Abstract
Purpose.
To investigate the relationship between serum 25-hydroxyvitamin D (25[OH]D) levels and nuclear cataract among participants of the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative (WHI) Observational Study (OS).
Methods.
Nuclear cataract was assessed from slit lamp photographs (2001–2004) taken 6 years after collecting serum analyzed for 25(OH)D levels at WHI baseline (1994–1998) in 1278 CAREDS participants age 50 to 79 years. Multivariate (age, iris color, smoking, pulse pressure) odds ratios (ORs) for nuclear cataract (nuclear opacities > level 4 or cataract extraction) by quintiles of serum 25(OH)D were estimated using logistic regression.
Results.
No significant association was observed between serum 25(OH)D and nuclear cataract among women of all ages (age-adjusted OR [95% confidence interval (CI)] 0.97 [0.65–1.45]). However, there was a significant age interaction (P for interaction = 0.04). There were no significant associations in the women 70 years or older. In women younger than 70 years, we observed an inverse association between serum 25(OH)D and nuclear cataract (multivariate adjusted ORs [95% CI] 0.54 [0.29–0.99] and 0.66 [0.36–1.20] for quintiles 4 and 5 vs. 1, respectively; P = 0.03). Further adjustment for 25(OH)D determinants (body mass index, vitamin D intake, and UVB exposure) attenuated this association.
Conclusions.
Serum 25(OH)D levels were unrelated to nuclear opacities in this study sample. However, exploratory analyses suggest a protective association in women younger than 70 years. Further investigations of the relationship between vitamin D and nuclear lens opacities are warranted.
Keywords: vitamin D, cataract, diet, cataract extraction
Serum 25-hydroxy vitamin D levels were unrelated to the presence of nuclear opacities, 6 years later, in postmenopausal women. Protective associations in women <70 years of age suggest that further investigations of the relationship between vitamin D and nuclear lens opacities are warranted.
Cataracts are the most commonly diagnosed age-related eye disease in the United States, affecting an estimated 31% of individuals between the ages of 65 and 74 and 53% of individuals 75 years and older.1 Nuclear cataracts are the most common subtype in Americans.2 The major nonmodifiable risk factors are older age and brown iris color.3–6 Modifiable risk factors include lifestyles that can increase inflammation and oxidative stress: smoking,7–12 alcohol use,8,13–15 obesity,16–20 dietary patterns associated with low levels of micronutrients and phytochemicals,21 and low levels of physical activity.22,23 Pathophysiologically, nuclear cataract has been linked to intraocular and systemic inflammation24,25 and diseases with strong inflammatory components.3,4,26,27
Vitamin D has been suggested to have anti-inflammatory properties.28,29 Adequate vitamin D status may therefore be protective against nuclear cataract. However, no previous studies have directly evaluated relationships between serum biomarkers of vitamin D status and nuclear cataract.
Our primary purpose was to investigate the association between serum 25-hydroxyvitamin D (25[OH]D) levels and nuclear cataract prevalence in postmenopausal women ages 50 to 79 residing in the northern United States using data from the Carotenoids in Age-Related Eye Disease Study (CAREDS). Our secondary aim was to evaluate to what extent serum C-reactive protein (CRP) levels, a marker of systemic inflammation, explained the potential association.
Methods
Study Sample
Individuals in this study were part of the 2005-member CAREDS population, an ancillary study of the Women's Health Initiative Observational Study (WHI-OS).30 The CAREDS consists of postmenopausal women ages 50 to 79 years, who were enrolled at 3 of the 40 WHI-designated sites (the University of Wisconsin in Madison, the University of Iowa in Iowa City, and the Kaiser Center for Health Research in Portland). We sampled 50% of the cohort by including women who had self-reported dietary intakes of lutein plus zeaxanthin that were either above the 78th or below the 28th percentiles, as assessed at WHI baseline (1994–1998). These participants were then followed-up an average of 6 years later as part of CAREDS (2001–2004). The details of the CAREDS and WHI studies are published elsewhere.21,31
Inclusion criteria included gradable nuclear lens photographs (n = 1821) and serum specimens analyzed for 25(OH)D (n = 1475) women. (Women who did not have either serum specimens and gradable nuclear lens photographs were not included, n = 613.) Exclusion criteria were a history of trauma to both eyes (n = 32), unreliable diet data (n = 1), self-reported cataract extraction before the age of 40 (n = 1), missing covariate data (n = 29), or factors that could impact the absorption of vitamin D from foods and supplements, including ulcerative colitis, part of intestines removed, or special malabsorptive diet (n = 51). This resulted in a final analysis dataset of 1278 individuals. Excluded women (n = 727) were slightly older on average compared with the included participants (70.3 vs. 69.3 years, P = 0.0013). There were no significant differences between the excluded and included groups with respect to age-standardized rates of cataract extraction (17.5% and 18.0%, P = 0.77), mean nuclear sclerosis score (3.6 vs. 3.6, P = 1.00), mean daily vitamin D intake from foods and supplements (11.6 vs. 12.1 μg, respectively; age-adjusted P = 0.11), or any of the covariates used in this study (data not shown). This sample permitted 80% power to detect a 16% or more change in odds ratios (ORs) corresponding to a 1 SD change in 25(OH)D. Institutional review board approval and informed consent were obtained. Research adhered to the tenets of the Declaration of Helsinki.
Serum Assays
Serum 25(OH)D was chosen for this analysis, as it is widely recognized as the appropriate measure of an individual's vitamin D status and reflects oral intake (diet and supplements) and cutaneous production.32 Serum samples were drawn at WHI baseline (1994–1998) after a 10-hour or longer fast and stored at −80°C30 and analyzed in nanomoles via the DiaSorin LIAISON chemiluminescence method (Heartland Assays, Inc., Ames, IA, USA).33 The coefficient of variation using blind duplicates was 8.9%. Serum 25(OH)D levels were then adjusted for month of blood draw, as previously described.33 Serum high-sensitivity (hs)-CRP samples were similarly obtained at WHI baseline (1994–1998) in 1126 of the 1278 women who had sufficient serum 25(OH)D samples and analyzed using the hs-CRP assay kit (DiaSorin, Stillwater, MN, USA).
