Abstract
Purpose.
To investigate the effects of IL-13 on goblet cell proliferation, differentiation, and expression of mucin and immunomodulatory genes.
Methods.
Explants were excised from the conjunctiva of young C57BL/6 mice. Cultures received 200 μL per week of either Keratinocyte media (KSFM) or KSFM supplemented with 10 ng/mL IL-13 and were incubated for 3 (D3), 7 (D7), or 14 (D14) days. Subsequently, cell proliferation was assessed or cultures were immunostained, collected for dot blot, or for reverse transcription (RT) and quantitative real-time PCR (qPCR) or for RT-PCR gene array.
Results.
The cultured conjunctival epithelium expressed goblet cell associated keratin 7 and mucins MUC5AC and MUC2 and when stimulated with IL-13 showed increased proliferation at D3 and D7 (P < 0.05) compared with control. MUC5AC expression was increased in the IL-13–treated group at D3 and D14 (P < 0.05). IL-13–treated cultures showed increased chemokine ligand 26 (CCL26), chloride channel calcium activated channel 3 (CLCA3), fas ligand (FasL), and Relm-β at D7. All conjunctival cultures expressed MUC2, and its expression was decreased at D3 (P < 0.05) and increased at D14 (P < 0.05) with IL-13 treatment.
Conclusions.
This study demonstrated that conjunctival goblet cells are IL-13 responsive cells that produce factors known to maintain epithelial barrier, stimulate mucin production, and modulate immune response in nonocular mucosa when treated with IL-13. The functional significance of IL-13–stimulated factors remains to be determined.
Keywords: conjunctiva, goblet cells, interleukin-13, cell culture
In the present study, we demonstrated the importance of the conjunctival goblet cells both as tear producing cells and as IL-13 regulated epithelial cells that function to maintain the epithelial barrier, produce mucins, and modulate immune response.
The conjunctiva covers two-thirds of the ocular surface and functions as a support tissue for cornea.1 The conjunctiva is covered by a stratified columnar epithelium that contains goblet cells, specialized secretory cells that produce mucins, large glycoproteins that contain multiple tandem repeats with O-linked oligosaccharides. These mucins lubricate the ocular surface, stabilize the tear film and provide a medium for holding growth and antimicrobial factors produced by the lacrimal glands on the ocular surface.2,3 Conjunctival goblet cells are surrounded by lymphocytes and dendritic cells and their density has been found to change in certain ocular surface immune/inflammatory conditions.4 Goblet cell density has been reported to decrease in aqueous tear deficiency, a condition where T helper 1 (Th1) and Th17 cells infiltrate the conjunctiva, and increase in atopic keratoconjunctivitis and vernal keratoconjunctivitis, predominantly Th2-mediated diseases.5–8
The mucus stimulating activity of the Th2 cytokine IL-13 has been reported to have a defensive role in the intestines by eliminating helminthic parasites and in the airways by protecting from particles or allergens.9,10 However, excessive IL-13 expression is associated with goblet cell hyperplasia and mucous hypersecretion, both in the gut and in the airways where it can result in airway obstruction.9,11,12 Interleukin-13 has two receptors, IL-13Rα1 and IL-13Rα2. Interleukin-13Rα1 combined with IL-4Rα forms the heterodimeric signaling receptor for IL-13.13,14 Interleukin-13Rα2 has higher affinity for IL-13 than IL-13Rα1, but no signaling function has been found for this receptor and its biological role is still under investigation. A soluble form of IL-13Rα2 has been found in vivo, suggesting that it may function as a decoy receptor that binds IL-13 and makes it inaccessible to the IL13Rα1, thus limiting its activity.15,16
Goblet cell density and MUC5AC production have been reported to decrease in aqueous tear deficiency and the balance between Th2 and Th1 cytokines in the tears of human dry eye patients and in the conjunctiva in experimental murine dry eye has been reported to shift toward Th1, reflected by a decrease in the Th2/Th1 cytokine ratio.17,18 Interleukin-13 has been found to have a homeostatic role in maintenance of filled goblet cells in the mouse conjunctiva and it is possible that loss of goblet cell produced factors that are modulated by IL-13 disrupts ocular surface homeostasis in dry eye.18 The effects IL-13 on conjunctival goblet cells have not been thoroughly investigated.
The purpose of the present study was to investigate whether the Th2 cytokine IL-13 can modulate proliferation, differentiation, and expression of mucin and immunomodulatory genes in cultured conjunctival epithelial cells as has been found in airway and intestinal epithelium.
Materials and Methods
All experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Conjunctival Cultures
Explants were excised from the forniceal conjunctiva of young adult, (6–8 week) female C57BL/6 mice and left for 15 minutes at 37°C in Keratinocyte SFM (10724-011; Gibco, Grand Island, NY, USA) supplemented with 3% fetal bovine serum defined (KSFM), 1.25 μg/mL amphotericin B (15290-018; Gibco), 0.5 μL/mL gentamicin (15750-060; Gibco), and 5 μg/mL dispase II (04942078001; Roche, Indianapolis, IN, USA). The explants were then plated one explant per well in 48-well plates and received 200 μL per week of either KSFM with 80 ng/mL mouse epidermal growth factor (EGF; 354001; BD, Franklin Lakes, NJ, USA) or KSFM supplemented with 10 ng/mL recombinant murine IL-13 (210-13; Peprotech, Rocky Hill, NJ, USA). Cultures were incubated for 3, 7, or 14 days. Subsequently cell proliferation/viability was assessed by WST-1 assay (11644807001; Roche), cultures were fixed in methanol or 4% paraformaldehyde and immunostained or collected for dot blot or for RNA extraction and transcription for reverse transcription (RT) and quantitative real-time PCR (qPCR) or for RT-PCR gene array.
WST-1 Assay
After 3, 7, or 14 days media and explants were removed and new media supplemented with cell proliferation reagent WST-1 (Roche), at a final concentration (1:10) was added and culture plates were placed in the incubator at 37°C. After 24 hours absorbance, delta was measured at wavelengths 440 and 690 with a plate reader (infinite M200; Techan, Durham, NC, USA).
Immunofluorescent Staining
The media was aspirated, explants removed and the cultures were fixed in cold methanol or 4% paraformaldehyde. Subsequently, the cultures were washed in PBS followed by 0.1% Triton X treatment for 10 minutes. After blocking with 20% goat serum, wheat germ agglutinin (WGA)-alexa fluor 488 lectin (W11261; Life Technologies, Grand Island, NY, USA) or the primary antibodies to MUC5AC (SC-20118/Abmart, 14906-1-4/C580_130917; Santa Cruz Biotechnology, San Diego, CA, USA), keratin 7 (K7; 9021; Abcam, Cambridge, MA, USA), K14 (130102; Abcam), Ki-67 (NB-600-1252; Novus, Littleton, CO, USA), chemokine ligand 26 (CCL26; bs-15513R; Bioss, Woburn, MA, USA), chloride channel calcium activated channel 3 (CLCA3; 46512; Abcam), fas ligand (FASL; SC-834; Santa Cruz Biotechnology), IL-33 (SC-98659; Santa Cruz Biotechnology), trefoil factor 3 (TFF3; PA5-21081; Fisher, Waltham, MA, USA), restin-like molecule β (Relm-β; NB-200-204; Novus), or MUC2 (SC-15334, Santa Cruz Biotechnology) were applied for 1 hour or overnight. The cultures were rinsed with PBS and the fluorescent secondary antibodies were applied to antibody treated wells. The cultures were then counter stained with Hoechst (33342 DNA dye; Life Technologies), 1 drop of gel mount was applied and finally the cultures were cover slipped with #1.5 (8-mm diameter) round cover glass (72296-08; Electron Microscopy Sciences, Hatfield, PA, USA).
