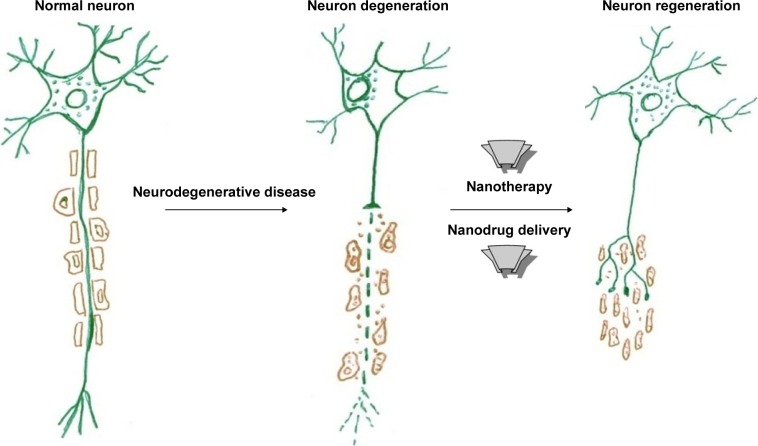
Neuroregeneration is the restoration of neurons that have deteriorated as a result of neurodegenerative diseases. Neuroregeneration denotes the regrowth or repair of degenerated nervous tissues and nerve cells involved in the production of new neurons, glia, axons, myelin, and synapses. The functional mechanism of neuroregeneration differs between the peripheral nervous system (PNS) and the central nervous system (CNS). While the PNS has an inherent capability for self-repair and regeneration, the CNS is unable to self-repair and regenerate.6 Neuroregeneration is significant clinically, because it is a regenerative mechanism involving the damaged neuron that provides mechanical support for regeneration (Figure 1).
Figure 1.
Application of nanotherapy to degenerated neuron for regeneration.
The development of nanomaterials, especially carbon nanotubes (CNTs) and graphene, which can advance neuronal growth, is likely to have a major impact on neuroregenerative techniques used in the future for neurodegenerative pathologies such as AD and PD. This paper reviews the most common degenerative pathologies and discusses the application of CNTs and graphene nanomaterials as tissue engineering scaffolds for neuroregeneration and nanodrug delivery aiming at neuroprotection and functional regeneration of neurons in the CNS.
Neurological disorders
The basic unit of the brain is the neuron, which consists of a cell body, dendrites, and a longer extension of the cell body known as the axon. The dendrites transmit signals from their tips toward the neuron cell body, whereas the axon carries messages away from the cell body toward the terminal end of the axon. The distal tips of the axon are used to communicate with other cells, eg, effector cells. Bundles of axons from a collection of neurons are called nerves, which transmit signals in the same direction and serve as an intermediary connection between the brain and effector cells. Thus, if there is any damage to the nerve, transmission of signals between the cell body and effector cells is interrupted, and the neurons are unable to convey signals effectively.
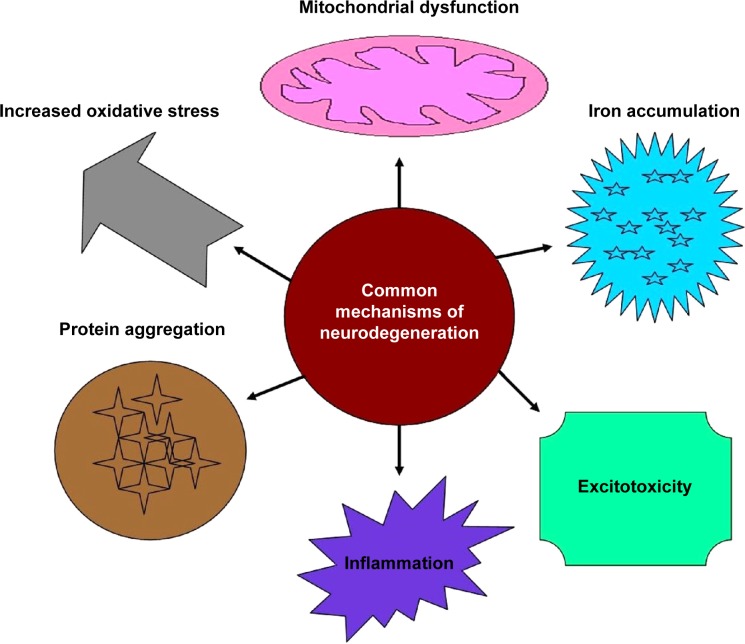
Neurological disorders result from functional abnormality or disturbance of the nervous system, are assessed by neurological examination, and are treated within the specialties of neurology and clinical neuropsychology. AD and PD are the most common types of neurodegenerative disease7 and are briefly outlined in this review. Common mechanisms of neurodegeneration include dysfunction of the mitochondria, increased oxidative stress levels, excitotoxicity, aggregation of proteins, accumulation of iron, and inflammatory changes (Figure 2).
Figure 2.
Common mechanisms involved in neurodegeneration.