Age-Related Nuclear Cataract Classification
Lens photography and eye examinations were conducted at the CAREDS study visits (2001–2004) 6 years after serum collection (1994–1998) using the standardized Early Treatment for Diabetic Retinopathy Study protocol34 that was modified for the Age-Related Eye Disease Study.35 Grading reliability has been previously reported.36 Both eyes were examined with slit lamp biomicroscopy. A single colored nonstereoscopic photograph using a central fixed slit beam from 12 to 6 o'clock (0.3 × 0.9 mm height and width, respectively, at a 45-degree angle) was taken of each eye with a modified Topcon slit lamp camera (Topcon, Paramus, NJ, USA). The nuclear sclerosis severity in the center of the lens was graded against a series of seven standard photographs, producing continuous scores on a decimal scale that can range from 0.9 to 7.1. The standard photographs were approximately linearly spaced with respect to optical density, as measured by a digital image processor. Two main factors are considered in grading nuclear sclerosis: (1) the opalescence of the nuclear landmarks, especially the sulcus, and (2) the definition of the nuclear surface bands. Severity of nuclear sclerosis was determined in eyes that had not previously undergone cataract extraction. Dates of cataract extraction in each eye, trauma to eyes, and physician-diagnosed histories of cataract were also queried at the time of lens photography.21
The primary outcome was nuclear cataract (NC), defined as a nuclear sclerosis severity score of 4 or greater in the worse eye and/or a history of cataract extraction in either eye. It was previously determined in a similar population that the incidence of cataract surgery was highest among people with photographically evident cataracts in the nuclear region of the lens,9 suggesting that NCs were likely in women who had received cataract extractions. Given the potential confounding effect of cortical and posterior subcapsular cataracts in the cataract extraction population, we compared the results of these two outcomes separately. Data trends were similar when evaluating these two outcomes separately (data not shown).
Covariates
Ultraviolet B (UVB) Exposure.
As part of CAREDS, participants reported their sunlight exposure for each city/town in which they resided from age 18 years to present. For each residence, participants reported the number of daytime hours (<1, 1–3, >3) spent in direct sunlight between 10 AM and 4 PM, in the months of April through September, during weekdays, leisure time, and vacations. Participants also documented if the main daytime activity was on the water for 3 hours or more (no, yes) and whether they used protective gear (hats, sunglasses, and protective lenses). These data permitted average annual ambient UVB exposure at WHI baseline (an average of 6 years before eye photography) and computation of estimated average annual ocular UVB exposure over a women's adult life using conversion coefficients from a previously published algorithm.37
Nonnutritional Covariates.
Demographic, lifestyle, and health history data were obtained from WHI entry questionnaires (education, ethnicity, smoking, physical activity, diet, height, weight, hormone replacement therapy use, alcohol use, pulse pressure, diabetes, hypertension, and cardiovascular disease). Age, family history of cataract (immediate family member aged <65 years when diagnosed), smoking, alcohol use, and diabetes were updated from CAREDS questionnaires. Iris color was classified from photographs taken at the CAREDS eye examination.
Nutritional Covariates From Foods and Supplementation.
At WHI baseline, nutrient intake from foods was estimated from a self-administered food frequency questionnaire,38 to assess usual dietary intake over the previous 3 months. An interviewer-administered form was used to collect information on the dose, frequency, and duration of current supplement use at WHI-OS baseline.38,39 Responses to these diet and supplement questionnaires were combined to estimate total intake of vitamins D, C,21 and E,40 and fat31 and lutein and zeaxanthin,31 which have been associated with decreased NC risk.
Statistical Analyses
Age-adjusted ORs and 95% confidence intervals (95% CI) for NC were computed by quintiles (Q) of serum 25(OH)D for each risk factor (PROC LOGISTIC in SAS version 9.1 statistical software; SAS Institute, Inc., Cary, NC, USA) with Q1 (low 25[OH]D concentrations are the reference group). The P for trend across continuous levels of serum 25(OH)D was also computed. We explored the possibility of an age interaction in the relationships of cataract to 25(OH)D before model building. The rationale was based on the observation of age interactions in relationships of nutritional variables to ocular endpoints previously noted in this cohort.33,41 A P for interaction of less than 0.10 was considered statistically significant. When present (P < 0.10), ORs were computed separately in two approximately equal age strata (<70 vs. ≥70 years).
Potential confounders for the relationship between serum 25(OH)D and NC included all measured variables biologically related to NC, including age, iris color, smoking, pulse pressure, physical activity, diabetes mellitus, oral or inhaled corticosteroid use, hormone therapy, family history of cataract, and several nutritional factors. Variables related to both serum 25(OH)D and NC from a univariate logistic regression (P < 0.20) were chosen as a priori confounders.
Further exploratory analyses were done to guide interpretation of the results. Multivariate models were adjusted for the strongest predictors of 25(OH)D levels, including exogenous sources of vitamin D (vitamin D intake from foods and supplements and baseline UVB at WHI) and body mass index (BMI).42,43 We also explored whether adjusting for hs-CRP, attenuated the association to evaluate potential evidence that the association might be explained by an anti-inflammatory mechanism. Last, we explored whether there was evidence that higher 25(OH)D levels might reflect two opposing influences; both the protective effect of adequate vitamin D status and the harmful cumulative effects of lifetime UVB exposure.