Dot Blot
A bicinchoninic acid (BCA) assay was initially performed to determine total protein concentration per sample. Based on the results, the volume that was equal to 1.5 μg protein per sample was used for the dot-blot assay.
The samples were either left as is (WGA), or were treated with 2.5 milliunits (mU; 0.2 μL) of neuraminidase (480716; Calbiochem, Billerica, MA, USA) and incubated at 37° for 2 hours (MUC5AC). RIPA buffer (R0278; Sigma-Aldrich Corp., St. Louis, MO, USA) was added to bring the total volume of each sample to 50 μL before proceeding to dot blot. Briefly, the membranes were prewet with PBS + 0.05% Tween 20 (TPBS) and applied to the dot-blot apparatus. Next, the samples were loaded and left to incubate on the apparatus for 1 hour, and subsequently washed for 30 minutes in Carbo-Free Blocking Solution (Cat. No. SP-5040; Vector Labs, Burlingame, CA, USA). The membranes were then incubated overnight with 10 μg/mL biotinylated lectin (biotinylated wheat germ agglutinin, Cat. No. B-1025; Vector Labs), anti-MUC5AC (SC-20118; Santa Cruz Biotechnology), or anti-MUC2 (SC-15334; Santa Cruz Biotechnology) at 48, washed with TPBS and incubated for 30 minutes with a VECTASTAIN ABC (peroxidase, Cat. No. PK-6100; Vector Labs) reagent for the lectin according to the kit instructions or secondary HRP-goat-anti-rabbit (656120; Invitrogen, Grand Island, NY, USA) for antibody-treated blots, then washed again with TPBS, and finally, developed and photographed. The mean intensity of the blots from the different samples was measured with NIS Elements (Nikon, Garden City, NY, USA).
Western Blot
Samples included mouse conjunctiva and colon that were prepared by dissection and placed in 150 μL RIPA buffer (R0278; Sigma-Aldrich Corp.). A BCA assay was performed to measure total protein concentration of each sample. Samples (25 μL, equal to a protein concentration 50–100 μg) were diluted with 1 part 2× sample buffer (2× Laemmli Sample Buffer, 161-0737; Bio-Rad Laboratories, Inc., Hercules, CA, USA), boiled for 5 minutes, and loaded on to the polyacrylamide gel. The gel (mini-PROTEAN TGX stain free Precast Gel 7.5%, 456-8024; Bio-Rad laboratories, Inc.) was run at constant current at 100V for approximately 90 minutes at room temperature before the proteins were transferred onto a PVDF membrane (Immobilion Transfer membranes, IVPH07850; Millipore, Billerica, MA, USA) at 20V over night at 4°C. Next, the membranes were incubated in 100 mM Tris-HCL, 0.9% NaCl, 0.1% Tween 20 (TTBS) with 5% fat-free milk for 60 minutes, followed by incubation in primary antibody MUC5AC or MUC2 for 3 hours at room temperature. Subsequently, the membranes were washed in TTBS and incubated in secondary HRP-goat-anti-rabbit (656120; Invitrogen), then washed again with TPBS, and finally, developed and photographed.
RT-PCR and RT-PCR Gene Array
For RT-PCR, total RNA from approximately 10 cultures per sample was extracted with an RNeasy Micro Kit (Qiagen, Valencia, CA, USA), cDNA synthesized by Ready-To-Go You-Prime First-Strand Beads according to manufacturer's instructions (27-9264-01; GE Healthcare, Pittsburgh, PA, USA). Reverse transcription–PCR was then either performed on a Step One Plus system (Applied Biosystems, Grand Island, NY, USA) or the cDNA (400 μg per sample) was loaded in duplicate on Custom TaqMan Gene Array 384-well Card Format 96b (Cat. No. 4342261; Life Technologies) and run by the Genomic and RNA Profiling Core (GARP) at Baylor College of Medicine on the ViiA 7 real-time PCR system (Applied Biosystems). A list of the genes included in the array is provided in Supplementary Table S1. The RT-PCR was run three independent times with at least five samples per group and time point and the RT-PCR gene array was run on two sets of samples. The results were analyzed by the comparative threshold cycle method and normalized by beta 2 microglobulin (B2M) as the control.
Statistical Analysis
Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. An unpaired t-test was used to compare control versus IL-13 treated cultures at days 3 (D3), 7 (D7), and 14 (D14). The statistical significance was set to P less than or equal to 0.05 and data are presented as mean ± SEM.
Results
Conjunctival Goblet Cell Culture
Growth kinetics were initially evaluated with different EGF concentrations ranging from 20 to 120 ng/mL to determine the optimal EGF concentration to obtain epithelial outgrowth from at least 90% of the explants. An EGF concentration of 80 ng/mL was found to give optimal growth and this concentration was used for the reminder of the experiments (data not shown).
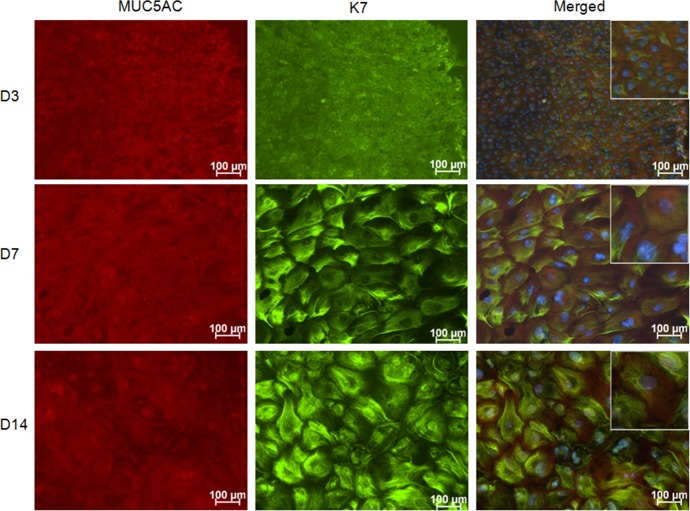
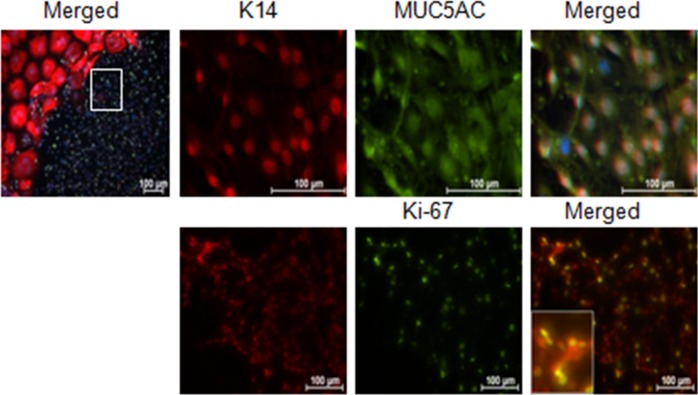
All cells (100%) growing from the explants stained positively for K7 and K14, confirming they are epithelium (Fig. 1). All cells also expressed the goblet cell–specific mucin MUC5AC as well as wheat germ agglutinin (WGA) lectin positive glycoproteins that are found in goblet cells. These criteria have been used to define goblet cells in previous published studies.19,20
Figure 1.