Alzheimer’s disease
AD is thought to have a genetic basis in approximately 70% of cases, and is characterized by loss of neurons and synapses in the cerebral cortex and subcortical areas. This loss of neurons may result in degeneration of the affected area, including the temporal lobe, parietal lobe, parts of the frontal cortex, and the cingulate gyrus.8 Accumulation of unusually folded amyloid beta and tau proteins in the brain leads to formation of plaques that cause AD, with the disease now being recognized as a protein misfolding condition, ie, a proteopathy.9 These plaques are made up of small peptides and amino acids called amyloid beta, which is part of the larger amyloid precursor protein that penetrates through the membrane of the neuron and is an important factor for the growth of the neuron and post-injury repair.10,11
Parkinson’s disease
Also known as idiopathic or primary parkinsonism, hypokinetic rigid syndrome, or paralysis agitans, PD is the second most widespread neurodegenerative disorder and manifests as bradykinesia, rigidity, resting tremor, and postural instability. PD is a degenerative pathology of the CNS and occurs as a result of death of dopamine-generating cells in the midbrain, mainly those in the substantia nigra. The cause of this cell death is unknown. Possible diagnostic approaches include detection of biomarkers and the molecular mechanisms leading to PD. Therapeutic goals for PD involve neuroprotection, eg, preventing injury caused by free radicals and lowering levels of metal ions in the brain by administration of chelators, as well as neuroregeneration, eg, activation of neuronal progenitor cells and more effective drug delivery systems that can penetrate the blood–brain barrier. Nanotechnology has a key role in developing these new diagnostic and therapeutic tools for PD.12 Accumulation of alpha-synuclein protein in the Lewy bodies of neurons, along with inadequate formation and activity of dopamine in neurons, is one of the major manifestations of the disease.13,14
Role of nanotechnology in neuroprotection
Preservation of the structure and function of neurons is called neuroprotection. This strategy is a popular choice in patients suffering from CNS disorders such as stroke, neurodegenerative diseases, and other brain injuries in order to halt, or reduce the loss of neurons. The mechanisms underlying neurodegeneration are the same in different CNS disorders despite their different signs and symptoms.15 Neuroprotective treatments often target oxidative stress and excitotoxicity that are related to disorders of the CNS. A combination of oxidative stress and excitotoxicity causes lysis of neurons, and preventing these is an essential aspect of neuroprotection. Glutamate antagonists and antioxidants are neuroprotective treatments that aim to limit excitotoxicity and oxidative stress, respectively.16 Neurons can be protected from degenerative disorders by using neuroprotective drugs. Steroids such as methylprednisolone can be used to prevent secondary damage and to reduce the neuroinflammation caused by spinal cord injury, and Sygen® (Fidia SpA, San Mauro Torinese, Italy), a recently discovered drug, appears to reduce the loss of nerve function.17
The advantages of nanotechnology in the treatment of PNS and CNS disorders are enormous, and may eventually offer the patient and clinician more treatment options. The combination of development of synthetic and characterization methods in chemistry and materials science, and improvement of nanoengineered applications in the nervous system, along with a detailed study of neurophysiology and neuropathology, should be done.18 CNTs and graphene are promising candidates in a number of areas of nanomedicine, especially in the field of neurology. The applications of CNTs and graphene in neuroregeneration and the nanotechnologies used for restoring neuronal cells are discussed in the following sections.
Carbon nanotubes
CNTs are allotropes of carbon with a cylindrical nanostructure. Nanotubes are part of the fullerene family, members of which have a long hollow structure made of sheets one atom thick. In the case of CNT, carbon is the atom present in these sheets. Once rolled, these sheets become nanotubes. The rolling angles are chiral and the radius of the tube determines the properties of the CNT. CNTs are further classified as single-walled nanotubes (SWNTs), which consist of a single sheet of carbon rolled up into a tubular-like structure, and multi-walled nanotubes (MWNTs), which consist of multiple layers of carbon sheets. In the future, CNT-based technologies are likely to be particularly useful for promoting functional recovery of neurons after brain damage due to the outstanding physical properties of these nanomaterials together with their recently documented ability to interface with neuronal circuits, synapses, and membranes.19
Pristine CNTs are insoluble in most aqueous solvents, but chemical modification or functionalization of the nanotube surface may improve their aqueous solubility, enabling their use in the treatment of CNS pathology.20 Non-functionalized CNTs decrease cell viability, and an inflammatory response has been observed with pristine CNTs. CNTs (0.5 mg) have toxic effects on the respiratory system, that include formation of granuloma in the lungs.21 In addition, aggregation of CNTs via van der Waals’ interactions can affect profibrogenic cellular responses and contribute to the pulmonary toxicity of CNTs in vivo.22 Oxidative stress is the main mechanism of toxicity, but agglomeration, chirality, and impurities are some of the structural and chemical characteristics contributing to the toxicity of CNTs,23 and it has been reported that functionalized CNTs do not have harmful effects.
The above limitations can be resolved by chemical modification of CNTs, making them a promising nanoengineered biomaterial for biomedical application.24,25 CNTs can be chemically functionalized covalently or non-covalently.26 The mechanism of non-covalent modification is mainly related to coating of the CNT using hydrophilic macromolecules such as surfactants, polymers, peptides, or single-stranded DNA. This coating or wrapping technology enables preservation of the aromatic structure of the CNT without harmful effects on their electronic characteristics.27 Covalent functionalization of CNTs involves chemical reactions, which can be achieved in two ways, ie, sidewall covalent conjugation of functional groups and oxidation of CNT followed by modification. The first approach, ie, sidewall covalent conjugation of organic functional groups, may be achieved by chemical reactions, particularly 1,3-dipolar cycloaddition of azomethine ylides. Georgakilas et al obtained highly soluble CNTs in aqueous solution by attaching amino functional groups to the tips and sidewalls of the CNTs.20 On the other hand, oxidation and further functionalization leads to purified and shorter CNTs. Esterification or amidation reactions further derivatize the attached carboxylic acid groups into other functional groups. Thus, chemical modification of CNTs is a powerful tool that can be used to design substrates that can control the growth and morphology of neuronal cells.