Results
Participant Characteristics
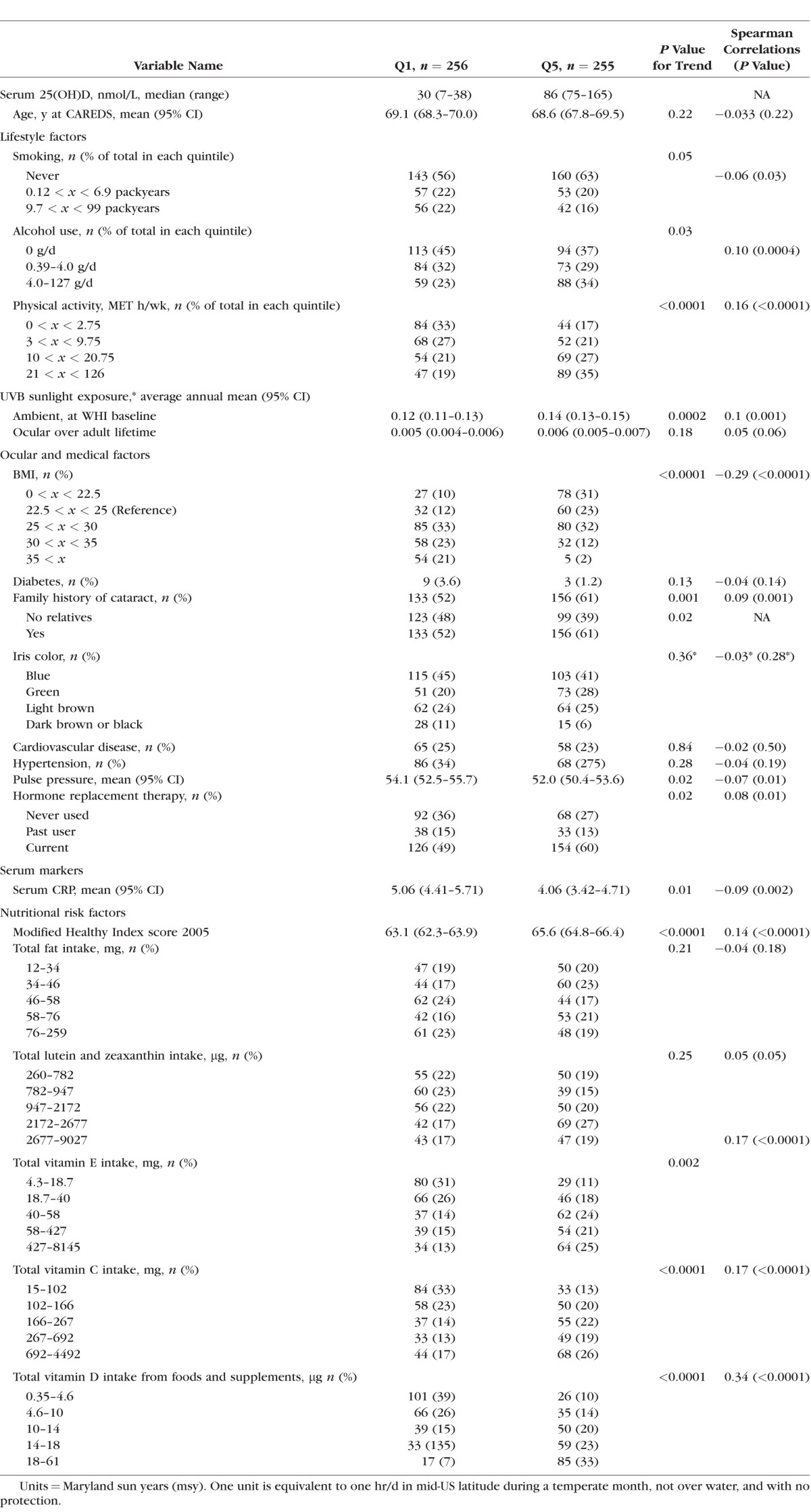
Of the 1278 participants included in the study, 98% were Caucasian. A total of 516 (40%) women had NC; 283 women had nuclear sclerosis score higher than 4, and an additional 233 women reported cataract extraction surgeries. The median (range) value of serum 25(OH)D in Q1 was 30 nM (7–38) and 86 nM (75–165) in Q5. Participants in the highest quintile of serum 25(OH)D compared with those in the lowest quintile were more physically active, had lower BMIs, higher self-reported annual ambient UVB exposure at WHI baseline, were more likely to have a family history of cataract, and were less likely to report alcohol use (Table 1). Highest quintile participants also had higher modified Health Eating Index 2005 (modified HEI 2005) scores, lower serum hs-CRP levels, and reported higher levels of vitamins C, D, and E intake from foods and supplements. Participant characteristics by 25(OH)D quintile in women younger than 70 and older than 70 years were similar (data not shown).
Table 1.
Risk Factors for NC (Nuclear Sclerosis > 4.0 or Cataract Surgery) by Quintiles of Serum 25(OH)D of 1278 Postmenopausal Women Ages 50 to 79 Years, at WHI Baseline (1994–1998) Unless Otherwise Noted
Associations Between Serum 25(OH)D and Cataract
In the age-adjusted model, there was no statistical association between NC and serum 25(OH)D in Q5 versus Q1 (OR 0.97, 95% CI 0.65–1.45, P trend = 0.58) (Table 2). There was a statistically significant age interaction (P for interaction = 0.04). Women younger than 70 years in Q5 had significantly lower odds of NC relative to women in Q1 (OR 0.62, 95% CI 0.34–1.11, P trend = 0.02), whereas women 70 years or older in Q5 had nonsignificant higher odds of NC relative to women in Q1 (OR 1.49, 95% CI 0.86–2.60, P trend = 0.17). This remained true after additional adjustment for multivariate confounders (age, iris color, smoking, pulse pressure) (Table 2; Fig.). Therefore, the remaining analyses were conducted in women younger than 70 years and 70 years or older separately.
Table 2.