MUC5AC and K7 immunostaining in control conjunctival explant cultures. The staining confirms that all cells growing from the mouse conjunctival explants express the goblet cell markers MUC5AC and K7. Hoechst (DNA stain), blue; MUC5AC, red; and K7, green.
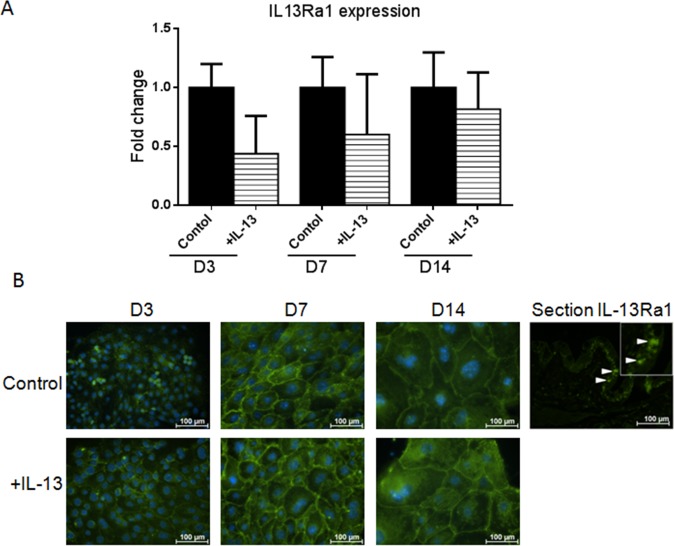
To determine if the cultured epithelium would be responsive to IL-13, expression of the IL-13 signaling receptor (IL-13Rα1) was evaluated by PT-PCR and immunostaining. Interleukin-13Rα1 was expressed by control and IL-13 treated cultures (Figs. 2A, 2B).
Figure 2.
Interleukin-13Rα1 expression in primary conjunctival goblet cell cultures. Change in mRNA transcripts over D14 in culture (A). Interleukin-13Rα1 immunostaining in control and IL-13 treated cells (B). Hoechst (DNA stain), blue; IL-13Rα1, green. Arrows, IL-13Ra1 stained goblet cells in conjunctival tissue section.
IL-13 Stimulates Conjunctival Epithelial Proliferation
First, viability of our cultures treated with IL-13 was investigated. Interleukin-13 concentrations between 10 and 100 ng/mL were used; viability was measured with a WST-1 assay and morphology of the cells was examined. No toxicity of IL-13 was noted and all concentrations of IL-13 increased proliferation of the cultured cells compared with control and this reached statistical significance at a concentration of 10 ng/mL (data not shown).
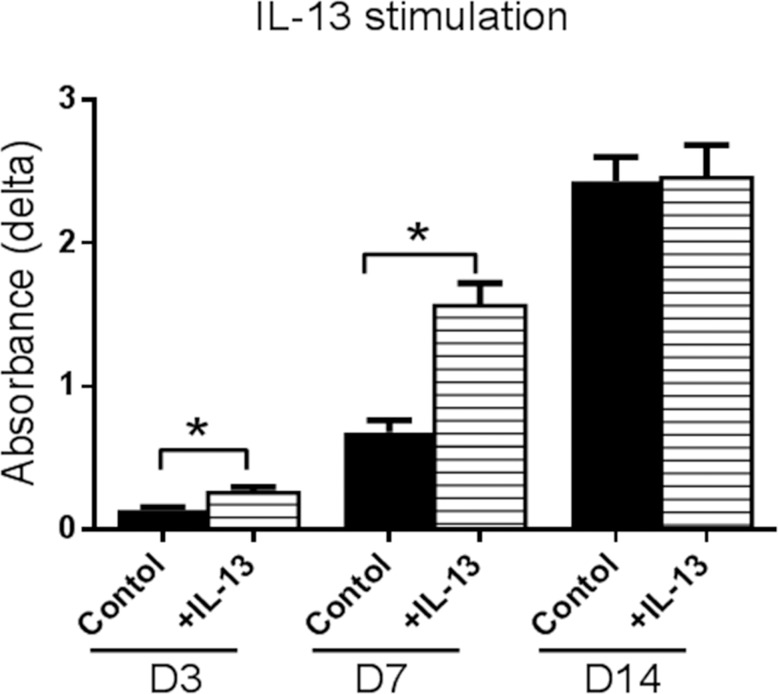
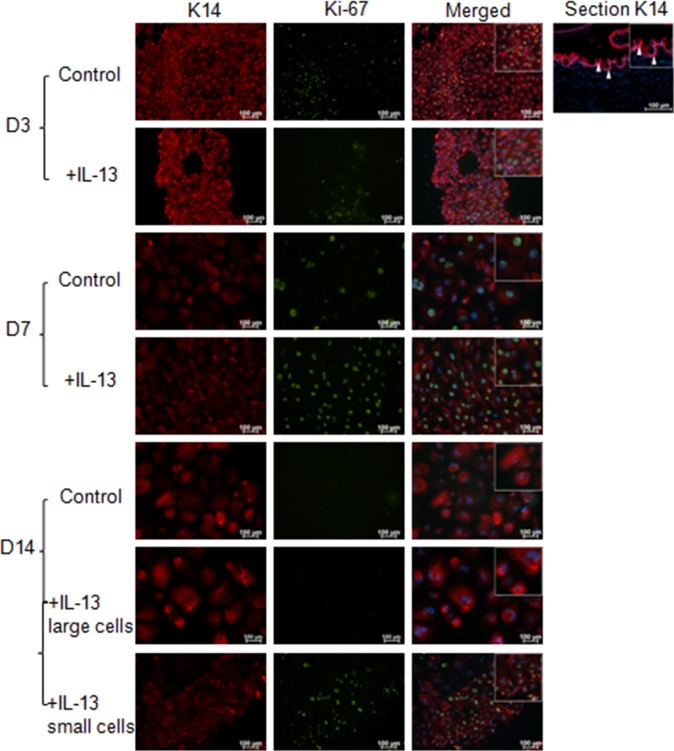
Cultures stimulated with IL-13 illustrated a statistically significant increase in proliferation at D3 and D7 compared with control (Fig. 3). This finding was confirmed by immunostaining for the proliferation marker Ki-67 and cytokeratin 14 (K14) that shows the greatest expression in the basal epithelial compartment in the mouse conjunctiva (Fig. 4). At D14, cells cultured in control media were Ki-67 negative; however, two populations of cells were observed in the IL-13–treated group. The majority of cells in the IL-13–treated group were large and KI-67 negative, while a population of smaller KI-67+ cells was noted in 25% of wells. These small cells also stained positively for the goblet cell markers K14 and MUC5AC (Fig. 5).
Figure 3.
Effects of IL-13 stimulation on proliferation of cultured conjuntival goblet cells measured by WST assay. Cultures stimulated with IL-13 showed a statistically significant increase in cell number at D3 and D7 compared with control. *P < 0.05.
Figure 4.