CNTs in neuroregeneration
Acute neurological disorders are a destructive actions that harm human biological functioning. Despite the abundance of published studies and established findings, the treatments available for neurodegenerative diseases are limited and more investigation is needed to explore the mechanisms involved in neurological disorders. A large number of CNS pathologies, in particular AD, PD, stroke, traumatic brain injury, and spinal cord injury may lead to failure of brain and spinal cord cells.28 The two most important approaches to promoting self-repair of damaged axonal connections are regrowth of axons and/or reorganization of the neuronal circuitry. Therefore, the requirements for successful regenerative engineering are preservation of neurons (neurorestoration), promotion of an environment conducive to regrowth of the degenerated neurons (neurogenesis), and reconnection of neuronal circuits, followed by promotion of the plasticity of neuronal tissue (neuroplasticity).29
The clear understanding of the nervous system and improvement of healing approaches for neurological intervention are believed to have a significant effect in neuroscience research due to the advancements in nanomedicine.30 Zhang et al have demonstrated the electrical conductive capacity and strong mechanical properties of CNTs, and shown the morphological characteristics of CNTs to be similar to those of neurons.31 In addition, the structure and dimensions of CNTs resemble those of certain neuronal structures, such as ion channels, signaling proteins, and elements of the neuronal cytoskeleton. The resemblance of CNTs to these neuronal elements may have an additional benefit with regard to enhancing neuronal interaction at the molecular level and accordingly improve management of the physiological activity of neurons and processing of neuronal information.32 Mattson et al described how hippocampal neurons in rats could grow on MWNT coated with 4-hydroxynonenal, a bioactive component, and confirmed the biocompatibility of CNT as a promising candidate for neuroregeneration.33 Neuronal growth patterns and properties, such as length, branching, and numbers of growth cones were based on the chemical modification techniques used to functionalize the CNT. Of the various chemical functionalization methods possible, MWNTs coated with poly(ethylene glycol) diamine resulted in a higher number of growth cones and neurite branches.34 In the same way, SWNTs coated with polyethylenimine (PEI) promoted growth of neurites and their branches to a greater extent than the PEI substrate alone.35 Matsumoto et al reported that MWNTs coated with nerve growth factor or brain-derived neurotrophic factor could induce growth of neurons on a CNT scaffold, and also controlled the differentiation and continued viability of the neurons.36 Lovat et al confirmed that elongation of dendrites and hippocampal neuron cell adhesion were enhanced by incorporating a purified MWNT substrate. Enhancement of dendrite elongation results in improved signal processing of neurons.37 Similarly, Mazzatenta et al suggested that purified SWNTs promote growth of hippocampal neurons and develop the neuronal circuits. Increased transmission of electrical signals within the neuronal network leads to notable growth of the neuronal circuit.38 Jan et al confirmed the compatibility of CNT substrates with neural stem cells (NSCs), based on cell viability and development of neuronal processes identical to those seen with the normally used growth substrate poly-L-ornithine.39 The efficacy of CNTs was demonstrated by successful delivery of NSCs to injured sites of the CNS and their differentiation into neurons.
Novel and improved nanoscaffolds have been developed to repair or improve the functioning of neural tissue by providing mechanical support to the neurons. Because of their electrical and mechanical characteristics, along with their neuronal biocompatibility, CNTs are considered to be promising candidates for neural tissue engineering.40 Roman et al demonstrated the applications of CNTs in the treatment of neurodegenerative disease. PEG-modified SWNTs were successfully used for neuroregeneration in a rat model of Sprague Dawley. The efficiency of the CNT technique was confirmed by a decrease in the volume of damaged tissue and an increase in the number of neurofilaments in the area surrounding the site of injury, along with functional healing of the rat hind limb. This study confirms that CNT-based substrates can promote ongoing regeneration of damaged neurons and neural tissues, giving rise to new perspectives in the field of neuroregeneration.41
CNTs in drug delivery
Neuroprotection could be achieved in the future by use of nanodrug delivery in chronic neurological disorders. Delivery of drugs across the blood–brain barrier is attained by the application of nanotechnology in therapeutic techniques, known as nanomedicine.42 Recent research shows that the nanomedicine required for neurodegenerative pathologies is much less than that needed for cancer and infectious diseases.43 Thus, emerging nanotechnologies for production of neurotrophin delivery systems are promising in terms of their ability to activate neurotrophin signaling for neuroprotection and neuroregeneration.44 Neurotrophins are proteins that were initially recognized as being factors related to the survival of sympathetic and sensory neurons. Neurotrophins are essential for the development and function of neurons in both the CNS and PNS, and can be delivered using CNTs.45
The use of CNTs as a delivery mechanism for the treatment of CNS pathology is based on their structural features, especially their improved solubility in physiological solvents (even though not a heterogeneous solution) due to their functionalization, large surface area, ability to be easily modified with drug molecules, and biocompatibility with neural systems. Zhang et al used SWNT modified with acetylcholine to treat AD. After gastric gavage, SWNT doses up to 300 mg/kg could enable delivery of drug into the lysosomes of neurons, thus demonstrating the effectiveness of this therapy.46 Dealing with brain tumors is a challenge regardless of the therapeutic advances made with the clear understanding of carcinogenesis. Anti-tumor drug molecules have low permeability across the blood–brain barrier, and this has opened up new possibilities for CNT-based approaches. In one study, a drug delivery system using CNTs considerably enhanced the effects of CpG oligodeoxynucleotide immunotherapy in the treatment of glioma (a tumor arising from the glial cells) and preventing tumors.47 CNT-based therapy would be useful in the treatment of a number of neurodegenerative pathologies. SWNT functionalized with amine groups via the amidation reaction enhances the tolerance of neurons to ischemic injury. Using this method, neurons are protected and their functions are regained with amine-modified SWNT without therapeutic or drug molecules. The mechanism via which amine-functionalized SWNTs protect neurons is as yet unclear.48 A study by Al-Jamal et al demonstrated the effectiveness of amino-functionalized MWNTs in delivery of small interfering RNA that decreased apoptosis at the injury site and promoted recovery in a rodent model of endothelin-1 stroke.49 The findings of research on the use of CNTs for neuroregeneration are summarized in Table 1.