Age-Adjusted and Multivariate ORs (CI) for NC (Nuclear Sclerosis > 4.0 or Cataract Surgery) by Quintiles of Serum 25(OH)D* in 1278 Postmenopausal Women Ages 50 to 79 in CAREDS
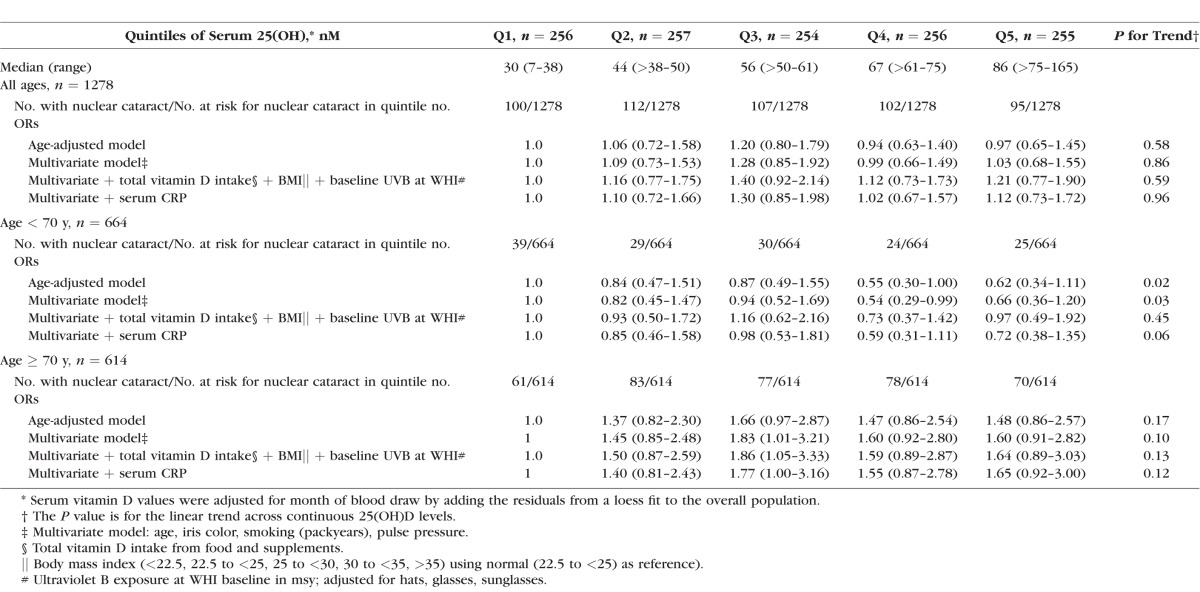
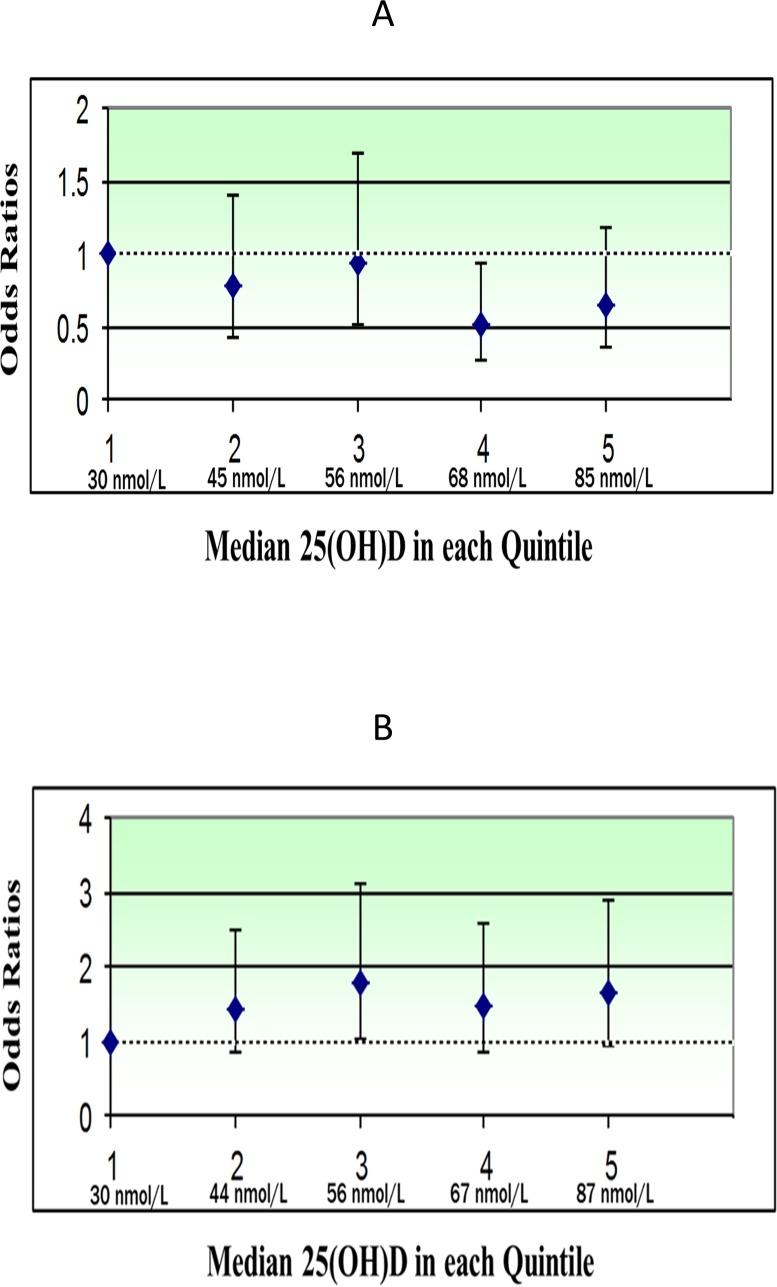
Figure.
Multivariable ORs (95% CIs) for NC in 25(OH)D quintiles 2 through 5, compared with quintile 1, by age group (values detailed in Table 2). P interaction for age group = 0.04. (A) Odds ratios of NC by quintiles of serum 25(OH)D in postmenopausal women younger than 70 years in CAREDS, a subset of the WHI. P trend equaled 0.03. (B) Odds ratios of NC by quintiles of serum 25(OH)D in postmenopausal women 70 years or older in CAREDS, a subset of the WHI. P trend equaled 0.10.
Among women younger than 70, multivariate adjusted ORs (95% CI) for NC were less than 1 in Q4 and Q5, relative to Q1 (OR 0.54, 95% CI 29–0.99 and OR 0.66, 95% CI 0.36–1.20, P for trend = 0.03, respectively). We then explored adjustment for exogenous sources of vitamin D (vitamin D intake from foods and supplements and UVB exposure) at WHI baseline, as previous analyses showed these variables explained the largest amount of variability in 25(OH)D in this sample; 9.5% and 7.0%, respectively.43 The ORs in Q4 and Q5 indeed weakened by 8% and 14%, respectively, and the P for trend became nonsignificant (OR 0.62, 95% CI 0.33–1.17, and OR 0.80, 95% CI 0.42–1.53; P for trend = 0.16, respectively). Likewise, adjustment for BMI, a strong predictor of vitamin D status, also weakened the multivariate ORs in Q4 and Q5 by 9% and 13% and the P for trend became nonsignificant (OR 0.63, 95% CI 0.34–1.19 and OR 0.7, 95% CI 0.42–1.48; P for trend = 0.16, respectively). Adjusting for physical activity, which explained only 2.5% of variability in levels of 25(OH)D in this sample,42 did not attenuate ORs (data not shown).
Further adjusting for total vitamin C, total vitamin E, total fat, total lutein and zeaxanthin or the modified HEI 2005 scores21 did not substantively modify ORs (data not shown). Adjusting for education, as a marker of socioeconomic status, also did not change the protective association between 25(OH)D and NC (data not shown).