K14 and Ki-67 immunostaining cultured conjuntival goblet cells. The increased proliferation with IL-13 stimulation at D3 and D7 detected by the WST assay was confirmed at the protein level with K14 (red) and Ki-67 (green) staining. At D14, the majority of the cultured cells were Ki-67 negative; however, in approximately 25% of the cultures a mixture of large, Ki-67 negative and small, Ki-67 positive cells were observed. Arrows, K14 epithelial staining in conjunctival tissue section.
Figure 5.
Higher magnification of small proliferating cells noted in the D14 IL-13–treated conjunctival goblet cell cultures (area surrounded by white box in upper left). Staining illustrates that these small cells are K14, MUC5AC, and KI-67 positive. Inset in bottom panel shows higher magnification of K14 and Ki-67 and dual positive cells. Hoechst (DNA stain), blue; K14, red; Ki-67, or MUC5AC, green.
IL-13 and Glycoprotein Production
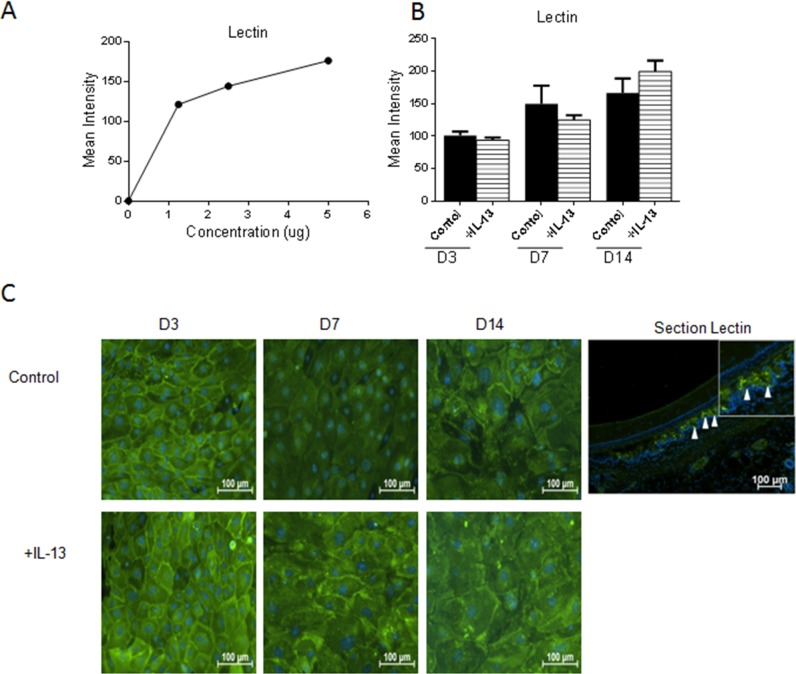
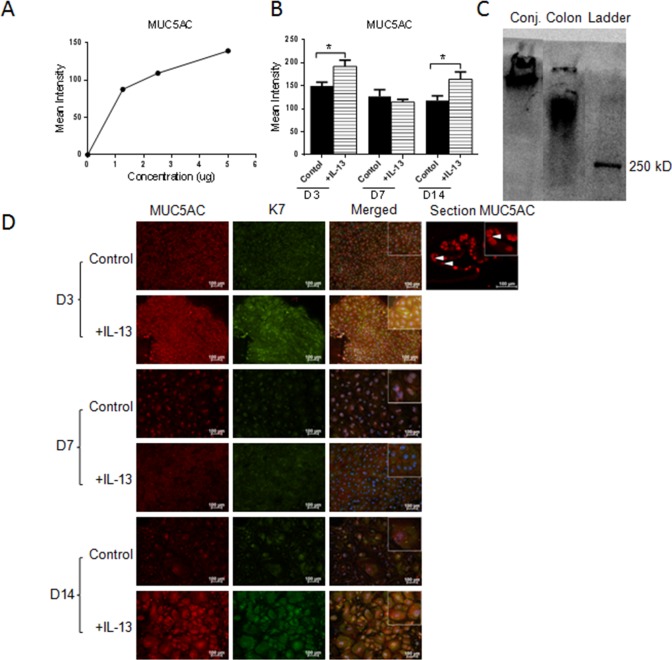
The following experiments investigated whether addition of IL-13 would increase glycoprotein production in general by assessing reactivity to the lectin wheat germ agglutinin (WGA) or to antibodies to the mucin glycoprotein, MUC5AC. First, concentration, mean intensity dependent dot-blot assays were performed to confirm the specificity of the lectin and antibody probes (Figs. 6A, 7A). No difference was found between the control and IL-13–treated groups for WGA by dot blot or staining (Figs. 6B, 6C). In contrast, MUC5AC expression was noted to increase in the IL-13–treated group at D3 and D14 by dot blot and immunostaining (Figs. 7B, 7D). Specific immunoreactivity of the MUC5AC antibody in mouse conjunctiva was confirmed by Western blot (Fig. 7C).
Figure 6.
Glycoprotein production in cultured conjunctival goblet cells in response to IL-13 treatment. Wheat germ agglutinin lectin showed a positive correlation between concentration and mean intensity in dot-blot assay (A). No difference was found in WGA staining between any of the groups (B, C). Hoechst (DNA stain), blue; WGA, green. Arrows, Lectin staining in goblet cells in conjunctival tissue section.
Figure 7.
MUC5AC production in cultured conjunctival goblet cells in response to IL-13 treatment. MUC5AC showed a positive correlation between concentration and mean intensity in dot blot assay (A). Increased expression of MUC5AC was seen at D3 and D14 in the IL-13 treated samples compared with control (B, D). Immunoreactivity of the MUC5AC antibody in mouse conjunctiva was confirmed by Western blot using colon as a positive control (C). Hoechst (DNA stain), blue; MUC5AC, red; WGA or K7, green. Arrows, MUC5AC staining in goblet cells in conjunctival tissue section. *P < 0.05.
IL-13 Stimulated Expression of Mucin and Immunomodulatory Genes
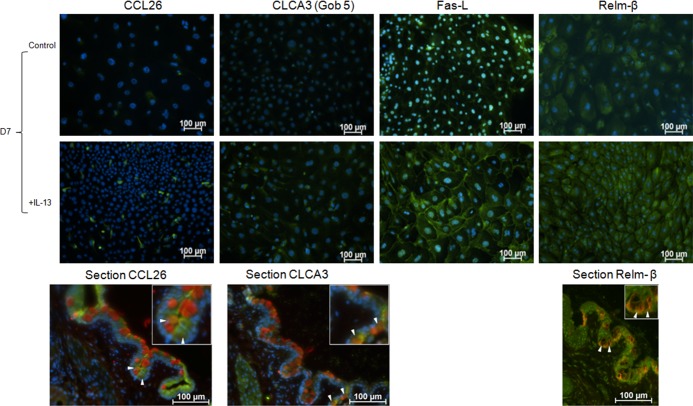
A PCR gene array of 95 genes that have been found to be expressed by goblet cells was performed to determine whether any of them are regulated by IL-13. The genes that showed a 2-fold or greater increase or decrease at day 7 in IL-13–treated cells relative to control are presented in the Table. Expression of genes of special interest with regard to their homeostatic or immunomodulatory functions that were found to increase in the PCR array, including chemokine ligand 26 (CCL26), chloride channel calcium activated channel 3 (CLCA3), fas ligand (FasL), and restin-like molecule beta (Relm-β) was confirmed by immunofluorescent staining of D7 cultures (Fig. 8).