Table 1.
Evidence for application of carbon nanotubes in neuroregeneration
| Type of CNT | Findings | References |
|---|---|---|
| SWNT, MWNT | Morphological characteristics of CNT were similar to neurons | 31 |
| SWNT, MWNT | CNT structural features resemble neuronal elements | 32 |
| MWCT coated with 4-hydroxynonenal | Compatible for nerve cell growth | 33 |
| MWNT coated with polyethylenediamine | Large numbers of growth cones and neurite branches | 34 |
| SWNT chemically functionalized with polyethylenimine | Promotes more neuronal branching than polyethylenimine alone | 35 |
| MWNT coated with nerve growth factors | Controls differentiation and survival of neurons | 36 |
| Purified MWNT | Promotes dendrite elongation and signal processing | 37 |
| Purified SWNT | Improves information processing of neurons | 38 |
| SWNT, MWNT | Compatible with neural stem cells, neural processes were developed | 39 |
| SWNT, MWNT | Effective delivery of neuronal stem cells, supports their differentiation into neurons | 40 |
| SWNT chemically modified with PEG | Successful regeneration of axon | 41 |
| SWNT wrapped up with acetylcholine | Safe delivery of drugs to the neuron | 46 |
| SWNT, MWNT | Effective drug delivery leads to enhanced anti-tumor protection | 47 |
| Amine-functionalized SWNT | Valuable treatment for neurodegenerative diseases, improves tolerance of neurons to ischemic injury | 48 |
| Amino-functionalized MWNT | Diminish apoptosis in affected area, advances behavioral recovery | 49 |
Abbreviations: CNT, carbon nanotube; MWNT, multi-walled nanotube; PEG, poly(ethylene glycol); SWNT, single-walled nanotube.
Graphene
Graphene, like CNT, is a form of nanocarbon. Graphene is composed of a single layer of carbon atoms arranged in a hexagonal honeycomb lattice, and has unique mechanical, optical, thermal, electronic, and magnetic properties. A recent analysis highlighted the unique properties of graphene and their promise in terms of opening up further research avenues in neurotherapeutics, including neuro-oncology, neuroimaging, neuroregeneration, functional neurosurgery, neurointensive care, spinal surgery, and peripheral nerve surgery.50 Initial standard testing has already demonstrated that new biosensors based on graphene technology have improved accuracy in the detection of glucose levels, DNA, peptides, and important enzymes involved in the synthesis of neurotransmitters, such as acetylcholinesterase.51
The large surface area available and the possibility of conjugating different biomolecules onto its surface makes graphene a suitable nanoscaffold for holding small-molecule drugs, genes, antibodies, proteins, DNAs, and small interfering RNAs.52 In addition, it is possible to modify the chemical structure of graphene by attachment of reactive functional groups, such as amino, carboxyl, hydroxyl, alkyl halogen, or azide groups. Graphene-based materials generally aggregate in aqueous medium containing salts, proteins, or other ions, and require chemical modification or functionalization to have the desired properties. Such functionalization enables researchers to change the basic electrical and optical properties of graphene. These modifications also allow conjugation of contrast agents, antibodies, peptides, ligands, drugs, and genes to the surface of graphene nanoparticles. These chemical modifications may provide the means for further customization of graphene, so that it may be used to detect other essential physiological changes associated with brain metabolism.53 It has been shown that amalgamation of specific functional chemical groups onto graphene may help otherwise insoluble drugs to become soluble, while regaining their anticancer activity. A study by Liu et al established that addition of a PEG chain to graphene oxide (GO) nanoparticles allows further noncovalent binding of hydrophobic drugs through π–π stacking.54 Thus, chemical functionalization to modify graphene plays a vital role in new drug therapies designed to overcome the blood–brain barrier when targeting the CNS.