We next explored whether the association of higher serum 25(OH)D with lower risk of NC may be explained by the anti-inflammatory properties of vitamin D, as assessed by serum hs-CRP. There was a modest weakening of the inverse association in those younger than 70 (OR in Q5 versus Q1 = 0.72, 95% CI 0.38–1.35; P trend = 0.06) (Table 2).
In women 70 years or older, the nonsignificant multivariate odds between serum 25(OH)D and NC in Q5 versus Q1 (OR 1.60, 95% CI 0.91–2.82; P trend = 0.10) did not change after further adjustment for predictors of vitamin D or serum hs-CRP (Table 2).
We also considered the presence of cortical opacities and their established adverse relationship with UVB exposure44–46 as a potential contributor to the positive trend, as participants were not distinguished based on the existence of both cataracts. However, we did not find a significant adverse association between UVB and cortical cataract or changes in the trend between serum 25(OH)D and NC in the older women when cortical cataracts were excluded (data not shown).
Exploratory Analyses of Accumulated UVB Exposure
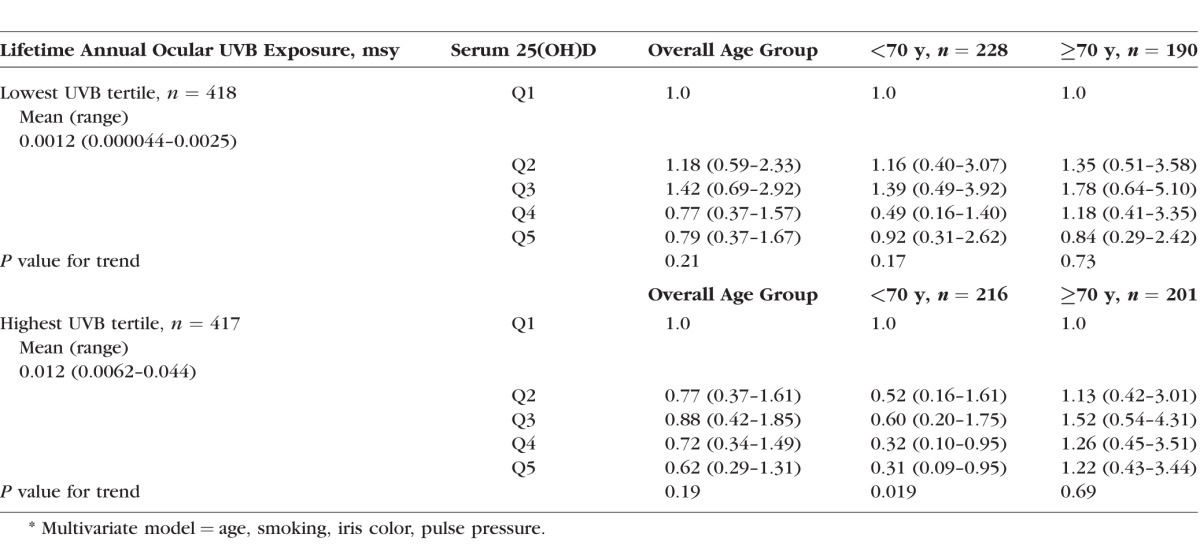
We hypothesized that with age, the cumulative harmful effects of UVB exposure to the lens might overshadow the beneficial effect of UVB on improving vitamin D status (through vitamin D production in the skin). Therefore, we might observe a net protective association through middle age and a net adverse association in old age, if cumulative lifetime UVB exposure were high. In order to explore this, we evaluated lifetime ocular UVB as a potential effect modifier in the relationship between serum 25(OH)D and NC (Table 3). We observed no significant interaction of the relationship between serum 25(OH)D and NC by tertiles of lifetime ocular UVB (P for interaction = 0.69). However, we observed data trends that supported our speculation: A direct trend of the relationship between 25(OH)D and NC was observed only in older women with the highest UVB exposure.
Table 3.
Multivariate* ORs (95% CI) for NC (Nuclear Sclerosis ≥ 4.0 or Cataract Surgery) by Quintiles of Serum 25(OH)D in the Lowest and Highest Lifetime Average Annual Ocular UVB Exposure Tertiles in Maryland Sunyears (msy) for 1278 Postmenopausal Women Ages 50 to 79 in CAREDS
Discussion
We observed no overall significant association between serum 25(OH)D and NC in a sample of postmenopausal, mostly Caucasian, women. However, we found a significant protective association in women who were younger than 70 years. This result may be a chance finding and requires replication in separate samples. Associations between vitamin D status and cataract risk are understudied; only one previous study has investigated associations between a blood biomarker of vitamin D status (reflecting all sources of vitamin D) and cataract.47 In this case-control study of 112 individuals between 40 and 70 years of age, the authors observed a protective association for any, cortical, or posterior subcapsular cataracts with high compared with low blood vitamin D concentrations in women younger than 70, similar to our study results.
Several studies support the biological plausibility that vitamin D adequacy protects against cataract development by suppressing inflammation. Cataract incidence and prevalence have been directly associated with intraocular and acute systemic inflammation via plasma CRP,25 IL-6,24 and soluble intercellular adhesion molecule-1.24 In addition, increased NC risk is observed in those with diseases that have inflammatory components, including arthritis48 and diabetes mellitus.3,4,26,27
Vitamin D has been shown to suppress proinflammatory and toxic agents at the cellular level, such as TNF-α,28 nitric oxide,28 and IL-2 (required for differentiation of T-helper 1 lymphocytes).29 Adequate vitamin D status has been related to lower rates of autoimmune diseases, including Type-1 diabetes, rheumatoid arthritis, and multiple sclerosis.49–52 Intraocularly, vitamin D decreases inflammation53 in corneal cells in the anterior segment of the eye. Both 25(OH)D and its active metabolite 1, 25(OH)2D, have been detected in aqueous and vitreous humor and enhance corneal epithelial barrier function.54
We explored the potential protection via an anti-inflammatory mechanism by adjusting for hs-CRP. However, the protective association between 25(OH)D and NC in the younger than 70 age group was only mildly attenuated, providing weak support for this hypothesis. Of note, serum CRP is a limited biomarker of systemic inflammation, reflecting the acute inflammation stage and not necessarily chronic inflammation. Serum CRP is not consistently related to NC risk with increased risk observed in the Physicians' Health Study,25 but not in the present study (data not shown) or the Singapore Malay study.55
Variables accounting for the largest degree of variability in serum 25(OH)D in this sample42,43 (vitamin D intake, UVB exposure, and BMI) attenuated the protective association observed in women younger than 70 years, whereas other predictors of healthy diets did not. Such evidence is consistent with a protective association. Previously indirect protective relationships between vitamin D intake and NC were observed in The Beaver Dam Eye Study: Reduced odds for NC were related to both milk drinking56 (a key source of vitamin D intake in the United States) and moderate levels of vitamin D intake from supplements.57 High vitamin D intake from supplements was associated with a decreased NC risk in smokers.57 However, the associations between vitamin D intake and broad cataract outcomes (cataract of any type and cataract extraction) have been inconsistent; no significant relationships with vitamin D intake were observed in two other studies.58,59
Obesity also has been related to cataract in previous studies16–20 and is strong predictor of poor vitamin D status in this sample, explaining 7% of variability in serum 25(OH)D.43 It may be that greater adipose tissue leads to more storage and thus lower 25(OH)D or that obesity may directly promote cataract development via direct oxidative stress and inflammation.60 Attenuation of our study findings after BMI adjustment may be due to confounding or its influence on 25(OH)D variability. It was not possible to distinguish between these two influences in this study.