Table.
Polymerase Chain Reaction Gene Array Data
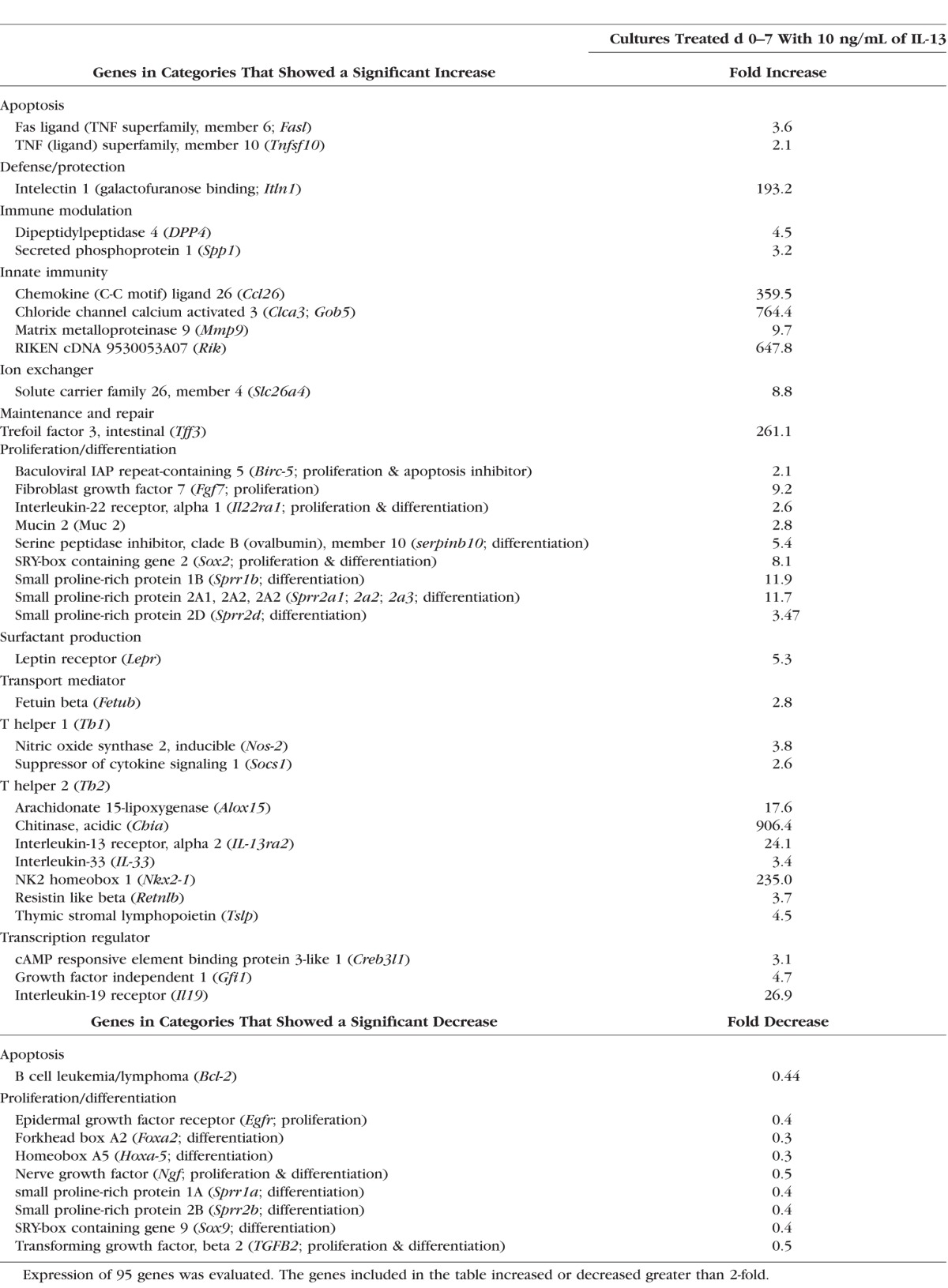
Figure 8.
Immunofluorescent staining of goblet cell produced products that showed marked changes with IL-13 stimulation in the PCR array performed on cultured conjunctival goblet cells. Hoechst (DNA stain), blue; CCL26, CLCA3, Fas-L, or Relm-β, green. In conjunctival tissue sections (bottom row): MUC5AC, Relm-β, red; CCL26, CLCA3, and K7, green (arrows indicate goblet cells).
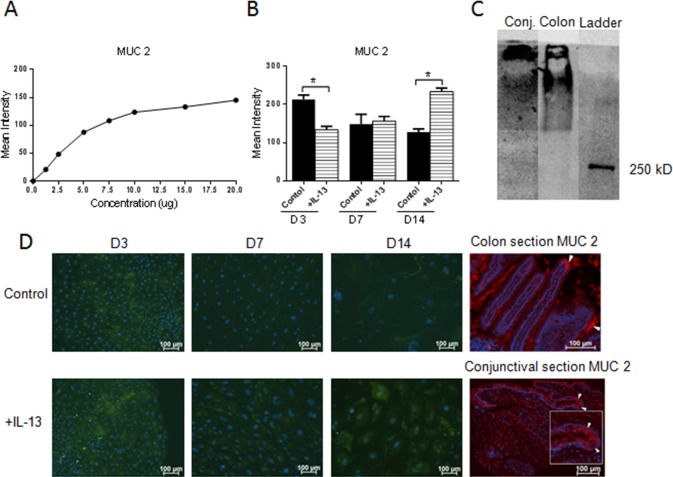
Lastly, we investigated if MUC2 glycoprotein was present in our samples and whether MUC2 production is regulated by IL-13 treatment. To confirm specificity of the MUC2 antibody, a concentration mean intensity dependent dot-blot assay was performed (Fig. 9A). All cultured conjunctival epithelial samples showed positive MUC2 expression. MUC2 expression was decreased at an early time point (D3) and increased at the late time point (D14) in IL-13–treated samples (Figs. 9B, 9D). Specific immunoreactivity of the MUC2 antibody in mouse conjunctiva was confirmed by Western blot using colon as a positive control (Fig. 9C).
Figure 9.
MUC2 glycoprotein production in cultured conjunctival goblet cells in response to IL-13 treatment in dot-blot assay. Positive correlation between concentration and mean intensity of positive colon control (A). In cultured cells, MUC2 expression decreased at D3, showed no difference at D7, and increased at D14 (B). Immunoreactivity of the MUC2 antibody in mouse conjunctiva was confirmed by Western blot using colon as a positive control (C). Immunofluorescent staining of MUC2, colon, and conjunctival sections were used as control (D). Hoechst (DNA stain), blue; MUC2 in culture, green; MUC2 in section, red. *P < 0.05. Arrow(s) in the colon and conjunctival sections indicate positively-stained goblet cells.
Discussion
This study investigated the effects of the Th2 cytokine IL-13 on conjunctival goblet cell cultures using a primary conjunctival epithelial explant culture system that was established using similar methodologies to those previously described for the mouse, rat, and the human.19,21,22 The unique aspect of this study was that the effects of IL-13 on goblet cell proliferation, differentiation, and production of mucin and immunomodulatory genes were studied from time of initiation of cultures.