Graphene in neuroregeneration
Studies in animal models of peripheral nerve transection indicate that electrical stimulation at lower frequencies of approximately 2 Hz has a favorable effect on neuronal regrowth.55 Because of the electrical characteristics of graphene, it is suitable for the design of electroactive scaffolds that may be able to transmit the externally applied electrical signal to promote neuroregeneration.56 It has been proposed that three-dimensional porous graphene scaffolds provide a unique environment for future neuroregenerative therapies relating to NSCs. These porous scaffolds have been shown to improve the differentiation of NSCs and functional neurons.57 Sahni et al showed high compatibility between immobilized graphene surfaces and neurons, which was confirmed by the response of the neuronal cells to the surface of graphene. Such graphene surfaces were adhered to uncoated poly (D-lysine) surfaces, with reduced cytotoxicity.58 In a culture model of the mouse hippocampus, it was shown that graphene scaffolds are not only compatible with a neural interface but also increase branching and regrowth of the neuronal circuit when compared with conventional strategies. Graphene scaffolds increased neuronal cell counts and the average length of the neuron 7 days after cell seeding when compared with neuron cultures on a polystyrene substrate. Western blot analysis also confirmed that graphene established higher levels of growth associated protein 43, an indicator of neuronal growth.59 Even though some studies have suggested that graphene scaffolds have satisfactory biocompatibility with neuronal elements, some biocompatibility issues remain, in particular regarding the use of granulated GO-based or graphene-based nanoparticles. Wang et al demonstrated that lower doses (0.10–0.25 mg) of soluble GO were not noticeably toxic in mice, but higher doses (approximately 0.40 mg) did cause some toxicity due to granuloma formation in the lung, liver, spleen, and kidneys.60 In addition, there was significant cytotoxicity when fibroblasts were cultured at higher doses of graphene (>50 mg/mL), with graphene particles penetrating into the lysosomes, mitochondria, endoplasm, and cell nuclei, causing poor cell adhesion. Another study suggested that the irregular edges and protrusions generally present in graphene microsheets may penetrate cells. These features of graphene raise some safety concerns because sharp edges may potentially lead to disruption of the cytoskeleton, impaired cell motility, and other harmful effects.61 Specific developments in neuroregeneration may result if the high penetration capacity of graphene layers is carefully explored in the experimental setting.
The brain–computer interface, also known as the brain–machine interface, is a leading field of research which significantly benefits graphene-based technologies. This technology attempts to combine neural signal processing and advanced robotic engineering to assist patients with stroke, spinal cord injury, or limb loss. Artificial patient-controlled prosthetic devices and artificial simulation of the CNS play a major role in this developing technology.62 The electrical fields not only stimulate nerves but also perform therapeutic function and help to regenerate central and peripheral nerves. The therapeutic role of electrical simulation has been experimentally proven to increase growth rates, promote alignment of astrocytes, initiate myelin repair, and improve the spinal cord’s function after injury.63
Graphene in drug delivery
The high specific surface area, π–π stacking, and electrostatic or hydrophobic interactions of graphene can be exploited to achieve high drug loading of poorly soluble drugs without compromising potency and efficiency. Graphene and GO, with their advantageous properties, have recently emerged as new and competitive drug delivery systems with the potential to be applied in systemic, targeted, and local drug delivery systems.64 More efficient and safe gene and drug delivery vectors are required to treat genetic disorders like PD, AD, cystic fibrosis, and cancer, and the DNA should be devoid of degradation while facilitating high transfection.65 The carbon allotrope graphene has been investigated as a vector for gene, gene-drug, and protein delivery. In one experiment, graphene was functionalized with the cationic polymer PEI and used for delivery of drugs. PEI is a non-viral gene vector that has strong electrostatic interaction with the negatively charged phosphates of RNA and DNA and may be chemically modified. Chemical modification of PEI leads to increased transfection efficacy and cell selectivity, and a decrease in cytotoxicity. However, the biocompatibility of PEI remains a limitation to its biomedical application.66 Chen et al used PEI-functionalized GO for gene delivery and found that the cytotoxicity of PEI-GO was much better than that of PEI alone. PEI-functionalized GO helped to achieve high transfection of gene-drug.67 In recent studies, graphene foams have been used as three-dimensional scaffolds in NSC cultures. These foams were found to be capable of electrically stimulating NSCs and to promote proliferation and differentiation of NSCs.57 The documented findings related applications of graphene in neuroregeneration are shown in Table 2.
Table 2.
Findings of the applications of graphene in neuroregeneration
| Form of graphene | Findings | References |
|---|---|---|
| Graphene scaffold | Allows conjugation with pharmacologically active molecules | 52 |
| Modified graphene nanoparticles | Customized graphene structure detects variations in brain metabolism | 53 |
| Graphene modified with PEG | Increases solubility and allows attachment of hydrophobic drugs | 54 |
| Graphene in electrical stimulation | Excellent electroconductive properties of graphene stimulates neuronal growth | 55 |
| Graphene scaffold | Electrical stimulation enhances neuroregeneration | 56 |
| Porous graphene scaffold | Enhances differentiation of neural stem cells | 57 |
| Immobilized graphene | Outstanding biocompatibility with neurons | 58 |
| Graphene scaffold | Improves neuronal outgrowth and length of neuron Higher level of growth protein |
59 |
| Graphene oxide | High doses causes chronic toxicity due to formation of granuloma | 60 |
| Graphene microsheets | Irregular edges penetrate the cell, leading to impaired cell motility and cytoskeletal disruption | 61 |
| Graphene in neuroprosthetics | Stimulates CNS artificially to regain neural signal processing and function | 62 |
| Graphene in electrical stimulation | Directs alignment of astrocytes and initiates myelin repair | 63 |
| Graphene and graphene oxide | Excellent targeted and local drug delivery due to their advantageous properties | 64 |
| Graphene | Protects DNA from degradation | 65 |
| Graphene functionalized with polyethylenimine | Increased transfection efficiency and reduced cytotoxicity | 66 |
| Graphene oxide functionalized with polyethylenimine | Increased transfection efficiency and reduced cytotoxicity | 67 |
| Graphene scaffold | Supports neural stem cell proliferation and differentiation | 57 |
Abbreviations: CNS, central nervous system; PEG, poly(ethylene glycol).