Implications for Relationships of Sunlight Exposure to NC
A protective influence of vitamin D status on NC development may explain why several studies have not observed a direct association between sun exposure and NC,61–64 despite the known effects of UVB on oxidative stress in the lens,44 and consistent reports of UVB as an adverse risk factor for cortical cataract.44–46 In the CAREDS sample, a nonsignificant inverse association between lifetime ocular UVB exposure and NC was observed.21 We speculate that the lack of deleterious associations between sun exposure and NC may result from a dual role of UVB on NC formation, both causing oxidative stress and damage to the lens and preventing cataract formation via higher levels of cutaneous vitamin D production.32
The balance between adverse and protective influences of UVB on NC formation might vary by latitude. This might explain the inconsistent relationships between light exposure and NC in previous studies. In three countries in the northern hemisphere in the mid to lower latitudes, adverse associations between UVB and NC risk were observed.65–67 Conversely, in the present study21 and in four other study populations,61–64 in latitudes north of areas of persons in the abovementioned cities,65–67 did not find an adverse association. It is possible that, over time, persons living in the mid to lower latitude cities were more likely than persons in northern latitudes to have experienced an excess of UVB exposure relative to needs for vitamin D production, such that adverse UV effects on the lens outweighed the benefits.
In our study, we speculated that the opposing influences of UVB on NC formation might vary by age. As individuals live longer, chronic UVB exposure may lead to direct accumulative oxidative damage to the cortical lens, outweighing the protective influence of vitamin D. Consistent with the hypothesis, the different directions of the serum 25(OH)D and NC relationship by age group only persisted in the highest UVB tertile. However, the sample size was small and CIs were large in these subsequent stratified analyses.
An alternative explanation for the age interaction could be a selective mortality (or “survivor”) bias. Vitamin D supplementation has been associated with falls prevention in older women; older women with higher vitamin D levels and cataracts may outlive those with lower vitamin D levels and cataract.68
Limitations
The longitudinal prevalence design of CAREDS is more likely to provide reliable estimates of causal associations of vitamin D status to NC in younger women, whose NCs are more likely to have developed after or near the time in which serum 25(OH)D was measured, than older women. These associations need to be confirmed in prospective cohort studies that better align the exposure assessment to the time in which lens opacities progress. The sample size limited the statistical power to assess a modifying influence of lifetime UV light exposure, which might have explained, in part, direct associations in women older than 70 years. An additional limitation is that we were unable to accurately distinguish between ocular and skin exposure to UVB.
Conclusions
In this study, 25(OH)D levels were unrelated to nuclear opacities. However, exploratory analyses suggest a protective association in women younger than 70 years. Further investigations of the relationship between vitamin D and nuclear lens opacities are warranted. If results of the analyses in women younger than 70 years are confirmed, given the high prevalence of vitamin D inadequacy,69–72 achieving vitamin D adequacy via foods, supplementation, or modest skin sunlight exposure may be a lifestyle modification beyond healthy diets21,31 that may lower NC risk and have significant public health impacts.
Acknowledgments
We thank the women who generously contributed their time to participate in CAREDS and all CAREDS investigators whose efforts over many years have provided the data used in the present analysis. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
The CAREDS is supported by the National Institutes of Health, National Eye Institute (Grants EY013018 and EY016886), and the Research to Prevent Blindness. The WHI is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Disclosure: P. Rao, None; A.E. Millen, Mushroom Council (F); K.J. Meyers, None; Z. Liu, None; R. Voland, None; S. Sondel, None; L. Tinker, None; R. Wallace, None; B.A. Blodi, None; N. Binkley, None; G. Sarto, None; J. Robinson, None; E. LeBlanc, None; J. Mares, None
References
- 1. Rein DB,, Zhang P,, Wirth KE,, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006; 124: 1754–1760. [DOI] [PubMed] [Google Scholar]
- 2. Congdon N,, Vingerling JR,, Klein BE,, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004; 122: 487–494. [DOI] [PubMed] [Google Scholar]
- 3. McCarty CA,, Mukesh BN,, Fu CL,, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999; 128: 446–465. [DOI] [PubMed] [Google Scholar]
- 4. Delcourt C,, Cristol JP,, Tessier F,, Leger CL,, Michel F,, Papoz L. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: the POLA study. Pathologies Oculaires Liees a l'Age. Am J Epidemiol. 2000; 151: 497–504. [DOI] [PubMed] [Google Scholar]
- 5. Risk factors associated with age-related nuclear and cortical cataract: a case-control study in the Age-Related Eye Disease Study, AREDS Report No. 5. Ophthalmology. 2001; 108: 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younan C,, Mitchell P,, Cumming RG,, Rochtchina E,, Wang JJ. Iris color and incident cataract and cataract surgery: the Blue Mountains Eye Study. Am J Ophthalmol. 2002; 134: 273–274. [DOI] [PubMed] [Google Scholar]