Of note is that virtually 100% of epithelial cells growing from the conjunctival explants expressed the recognized goblet cell markers K7 and MUC5AC. In agreement with what has previously been reported, the conjunctival epithelium expresses IL-13Ra1 and is responsive to IL-13.19
This study represents the first attempt to determine the direct effect of IL-13 on proliferation, differentiation, and expression of mucins and immunomodulatory genes by primary nonpassaged goblet cells in vitro, without stimulation with cholinergic agonists to elicit a response. Another novel aim of this study was to explore the effects of IL-13 on primary cultures over a 14-day period. A previously published study used different aged mice and only treated cultures with inflammatory/immune cytokines, including IL-13 after they had grown for 2 weeks.19 In contrast, we only used one strain of mice (C57BL/6), added IL-13 at the time of culture initiation, and studied proliferation and differentiation systematically at early (D3 and D7) and later (D14) time points. Interestingly, our study demonstrated that the most prominent effects of IL-13 on proliferation, differentiation, and gene expression occur early, before or at D7, indicating that less differentiated cells are more responsive to IL-13. All the cells growing out from the explants in this model are positive for the goblet cell specific markers MUC5AC and K7. Another unique observation found in approximately 25% of the IL-13 treated cultures at 2 weeks was a population of small Ki-67-, K14-, and MUC5AC-positive cells from the edge of the epithelial outgrowth. This finding implies that IL-13 can stimulate growth of a second wave of progenitor-like MUC5AC-positive cells from the cultured cells.
Interleukin-13 has previously been reported to stimulate goblet cell differentiation and increase mucous secretion in airway epithelium.23,24 Therefore, we determined whether IL-13 stimulation would have the same effect in primary conjunctival cultures by evaluating production of WGA lectin positive glycoproteins and MUC5AC. No change was found in lectin binding in IL-13–treated cultures. One possible explanation for the increase in MUC5AC production without an increase in lectin positive glycoproteins could be that the total amount of glycoprotein is approximately the same, but the composition of the glycoprotein changes with IL-13 stimulation at certain time points.
In agreement with previously published data in primary airway cultures, but in contrast to findings in mouse conjunctival cultures, our study demonstrated that IL-13 increased MUC5AC expression at D3 and D14.19,23,24 It is possible that a longer stimulation than 24 hours is needed for a response, as our cultures were stimulated up to 14 days. However; this doesn't explain why MUC5AC expression was not increased in our cultures at D7. One theoretical explanation is that the goblet cells from the initial outgrowth at D7 are mature and that the second wave of small immature cells observed at D14 in some IL-13–treated samples could be responsible for the rebound in mucin production at this time point.
The conjunctiva is the most immunologically active tissue on the ocular surface and a variety of conjunctival reactions can be provoked by allergic, infectious or inflammatory stimuli. A PCR array of 95 goblet cell associated genes with known protective and immunomodulatory functions was performed to investigate which genes respond to IL-13 stimulation. Among the 95 tested genes, 43 (46%) showed at least 2-fold increased or decreased expression compared with control. Immunofluorescent staining was performed to confirm the gene products that have been found to be important for mucosal homeostasis in the gut or airways.
Our study found that conjunctival goblet cells produce a number of proteins with potential for regulating inflammation, stimulating mucin production and maintaining health of the ocular surface epithelium. One gene identified was CCL26, a chemotaxin also known as Eotaxin-3 that binds to the chemokine receptor CXCR1 expressed on eosinophils, basophils, certain dendritic cells, and Th2 lymphocytes.25–27 Interleukin-13 was previously found to stimulate production of CCL26 by a human lung epithelial cell line in a time-dependent manner.28 These findings were confirmed in conjunctival goblet cell cultures in which IL-13 increased CCL26 mRNA expression at D7 and immunostaining at D7. It is possible that CCL26 produced by conjunctival goblet cells could be chemotactic for intraepithelial dendritic cells or CD4+ T cells under homeostatic conditions and eosinophils and basophils in allergic inflammation.
Another gene of interest that increased over 500-fold in IL-13–stimulated samples was CLCA3 (also known as Gob-5). Chloride channel calcium activated channel 3 has previously been found to localize around mucin granules. In addition, CLCA3 has been used as a marker of goblet cell hyperplasia and secretory activity.29–31 Chloride channel calcium activated channel 3 mRNA expression has shown to increase after IL-13 stimulation and it has also been suggested that CLCA3 plays an important role in allergic inflammation of the lung as a downstream mediator of IL-13 and STAT-6.32–34 Similar to airway epithelium, IL-13 increased mRNA expression and immunostaining in cultured conjunctival goblet cells and could potentially serve as a marker of IL-13–stimulated goblet cell secretory activity in Th2-mediated conjunctival inflammation.
Increased expression of FasL was noted after 7 days in IL-13–treated conjunctival goblet cell cultures and a similar response to IL-13 has been previously reported in airway epithelium. Binding of Fas (CD95) to its ligand, FasL (CD95L) is an essential mechanism of cell-mediated apoptosis that can limit Th1- and Th2-mediated immune reactions.35–41 Increased FasL expression has been found in Barrett's esophagus, a precancerous condition with increased goblet cells and in severe asthma, a Th2-mediated condition associated with goblet cell hyperplasia.39,42 Interleukin-13–mediated FasL expression could potentially have a homeostatic role in vivo by stimulating apoptosis of T helper cells that infiltrate the conjunctiva. One example of such cells would be the IFN-γ producing Th1 cells noted in aqueous tear deficiency that have shown to induce apoptosis and loss of conjunctival goblet cells.43
We also observed that IL-13 stimulated production of two factors by conjunctival goblet cells that are regulated by IL-13 in intestinal goblet cells, trefoil factor 3 (TFF3) and Restin-like molecule β (RELM β) that function in mucosal repair and maintenance of epithelial barrier function, respectively. Trefoil factor 3 was noted to be the second most abundant product of intestinal goblet cells and it functions in epithelial repair, mucosal protection, and stabilization of the mucin layer.44–47 Trefoil factor 3 was found to decrease proliferation and increase cell migration in an experimental corneal epithelial healing model.48 Trefoil factor 3 and colonic mucin glycoproteins were found to have synergy in preventing inflammation induced mucosal epithelial barrier dysfunction with the combination having greater effect than TFF3 alone.47 It is possible that TFF3 has similar ocular surface protective functions and these may be compromised in aqueous tear deficiency where the number of mucin filled goblet cells decreases.5,48 RELM β contributes to maintenance of mucosal barrier in the gut and it may have similar functions on the ocular surface.49,50