Discussion
Neuroprotection and neuroregeneration are the subject of a vast and dynamic field of research. The elderly population is the main target in the case of neurodegeneration, with ageing of the brain as a principal factor. Study of the cellular and molecular mechanisms involved in the neurodegeneration and neuroregeneration of the ageing brain could unmask new therapeutic approaches to reduce the degradation of neurons. Successful management of AD and PD may be achieved by developing novel and efficient therapies that sustain the self-repair ability of the brain.
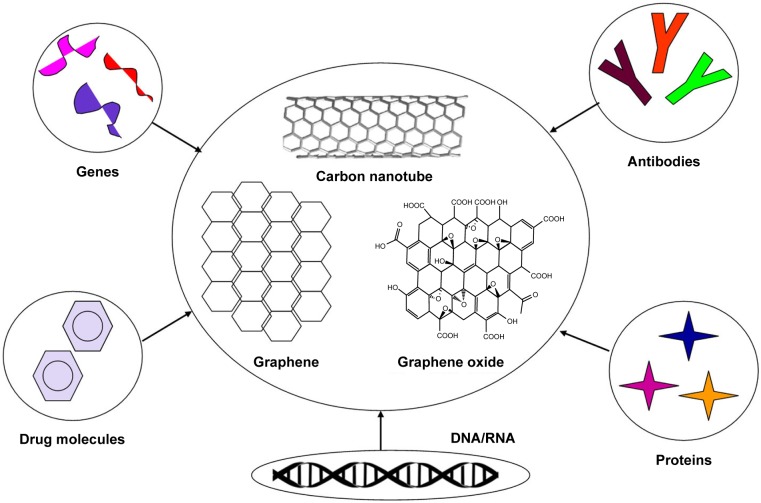
On the basis of the studies reported in the literature, a wide range of nanotechnologies has been designed to assess the functional outcomes of CNTs and graphene when used as scaffolds to provide mechanical support for neural tissues, and to deliver drugs, nerve growth factors, antibodies, and proteins to a particular area of the brain in AD and PD to stimulate regeneration of neurons (Figure 3). Nanotechnological solutions based on CNT and graphene could be expensive to implement for neuroprotection and neuroregeneration, so researchers should be looking at the cost-effectiveness of treatments for neural disorders that involve use of CNT and graphene.
Figure 3.
Overall scheme of carbon nanotube, graphene, and graphene oxide for nanotherapeutic drug delivery.
Some in vivo studies have assessed the toxic effects of CNTs that have accidentally penetrated the body and probably translocated in the CNS. CNT have been reported to have toxic effects on dorsal root ganglion neurons and to induce membrane damage.68,69 On the other hand, the irregular edges of graphene may penetrate the cells, leading to cytoskeletal disruption, with higher doses causing granuloma in the lungs, kidneys, and liver. In addition to these limitations, use of CNT and graphene is steadily increasing worldwide. The potentially toxic outcomes of using CNT and graphene need to be studied in depth in mouse hippocampal neuron models before we embark on clinical trials, to prevent adverse clinical and environmental effects.
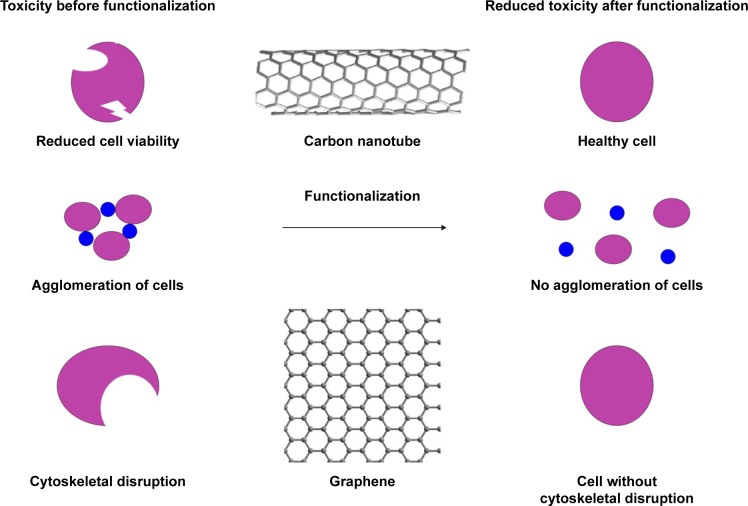
Functionalized CNTs provide enhanced solubility, and improve biocompatibility and mechanical properties. The π–π stacking and large surface area of modified graphene potentially enable it to be used for local and targeted drug delivery in AD and PD, and with reduced toxicity. The improved cytocompatibility of CNTs and graphene after functionalization is shown in Figure 4. Toxicity issues must be overcome to fulfill the promise of CNTs and graphene for nanotechnological application in neuroscience. Further research may show that the effectiveness of nanotechnologies can outweigh their risks, and the next decade will present huge scope for developing and delivering technologies in the field of neuroscience.
Figure 4.
Improved cytocompatibility of carbon nanotube and graphene after functionalization.