- 7. West S,, Munoz B,, Schein OD,, et al. Cigarette smoking and risk for progression of nuclear opacities. Arch Ophthalmol. 1995; 113: 1377–1380. [DOI] [PubMed] [Google Scholar]
- 8. Cumming RG,, Alcohol Mitchell P. smoking, and cataracts: the Blue Mountains Eye Study. Arch Ophthalmol. 1997; 115: 1296–1303. [DOI] [PubMed] [Google Scholar]
- 9. Klein BE,, Klein R,, Moss SE. Incident cataract surgery: the Beaver Dam eye study. Ophthalmology. 1997; 104: 573–580. [DOI] [PubMed] [Google Scholar]
- 10. Weintraub JM,, Willett WC,, Rosner B,, Colditz GA,, Seddon JM,, Hankinson SE. Smoking cessation and risk of cataract extraction among US women and men. Am J Epidemiol. 2002; 155: 72–79. [DOI] [PubMed] [Google Scholar]
- 11. Kelly SP,, Thornton J,, Edwards R,, Sahu A,, Smoking Harrison R. and cataract: review of causal association. J Cataract Refract Surg. 2005; 31: 2395–2404. [DOI] [PubMed] [Google Scholar]
- 12. Tan JS,, Wang JJ,, Younan C,, Cumming RG,, Rochtchina E,, Smoking Mitchell P. and the long-term incidence of cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2008; 15: 155–161. [DOI] [PubMed] [Google Scholar]
- 13. Klein BE,, Klein RE,, Lee KE. Incident cataract after a five-year interval and lifestyle factors: the Beaver Dam Eye Study. Ophthalmic Epidemiol. 1999; 6: 247–255. [DOI] [PubMed] [Google Scholar]
- 14. Morris MS,, Jacques PF,, Hankinson SE,, Chylack LT,, Jr,, Willett WC,, Taylor A. Moderate alcoholic beverage intake and early nuclear and cortical lens opacities. Ophthalmic Epidemiol. 2004; 11: 53–65. [DOI] [PubMed] [Google Scholar]
- 15. Lindblad BE,, Hakansson N,, Philipson B,, Wolk A. Alcohol consumption and risk of cataract extraction: a prospective cohort study of women. Ophthalmology. 2007; 114: 680–685. [DOI] [PubMed] [Google Scholar]
- 16. Glynn RJ,, Christen WG,, Manson JE,, Bernheimer J,, Hennekens CH. Body mass index. An independent predictor of cataract. Arch Ophthalmol. 1995; 113: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 17. Hiller R,, Podgor MJ,, Sperduto RD,, et al. A longitudinal study of body mass index and lens opacities. The Framingham Studies. Ophthalmology. 1998; 105: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 18. Caulfield LE,, West SK,, Barron Y,, Cid-Ruzafa J. Anthropometric status and cataract: the Salisbury Eye Evaluation project. Am J Clin Nutr. 1999; 69: 237–242. [DOI] [PubMed] [Google Scholar]
- 19. Schaumberg DA,, Glynn RJ,, Christen WG,, Hankinson SE,, Hennekens CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. 2000; 72: 1495–1502. [DOI] [PubMed] [Google Scholar]
- 20. Kuang TM,, Tsai SY,, Hsu WM,, Cheng CY,, Liu JH,, Chou P. Body mass index and age-related cataract: the Shihpai Eye Study. Arch Ophthalmol. 2005; 123: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 21. Mares JA,, Voland R,, Adler R,, et al. Healthy diets and the subsequent prevalence of nuclear cataract in women. Arch Ophthalmol. 2010; 128: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paunksnis A,, Kusleika S,, Kusleikaite M. The relationship of the intensity of lens opacity with physical activity. Medicina (Kaunas). 2006; 42: 738–743. [PubMed] [Google Scholar]
- 23. Williams PT. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009; 50: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klein BE,, Klein R,, Lee KE,, Knudtson MD,, Tsai MY. Markers of inflammation vascular endothelial dysfunction, and age-related cataract. Am J Ophthalmol. 2006; 141: 116–122. [DOI] [PubMed] [Google Scholar]
- 25. Schaumberg DA,, Ridker PM,, Glynn RJ,, Christen WG,, Dana MR,, Hennekens CH. High levels of plasma C-reactive protein and future risk of age-related cataract. Ann Epidemiol. 1999; 9: 166–171. [DOI] [PubMed] [Google Scholar]
- 26. McCarty CA,, Nanjan MB,, Taylor HR. Attributable risk estimates for cataract to prioritize medical and public health action. Invest Ophthalmol Vis Sci. 2000; 41: 3720–3725. [PubMed] [Google Scholar]
- 27. Leske MC,, Wu SY,, Nemesure B,, Hennis A. Risk factors for incident nuclear opacities. Ophthalmology. 2002; 109: 1303–1308. [DOI] [PubMed] [Google Scholar]
- 28. Lefebvre d'Hellencourt C, Montero-Menei CN, Bernard R, Couez D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J Neurosci Res. 2003; 71: 575–582. [DOI] [PubMed] [Google Scholar]
- 29. Manolagas SC,, Werntz DA,, Tsoukas CD,, Provvedini DM,, Vaughan JH. 125-Dihydroxyvitamin D3 receptors in lymphocytes from patients with rheumatoid arthritis. J Lab Clin Med. 1986; 108: 596–600. [PubMed] [Google Scholar]
- 30. Anderson GL,, Manson J,, Wallace R,, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003; 13: S5–S17. [DOI] [PubMed] [Google Scholar]
- 31. Moeller SM,, Voland R,, Tinker L,, et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an Ancillary Study of the Women's Health Initiative. Arch Ophthalmol. 2008; 126: 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 33. Millen AE,, Voland R,, Sondel SA,, et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch Ophthalmol. 2011; 129: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Early Treatment Diabetic Retinopathy Research Group. Early Treatment Diabetic Retinopathy Study. Manual of Operations. Baltimore: ETDRS Coordinating Center, University of Maryland School of Medicine; 1980.