Sterile alpha motif (SAM)–pointed domain containing ETS transcription factor (SPDEF) has been reported to play an essential role for goblet cell differentiation on the ocular surface.51 SAM-pointed domain containing ETS transcription factor was expressed by our cultured cells but did not significantly change at the early stage (D0–D7) in response to IL-13. One possible explanation is that all of the cells that grow in our cultures initiated from young adult mice express goblet cell markers, suggesting that SPDEF regulates lineage differentiation in more immature conjunctival epithelial cells than the ones that grow from our explant. Another possible explanation is that the stimulatory effects of IL-13 on our cultures resulted from reduced suppressive effects of transcription factor FOXA2. Interleukin-13 decreased expression of the transcription factor forkhead box A2 (FOXA2) by 66% in the cultured conjunctival goblet cells. FOXA2 is recognized to suppress goblet cell formation and MUC5AC expression in airway epithelial cells and IL-13 has been found to downregulate this transcription factor in these cells.52–54
MUC2 is a soluble gel–forming mucin produced by goblet cells (GCs) that has been detected on the ocular surface by several methods, including RT-PCR and Western blot.55–57 Changes in MUC2 expression have been noted in patients with ocular surface diseases. Corrales et al.58 reported a 50% decreased expression in the conjunctiva of patients with aqueous tear deficiency. Berry and colleagues59 noted a change in MUC2 buoyant density and hydrodynamic volume of MUC2 collected from the ocular surface of dry eye patients with symptoms and no signs compared with a control group and the expression of MUC2 in the conjunctival epithelium was reported to increase in patients with atopic keratoconjunctivitis, a condition with elevated tear IL-13 levels, with the most severe corneal epithelial disease.60,61 To our knowledge, the current study is the first to confirm the stimulatory effects of IL-13 stimulation on MUC2 production by cultured conjunctival goblet cells. Interleukin-13 was noted to increase MUC2 expression in cultured bronchial epithelium at day 14 and after 1 day in cultured human colon cancer cell lines.62,63 We found that IL-13 increased MUC2 mRNA at day 7 and protein expression at day 14 in our primary conjunctival epithelial cells cultures. MUC2 has been found to be a key factor maintaining immune tolerance in the gut by programming conventional dendritic cells from mice and humans to produce factors such as retinoic acid, IL-10, and TGF-β1 that promote differentiation of naïve CD4+ T cells into Tregs, while suppressing nuclear factor κB (NFκB) activation and production of IL-12, an immunogenic cytokine that promotes generation of IFN-γ producing Th1 cells.64
In conclusion, our study demonstrated that IL-13 stimulates conjunctival goblet cell proliferation, MUC5AC and MUC2 glycoprotein production, and expression of immunomodulatory genes. As has already been shown in the gut and the airways, this study demonstrated that conjunctival goblet cells function not only as mucin producing cells, but also as components of the innate ocular surface immune system that are capable of producing mucus and other factors with potential to maintain the epithelial barrier and to modulate ocular surface immune response in response to the Th2 cytokine IL-13.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (NIH) Grant EY011915 (SCP; Bethesda, MD, USA), National Eye Institute/NIH Core Grant EY-002520, Research to Prevent Blindness (New York, NY, USA), Oshman Foundation (Houston, TX, USA), William Stamps Farish Fund (Houston, TX, USA), and Hamill Foundation (Houston, TX, USA).
Disclosure: J. Tukler Henriksson, None; T.G. Coursey, None; D.B. Corry, None; C.S. De Paiva, None; S.C. Pflugfelder, None
References
- 1. Watsky MA,, Jablonski MM,, Edelhauser HF. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988; 7: 483–486. [DOI] [PubMed] [Google Scholar]
- 2. Govindarajan B,, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010; 90: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004; 78: 379–388. [DOI] [PubMed] [Google Scholar]
- 4. Zhang X,, Volpe EA,, Gandhi NB,, et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One. 2012; 7: e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pflugfelder SC,, Tseng SCG,, Yoshino K,, Monroy D,, Felix C,, Reis BL. Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology. 1997; 104: 223–235. [DOI] [PubMed] [Google Scholar]
- 6. Pflugfelder SC,, Corrales RM,, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res. 2013; 117: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roat MI,, Ohji M,, Hunt LE,, Thoft RA. Conjunctival epithelial cell hypermitosis and goblet cell hyperplasia in atopic keratoconjunctivitis. Am J Ophthalmol. 1993; 116: 456–463. [DOI] [PubMed] [Google Scholar]
- 8. Aragona P,, Romeo GF,, Puzzolo D,, Micali A,, Ferreri G. Impression cytology of the conjunctival epithelium in patients with vernal conjunctivitis. Eye (Lond). 1996; 10 (pt 1): 82–85. [DOI] [PubMed] [Google Scholar]
- 9. Mannon P,, Reinisch W. Interleukin 13 and its role in gut defence and inflammation. Postgrad Med J. 2013; 89: 448–456. [DOI] [PubMed] [Google Scholar]
- 10. Laoukili J,, Perret E,, Willems T,, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001; 108: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu D,, Ahrens R,, Osterfeld H,, et al. Interleukin-13 (IL-13)/IL-13 receptor alpha1 (IL-13Ralpha1) signaling regulates intestinal epithelial cystic fibrosis transmembrane conductance regulator channel-dependent Cl- secretion. J Biol Chem. 2011; 286: 13357–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kondo M,, Tamaoki J,, Takeyama K,, et al. Elimination of IL-13 reverses established goblet cell metaplasia into ciliated epithelia in airway epithelial cell culture. Allergol Int. 2006; 55: 329–336. [DOI] [PubMed] [Google Scholar]
- 13. Mannon P,, Reinisch W. Interleukin 13 and its role in gut defence and inflammation. Postgrad Med J. 2013; 89: 448–456. [DOI] [PubMed] [Google Scholar]
- 14. Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003; 111: 677–690. [DOI] [PubMed] [Google Scholar]
- 15. Kasaian MT,, Raible D,, Marquette K,, et al. IL-13 antibodies influence IL-13 clearance in humans by modulating scavenger activity of IL-13Ralpha2. J Immunol. 2011; 187: 561–569. [DOI] [PubMed] [Google Scholar]
- 16. Zhang JG,, Hilton DJ,, Willson TA,, et al. Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein. Evidence that it is distinct from the cloned Il-13 receptor and Il-4 receptor alpha-chains. J Biol Chem. 1997; 272: 9474–9480. [DOI] [PubMed] [Google Scholar]
- 17. Lam H,, Bleiden L,, de Paiva CS,, Farley W,, Stern ME,, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009; 147: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Paiva CS,, Raince JK,, McClellan AJ,, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011; 4: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Contreras-Ruiz L,, Ghosh-Mitra A,, Shatos MA,, Dartt DA,, Masli S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013; 2013: 636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Songhet P,, Barthel M,, Stecher B,, et al. Stromal IFN-gammaR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PLoS One. 2011; 6: e22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shatos MA,, Rios JD,, Tepavcevic V,, Kano H,, Hodges R,, Dartt DA. Isolation characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001; 42: 1455–1464. [PubMed] [Google Scholar]
- 22. Shatos MA,, Rios JD,, Horikawa Y,, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003; 44: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 23. Kondo M,, Tamaoki J,, Takeyama K,, Nakata J,, Nagai A. Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol. 2002; 27: 536–541. [DOI] [PubMed] [Google Scholar]
- 24. Laoukili J,, Perret E,, Willems T,, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001; 108: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daugherty BL,, Siciliano SJ,, DeMartino JA,, Malkowitz L,, Sirotina A,, Springer MS. Cloning expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996; 183: 2349–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sallusto F,, Mackay CR,, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997; 277: 2005–2007. [DOI] [PubMed] [Google Scholar]
- 27. Niess JH,, Brand S,, Gu X,, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005; 307: 254–258. [DOI] [PubMed] [Google Scholar]
- 28. Banwell ME,, Tolley NS,, Williams TJ,, Mitchell TJ. Regulation of human eotaxin-3/CCL26 expression: modulation by cytokines and glucocorticoids. Cytokine. 2002; 17: 317–323. [DOI] [PubMed] [Google Scholar]
- 29. Leverkoehne I,, Gruber AD. The murine mCLCA3 (alias gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J Histochem Cytochem. 2002; 50: 829–838. [DOI] [PubMed] [Google Scholar]
- 30. Long AJ,, Sypek JP,, Askew R,, et al. Gob-5 contributes to goblet cell hyperplasia and modulates pulmonary tissue inflammation. Am J Respir Cell Mol Biol. 2006; 35: 357–365. [DOI] [PubMed] [Google Scholar]
- 31. Kondo M,, Nakata J,, Arai N,, et al. Niflumic acid inhibits goblet cell degranulation in a guinea pig asthma model. Allergol Int. 2012; 61: 133–142. [DOI] [PubMed] [Google Scholar]
- 32. Long AJ,, Sypek JP,, Askew R,, et al. Gob-5 contributes to goblet cell hyperplasia and modulates pulmonary tissue inflammation. Am J Respir Cell Mol Biol. 2006; 35: 357–365. [DOI] [PubMed] [Google Scholar]
- 33. Lee CG,, Homer RJ,, Cohn L,, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002; 277: 35466–35474. [DOI] [PubMed] [Google Scholar]
- 34. Zhou Y,, Dong Q,, Louahed J,, et al. Characterization of a calcium-activated chloride channel as a shared target of Th2 cytokine pathways and its potential involvement in asthma. Am J Respir Cell Mol Biol. 2001; 25: 486–491. [DOI] [PubMed] [Google Scholar]
- 35. Vignola AM,, Chanez P,, Chiappara G,, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999; 103: 563–573. [DOI] [PubMed] [Google Scholar]
- 36. Wadsworth SJ,, Atsuta R,, McIntyre JO,, Hackett TL,, Singhera GK,, Dorscheid DR. IL-13 and TH2 cytokine exposure triggers matrix metalloproteinase 7-mediated Fas ligand cleavage from bronchial epithelial cells. J Allergy Clin Immunol. 2010; 126: 366–374, 374. [DOI] [PubMed] [Google Scholar]
- 37. Druilhe A,, Wallaert B,, Tsicopoulos A,, et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998; 19: 747–757. [DOI] [PubMed] [Google Scholar]
- 38. Jayaraman S,, Castro M,, O'Sullivan M,, Bragdon MJ,, Holtzman MJ. Resistance to Fas-mediated T cell apoptosis in asthma. J Immunol. 1999; 162: 1717–1722. [PubMed] [Google Scholar]
- 39. Younes M,, Lechago J,, Ertan A,, Finnie D,, Younes A. Decreased expression of Fas (CD95/APO1) associated with goblet cell metaplasia in Barrett's esophagus. Hum Pathol. 2000; 31: 434–438. [DOI] [PubMed] [Google Scholar]
- 40. Watanabe N,, Arase H,, Kurasawa K,, et al. Th1 and Th2 subsets equally undergo Fas-dependent and -independent activation-induced cell death. Eur J Immunol. 1997; 27: 1858–1864. [DOI] [PubMed] [Google Scholar]
- 41. Hahn S,, Stalder T,, Wernli M,, et al. Down-modulation of CD4+ T helper type 2 and type 0 cells by T helper type 1 cells via Fas/Fas-ligand interaction. Eur J Immunol. 1995; 25: 2679–2685. [DOI] [PubMed] [Google Scholar]
- 42. Druilhe A,, Wallaert B,, Tsicopoulos A,, et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998; 19: 747–757. [DOI] [PubMed] [Google Scholar]
- 43. Zhang X,, de Paiva CS,, Su Z,, Volpe EA,, Li DQ,, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014; 118: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taupin D,, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003; 4: 721–732. [DOI] [PubMed] [Google Scholar]
- 45. Kjellev S. The trefoil factor family—small peptides with multiple functionalities. Cell Mol Life Sci. 2009; 66: 1350–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Albert TK,, Laubinger W,, Muller S,, et al. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res. 2010; 9: 3108–3117. [DOI] [PubMed] [Google Scholar]
- 47. Kindon H,, Pothoulakis C,, Thim L,, Lynch-Devaney K,, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995; 109: 516–523. [DOI] [PubMed] [Google Scholar]
- 48. Schulze U,, Hampel U,, Sel S,, et al. Trefoil factor family peptide 3 (TFF3) is upregulated under experimental conditions similar to dry eye disease and supports corneal wound healing effects in vitro. Invest Ophthalmol Vis Sci. 2014; 55: 3037–3042. [DOI] [PubMed] [Google Scholar]
- 49. Krimi RB,, Kotelevets L,, Dubuquoy L,, et al. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm Bowel Dis. 2008; 14: 931–941. [DOI] [PubMed] [Google Scholar]
- 50. Nair MG,, Guild KJ,, Du Y,, et al. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008; 181: 4709–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marko CK,, Menon BB,, Chen G,, Whitsett JA,, Clevers H,, Gipson IK. SPDEF null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013; 183: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turner J,, Jones CE. Regulation of mucin expression in respiratory diseases. Biochem Soc Trans. 2009; 37: 877–881. [DOI] [PubMed] [Google Scholar]
- 53. Wan H,, Kaestner KH,, Ang SL,, et al. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development. 2004; 131: 953–964. [DOI] [PubMed] [Google Scholar]
- 54. Zhen G,, Park SW,, Nguyenvu LT,, et al. IL-13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol. 2007; 36: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McKenzie RW,, Jumblatt JE,, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000; 41: 703–708. [PubMed] [Google Scholar]
- 56. Corrales RM,, Calonge M,, Herreras JM,, Saez V,, Chaves FJ. Human epithelium from conjunctival impression cytology expresses MUC7 mucin gene. Cornea. 2003; 22: 665–671. [DOI] [PubMed] [Google Scholar]
- 57. Spurr-Michaud S,, Argueso P,, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007; 84: 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Corrales RM,, Narayanan S,, Fernandez I,, et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Invest Ophthalmol Vis Sci. 2011; 52: 8363–8369. [DOI] [PubMed] [Google Scholar]
- 59. Berry M,, Ellingham RB,, Corfield AP. Human preocular mucins reflect changes in surface physiology. Br J Ophthalmol. 2004; 88: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dogru M,, Okada N,, Asano-Kato N,, et al. Alterations of the ocular surface epithelial mucins 1, 2, 4 and the tear functions in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2006; 36: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 61. Leonardi A,, Curnow SJ,, Zhan H,, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006; 36: 777–784. [DOI] [PubMed] [Google Scholar]
- 62. Yoshisue H,, Hasegawa K. Effect of MMP/ADAM inhibitors on goblet cell hyperplasia in cultured human bronchial epithelial cells. Biosci Biotechnol Biochem. 2004; 68: 2024–2031. [DOI] [PubMed] [Google Scholar]
- 63. Iwashita J,, Sato Y,, Sugaya H,, Takahashi N,, Sasaki H,, Abe T. mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-alpha through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol Cell Biol. 2003; 81: 275–282. [DOI] [PubMed] [Google Scholar]
- 64. Shan M,, Gentile M,, Yeiser JR,, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science. 2013; 342: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.