Acknowledgments
This work was supported in part by the Ministry of Higher Education Malaysia (grant R.J130000.7809.4F444, reference PY/2014/03167).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 2.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajetunmobi A, Prina-Mello A, Volkov Y, Corvin A, Tropea D. Nanotechnologies for the study of the central nervous system. Prog Neurobiol. 2014;123:18–36. doi: 10.1016/j.pneurobio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Stabenfeldt SE, Garcia AJ, Laplaca MC. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77:718–725. doi: 10.1002/jbm.a.30638. [DOI] [PubMed] [Google Scholar]
- 5.Prang P, Müller R, Eljaouhari A, et al. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Kandel E, Schwartz J, Jessell TM. Principles of Neural Science. 4th ed. New York, NY, USA: McGraw-Hill; 2000. Medical. [Google Scholar]
- 7.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 8.Wenk GL. Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 9.Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- 10.Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 12.Fluri F. Nanomedical approaches in Parkinson’s disease. Eur J Nanomed. 2009;2:48–53. [Google Scholar]
- 13.Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 14.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidl SE, Potashkin JA. The promise of neuroprotective agents in Parkinson’s disease. Front Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zádori D, Klivényi P, Szalárdy L, Fülöp F, Toldi J, Vécsei L. Mitochondrial disturbances, excitotoxicity, neuroinflammation and kynurenines: novel therapeutic strategies for neurodegenerative disorders. J Neurol Sci. 2012;322:187–191. doi: 10.1016/j.jns.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Brown University. Division of Biology and Medicine Nerve Regeneration. [Accessed June 21, 2015]. Available from http://biomed.brown.edu/Courses/BI108/BI108_2001_Groups/Nerve_Regeneration/Introduction/Introduction.htm.
- 18.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood–brain barrier. Drug Dev Ind Pharm. 2002;28:1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 19.Fabbro A, Prato M, Ballerini L. Carbon nanotubes in neuroregeneration and repair. Adv Drug Deliv Rev. 2013;65:2034–2044. doi: 10.1016/j.addr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. Organic functionalization of carbon nanotubes. J Am Chem Soc. 2002;124:760–761. doi: 10.1021/ja016954m. [DOI] [PubMed] [Google Scholar]
- 21.Tang S, Tang Y, Zhong L, et al. Short- and long-term toxicities of multi-walled carbon nanotubes in vivo and in vitro. J Appl Toxicol. 2012;32:900–912. doi: 10.1002/jat.2748. [DOI] [PubMed] [Google Scholar]
- 22.Fisher C, Rider AE, Han ZJ, Kumar S, Levchenko I, Ostrikov K. Applications and nanotoxicity of carbon nanotubes and graphene in biomedicine. J Nanomater. 2012;2012:31518. [Google Scholar]
- 23.Rodriguez-Yañez Y, Muñoz B, Albores A. Mechanisms of toxicity by carbon nanotubes. Toxicol Mech Method. 2013;23:178–195. doi: 10.3109/15376516.2012.754534. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Zhu Y, Liao S, Li J. Carbon nanotubes reinforced composites for biomedical applications. Biomed Res Int. 2014;2014:518609. doi: 10.1155/2014/518609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyawaki J, Yudasaka M, Azami T, Kubo Y, Iijima S. Toxicity of single-walled carbon nanohorns. ACS Nano. 2008;2:213–226. doi: 10.1021/nn700185t. [DOI] [PubMed] [Google Scholar]
- 26.Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chem Rev. 2006;106:1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Jagota A, Semke ED, et al. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 28.Nunes A, Al-Jamal K, Nakajima T, Hariz M, Kostarelos K. Application of carbon nanotubes in neurology: clinical perspectives and toxicological risks. Arch Toxicol. 2012;86:1009–1020. doi: 10.1007/s00204-012-0860-0. [DOI] [PubMed] [Google Scholar]
- 29.Ellis-Behnke R. Nano neurology and the four P’s of central nervous system regeneration: preserve, permit, promote, plasticity. Med Clin North Am. 2007;91:937–962. doi: 10.1016/j.mcna.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Gilmore JL, Yi X, Quan L, Kabanov AV. Novel nanomaterials for clinical neuroscience. J Neuroimmune Pharmacol. 2008;3:83–94. doi: 10.1007/s11481-007-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Webster TJ. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 32.Cellot G, Cilia E, Cipollone S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4:126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14:175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 34.Hui Hu YN, Vedrana M, Haddon RC, Parpura V. Chemically functionalized carbon nanotubes as substrates for neuronal growth. Nano Lett. 2004;4:507–511. doi: 10.1021/nl035193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui Hu YN, Ni YC, Mandal SK, et al. Polyethyleneimine functionalized single walled carbon nanotubes as a substrate for neuronal growth. J Phys Chem B. 2005;109:4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Sato C, Naka Y, Kitazawa A, Whitby RL, Shimizu N. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J Biosci Bioeng. 2007;103:216–220. doi: 10.1263/jbb.103.216. [DOI] [PubMed] [Google Scholar]
- 37.Lovat V, Pantarotto D, Lagostena L, et al. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5:1107–1110. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- 38.Mazzatenta A, Giugliano M, Campidelli S, et al. Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. J Neurosci. 2007;27:6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jan E, Hendricks JL, Husaini V, et al. Layered carbon nanotube poly-electrolyte electrodes outperform traditional neural interface materials. Nano Lett. 2009;9:4012–4018. doi: 10.1021/nl902187z. [DOI] [PubMed] [Google Scholar]
- 40.Tran PA, Zhang LJ, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61:1097–1114. doi: 10.1016/j.addr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Roman JA, Niedzielko TL, Haddon RC, Parpura V, Floyd CL. Single-walled carbon nanotubes chemically functionalized with polyethylene glycol promote tissue repair in a rat model of spinal cord injury. J Neurotrauma. 2011;28:2349–2362. doi: 10.1089/neu.2010.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma HS, Muresanu DF, Sharma A. Novel therapeutic strategies using nanodrug delivery, stem cells and combination therapy for CNS trauma and neurodegenerative disorders. Expert Rev Neurother. 2013;13:1085–1088. doi: 10.1586/14737175.2013.836297. [DOI] [PubMed] [Google Scholar]
- 43.Kanwar JR, Sun X, Punj V, et al. Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomedicine. 2012;8:399–414. doi: 10.1016/j.nano.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Tan J, Wang Y, Yip X, Glynn F, Shepherd RK, Caruso F. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv Mater. 2012;24:3362–3366. doi: 10.1002/adma.201200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 46.Zhang YG, Yang Z, Yang YL, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6:427–441. doi: 10.1016/j.nano.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhao DC, Alizadeh D, Zhang LY, et al. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin Cancer Res. 2011;17:771–782. doi: 10.1158/1078-0432.CCR-10-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee HJ, Park J, Yoon OJ, et al. Amine-modified single walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. 2011;6:121–125. doi: 10.1038/nnano.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Jamal KT, Gherardini L, Bardi G, et al. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci U S A. 2011;108:10952–10957. doi: 10.1073/pnas.1100930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattei TA, Rehman AA. Technological developments and future perspectives on graphene-based metamaterials: a primer for neurosurgeons. Neurosurgery. 2014;74:499–516. doi: 10.1227/NEU.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 51.Zhai C, Guo Y, Sun X, et al. An acetylcholinesterase biosensor based on graphene-gold nanocomposite and calcined layered double hydroxide. Enzyme Microb Technol. 2014;58–59:8–13. doi: 10.1016/j.enzmictec.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Star A, Vidal S. Sweet carbon nanostructures: carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem Soc Rev. 2013;42:4532–4542. doi: 10.1039/c2cs35396b. [DOI] [PubMed] [Google Scholar]
- 53.Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2013;43:744–764. doi: 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- 54.Liu Z, Robinson JT, Sun X, Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang WJ, Zhu H, Li F, Wan LD, Li HC, Ding WL. Electrical stimulation promotes motor nerve regeneration selectivity regardless of end-organ connection. J Neurotrauma. 2009;26:641–649. doi: 10.1089/neu.2008.0758. [DOI] [PubMed] [Google Scholar]
- 56.Zhou K, Thouas GA, Bernard CC, et al. Method to impart electro- and biofunctionality to neural scaffolds using graphene-polyelectrolyte multilayers. ACS Appl Mater Interfaces. 2012;4:4524–4531. doi: 10.1021/am3007565. [DOI] [PubMed] [Google Scholar]
- 57.Li N, Zhang Q, Gao S, et al. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci Rep. 2013;3:1604. doi: 10.1038/srep01604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahni D, Jea A, Meta JA, et al. Biocompatibility of pristine graphene for neuronal interface. J Neurosurg Pediatr. 2013;11:575–583. doi: 10.3171/2013.1.PEDS12374. [DOI] [PubMed] [Google Scholar]
- 59.Li N, Zhang X, Song Q, et al. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32:9374–9382. doi: 10.1016/j.biomaterials.2011.08.065. [DOI] [PubMed] [Google Scholar]
- 60.Wang K, Ruan J, Song H, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:8. doi: 10.1007/s11671-010-9751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Yuan H, von dem Bussche A, et al. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc Natl Acad Sci U S A. 2013;110:12295–12300. doi: 10.1073/pnas.1222276110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee B, Liu CY, Apuzzo ML. A primer on brain-machine interfaces, concepts, and technology: a key element in the future of functional neurorestoration. World Neurosurg. 2013;79:457–471. doi: 10.1016/j.wneu.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 63.Royo-Gascon N, Wininger M, Scheinbeim JI, Firestein BL, Craelius W. Piezoelectric substrates promote neurite growth in rat spinal cord neurons. Ann Biomed Eng. 2013;41:112–122. doi: 10.1007/s10439-012-0628-y. [DOI] [PubMed] [Google Scholar]
- 64.Liu J, Cui L, Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9:9243–9257. doi: 10.1016/j.actbio.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 65.Kim H, Namgung R, Singha K, Oh IK, Kim WJ. Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjug Chem. 2011;22:2558–2567. doi: 10.1021/bc200397j. [DOI] [PubMed] [Google Scholar]
- 66.Feng L, Zhang S, Liu Z. Graphene based gene transfection. Nanoscale. 2011;3:1252–1257. doi: 10.1039/c0nr00680g. [DOI] [PubMed] [Google Scholar]
- 67.Chen B, Liu M, Zhang L, Huang J, Yao J, Zhang Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J Mater Chem. 2011;11:7736–7741. [Google Scholar]
- 68.Belyanskaya L, Weigel S, Hirsch C, et al. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology. 2009;30:702–711. doi: 10.1016/j.neuro.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Gavello D, Vandael DH, Cesa R, Premoselli F, Marcantoni A, et al. Altered excitability of cultured chromaffin cells following exposure to multi-walled carbon nanotubes. Nanotoxicology. 2012;6:47–60. doi: 10.3109/17435390.2011.553294. [DOI] [PubMed] [Google Scholar]