- 35. The age-related eye disease study (AREDS) system for classifying cataracts from photographs: AREDS report no. 4. Am J Ophthalmol. 2001; 131: 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Group A-REDSR. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119: 1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Duncan DD,, Munoz B,, West SK. Assessment of ocular exposure to visible light for population studies. Dev Ophthalmol. 2002; 35: 76–92. [DOI] [PubMed] [Google Scholar]
- 38. Patterson RE,, Kristal AR,, Tinker LF,, Carter RA,, Bolton MP,, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999; 9: 178–187. [DOI] [PubMed] [Google Scholar]
- 39. Patterson RE,, Kristal AR,, Levy L,, McLerran D,, White E. Validity of methods used to assess vitamin and mineral supplement use. Am J Epidemiol. 1998; 148: 643–649. [DOI] [PubMed] [Google Scholar]
- 40. Jacques PF,, Taylor A,, Moeller S,, et al. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Ophthalmol. 2005; 123: 517–526. [DOI] [PubMed] [Google Scholar]
- 41. LaRowe TL,, Mares JA,, Snodderly DM,, Klein ML,, Wooten BR,, Chappell R. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study. An ancillary study of the Women's Health Initiative. Ophthalmology. 2008; 115: 876–883.e1. [DOI] [PubMed] [Google Scholar]
- 42. Millen AE,, Wactawski-Wende J,, Pettinger M,, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010; 91: 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engelman CD,, Meyers KJ,, Iyengar SK,, et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J Nutr. 2013; 143: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beebe DC,, Holekamp NM,, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010; 44: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gallagher RP,, Lee TK. Adverse effects of ultraviolet radiation: a brief review. Prog Biophys Mol Biol. 2006; 92: 119–131. [DOI] [PubMed] [Google Scholar]
- 46. Robman L,, Taylor H. External factors in the development of cataract. Eye (Lond). 2005; 19: 1074–1082. [DOI] [PubMed] [Google Scholar]
- 47. Jacques PF,, Hartz SC,, Chylack LT,, Jr, McGandy RB,, Sadowski JA. Nutritional status in persons with and without senile cataract: blood vitamin and mineral levels. Am J Clin Nutr. 1988; 48: 152–158. [DOI] [PubMed] [Google Scholar]
- 48. Mukesh BN,, Le A,, Dimitrov PN,, Ahmed S,, Taylor HR,, McCarty CA. Development of cataract and associated risk factors: the Visual Impairment Project. Arch Ophthalmol. 2006; 124: 79–85. [DOI] [PubMed] [Google Scholar]
- 49. Cantorna MT,, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004; 229: 1136–1142. [DOI] [PubMed] [Google Scholar]
- 50. Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol. 2006; 92: 65–79. [DOI] [PubMed] [Google Scholar]
- 51. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004; 79: 362–371. [DOI] [PubMed] [Google Scholar]
- 52. Mark BL,, Carson JA. Vitamin D and autoimmune disease—implications for practice from the multiple sclerosis literature. J Am Diet Assoc. 2006; 106: 418–424. [DOI] [PubMed] [Google Scholar]
- 53. Xue ML,, Zhu H,, Thakur A,, Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002; 80: 340–345. [DOI] [PubMed] [Google Scholar]
- 54. Yin Z,, Pintea V,, Lin Y,, Hammock BD,, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011; 52: 7359–7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Boey PY,, Tay WT,, Lamoureux E,, et al. C-reactive protein and age-related macular degeneration and cataract: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2010; 51: 1880–1885. [DOI] [PubMed] [Google Scholar]
- 56. Mares-Perlman JA,, Brady WE,, Klein BE,, et al. Diet and nuclear lens opacities. Am J Epidemiol. 1995; 141: 322–334. [DOI] [PubMed] [Google Scholar]
- 57. Mares-Perlman JA,, Klein BE,, Klein R,, Ritter LL. Relation between lens opacities and vitamin and mineral supplement use. Ophthalmology. 1994; 101: 315–325. [DOI] [PubMed] [Google Scholar]
- 58. Tavani A,, Negri E,, La Vecchia C. Food and nutrient intake and risk of cataract. Ann Epidemiol. 1996; 6: 41–46. [DOI] [PubMed] [Google Scholar]
- 59. Appleby PN,, Allen NE,, Key TJ. Diet vegetarianism, and cataract risk. Am J Clin Nutr. 2011; 93: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 60. Vincent HK,, Innes KE,, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007; 9: 813–839. [DOI] [PubMed] [Google Scholar]
- 61. Cruickshanks KJ,, Klein BE,, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am J Public Health. 1992; 82: 1658–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. West SK,, Duncan DD,, Munoz B,, et al. Sunlight exposure and risk of lens opacities in a population-based study: the Salisbury Eye Evaluation project. JAMA. 1998; 280: 714–718. [DOI] [PubMed] [Google Scholar]
- 63. Taylor HR,, West SK,, Rosenthal FS,, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988; 319: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 64. Delcourt C,, Carriere I,, Ponton-Sanchez A,, Lacroux A,, Covacho MJ,, Papoz L. Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: the Pathologies Oculaires Liees a l'Age (POLA) study. Arch Ophthalmol. 2000; 118: 385–392. [DOI] [PubMed] [Google Scholar]
- 65. Hayashi LC,, Hayashi S,, Yamaoka K,, Tamiya N,, Chikuda M,, Yano E. Ultraviolet B exposure and type of lens opacity in ophthalmic patients in Japan. Sci Total Environ. 2003; 302: 53–62. [DOI] [PubMed] [Google Scholar]
- 66. Neale RE,, Purdie JL,, Hirst LW,, Green AC. Sun exposure as a risk factor for nuclear cataract. Epidemiology. 2003; 14: 707–712. [DOI] [PubMed] [Google Scholar]
- 67. Pastor-Valero M,, Fletcher AE,, de Stavola BL,, Chaques-Alepuz V. Years of sunlight exposure and cataract: a case-control study in a Mediterranean population. BMC Ophthalmol. 2007; 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murad MH,, Elamin KB,, AbuElnour NO,, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011; 96: 2997–3006. [DOI] [PubMed] [Google Scholar]
- 69. Looker AC,, Dawson-Hughes B,, Calvo MS,, Gunter EW,, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002; 30: 771–777. [DOI] [PubMed] [Google Scholar]
- 70. Calvo MS,, Whiting SJ. Prevalence of vitamin D insufficiency in Canada and the United States: importance to health status and efficacy of current food fortification and dietary supplement use. Nutr Rev. 2003; 61: 107–113. [DOI] [PubMed] [Google Scholar]
- 71. Hanley DA,, Davison KS. Vitamin D insufficiency in North America. J Nutr. 2005; 135: 332–337. [DOI] [PubMed] [Google Scholar]
- 72. Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed]