Abstract
Objectives
This study sought to investigate plasma levels of glucose and free fatty acids (FFA) and their relationship with adrenergic activation and insulin resistance (IR) in patients with advanced congestive heart failure (CHF).
Background
Adrenergic activation and IR are hallmarks of advanced heart failure. The resulting changes in fuel substrate availability and their implications for exercise capacity have not been elucidated.
Methods
Subjects with CHF underwent maximal exercise testing. Plasma glucose, FFA, insulin, and norepinephrine (NE) levels were measured at rest and at peak exercise. Beta-receptor sensitivity to NE was assessed using the Chronotropic Responsiveness Index (CRI). Homeostasis Model Assessment Index >2.5 defined IR. Left ventricular ejection fraction was estimated by 2-dimensional echocardiography.
Results
Ninety-six subjects were enrolled. CHF subjects without IR (CHF/No-IR), but not those with IR (CHF/IR), significantly increased glucose and insulin in response to exercise. Only CHF/No-IR subjects increased FFA in response to exercise (0.14 ± 0.27 mmol/l; p = 0.027). NE increased significantly less with exercise, and CRI was lower in CHF/IR subjects compared with CHF/No-IR subjects (1.3 ± 1.4 vs. 2.5 ± 2.1; 6.4 ± 2.6 vs. 8.5 ± 3.4; p = 0.069). CRI correlated with the exercise-induced increase in FFA (r = 0.41; p < 0.005). These results stayed the same after excluding diabetic patients from the CHF/IR group.
Conclusions
Circulating FFA levels increased during exercise in CHF subjects without IR, but not in those with IR or DM. Increased FFA availability during exercise may represent a catecholamine-dependent compensatory fuel shift in CHF.
Keywords: free fatty acids, glucose, heart failure, insulin resistance
In congestive heart failure (CHF), profound changes in the rate of whole-body free fatty acids (FFA) and carbohydrate oxidation take place, although studies examining the direction of such fuel shifts, particularly in the failing human heart, have not led to clear conclusions (1). Some investigators have reported a shift away from FFA utilization (2), whereas others have reported a shift towards FFA utilization (3). Few studies have examined overall fuel utilization in patients with CHF during exercise and in vivo (4,5). In contrast to the prevailing paradigm that glucose is the preferred fuel in humans with CHF, at least 1 study suggests that FFA utilization is critically linked to reduced cardiac efficiency (5).
Overall fuel source utilization is governed by substrate availability and the organism’s ability to use the substrate, which, in patients with CHF, may be affected by comorbid conditions such as diabetes (DM) or insulin resistance (IR), as well as pharmacotherapy and adrenergic activation (6,7). The availability of circulating fuel substrates in subjects with CHF receiving standard medical therapy during exercise has not been studied in regard to their status of IR. Accordingly, we examined resting and peak exercise levels of glucose, insulin, and norepinephrine (NE), as well as FFA, in subjects with advanced CHF on optimal medical therapy.
Methods
Patient population
Subjects with CHF were recruited from consecutive patients referred for a cardiopulmonary exercise test at New York Presbyterian Hospital–Columbia University Medical Center and were included if the following criteria were met: CHF, New York Heart Association class II to IV symptoms, left ventricular ejection fraction (LVEF) ≤40%, and optimal medical therapy including angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and beta-blocker for at least 3 months unless contraindicated. Subjects were considered diabetic if this diagnosis had been made previously and they were taking antidiabetic medications. Nondiabetic subjects were categorized as insulin resistant if the Homeostasis Model Assessment (HOMA) Index was >2.5. Subjects were excluded if they had been hospitalized within 3 months for cardiovascular diseases or had noncardiac exercise-limiting illnesses, such as moderate-to-severe arthritis, peripheral vascular disease, or chronic obstructive pulmonary disease. Study approval was obtained from the institutional review board, and all subjects signed informed written consent.
Study protocol
Subjects were asked to withhold breakfast on the morning of the test and take their medication with water. After 20 min of supine rest, an 18- or 20-ga angio-catheter was inserted into a forearm vein, and blood for biochemical assessments was drawn. Subjects underwent a symptom-limited cardiopulmonary exercise test, and blood was again drawn immediately upon cessation of exercise. Samples were stored on ice after collection and promptly centrifuged at 1,550 g for 10 min. After separation, plasma aliquots were stored frozen at −80°C.
Study procedures
CARDIOPULMONARY EXERCISE TESTING
Peak oxygen consumption (pVO2) in milliliters per kilogram per min (ml/kg/min) was assessed during graded treadmill exercise. Work rate increased continuously as a ramp function by augmenting the speed and grade of the treadmill according to the Naughton protocol (8). Heart rate and electrocardiograms were recorded continuously throughout the test, and blood pressure was measured at rest, every 2 min during exercise, and immediately upon cessation of exercise. Expired gases were collected throughout exercise, and oxygen consumption was calculated on a breath-by-breath basis using a metabolic cart (Medgraphics, St. Paul, Minnesota). Subjects exercised to exhaustion, and pVO2 was defined as the highest value of oxygen uptake attained in the final 20 s of exercise.
Chronotropic Responsiveness Index (CRI), a measure of post-synaptic beta-receptor responsiveness to NE, was calculated using the following formula: (peak heart rate — baseline heart rate)/Log (peak norepinephrine — baseline norepinephrine) (9).
ECHOCARDIOGRAPHY
Echocardiograms were performed within 6 months of the VO2 study, and results were gathered from online charts. LVEF was estimated using 2-dimensional parasternal long, parasternal short, apical long, and apical short images.
BIOCHEMICAL ANALYSES
Plasma glucose was determined with a glucose oxidase method (GM7 Analyser, Analox Instruments, Hammersmith, United Kingdom). Insulin levels were determined in heparinized plasma by 2-site chemiluminescent immunometric assay (Diagnostic Products Corp., Los Angeles, California). Serum FFAs were determined with an enzymatic colorimetric method (Nefa C test, Wako Chemicals, Neuss, Germany). NE was determined by high-performance liquid chromatography separation and tandem mass spectrometric detection (API 4000, Applied Biosystems, Carlsbad, California) in the first 55 CHF patients.
We estimated IR using the HOMA Index, which is defined as fasting insulin (μU/ml) times fasting glucose (mmol/l) divided by 22.5 (10). A HOMA index >2.5 defined IR (11). Subjects using exogenous insulin were excluded from the HOMA index calculations.
Statistical analysis
Continuous variables are expressed as mean ± SD. Continuous variables were explored for normal distribution according to histograms and the Shapiro-Wilk test. In Tables 1 and 2, the comparison of quantitative variables between the CHF groups was performed using the Student t test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. Categorical variables are expressed as number and percentage, and the chi-square test was used for comparisons with Fisher exact test when appropriate. In Figures 1 and 2, for the comparison of the change in glucose or FFA in response to exercise between diabetic patients, CHF subjects with IR (CHF/IR), and CHF subjects without IR (CHF/No-IR), we used the Kruskal-Wallis test. In Figure 3, for the comparison of the change in FFA in response to exercise according to the pVO2 in quartiles, we used the Jonckheere-Terpstra test for trend. For the comparison between resting and exercise measurements (response to exercise), we used the paired-samples Wilcoxon test for non-normally distributed variables. Bivariate correlations were analyzed by the Pearson correlation coefficient, and the significance test was 2-tailed. For correlation analyses that involved the HOMA Index, we excluded patients with insulin-dependent DM because we did not control the administration of exogenous insulin before the study and the possible influence of this exogenous insulin on the HOMA Index. We performed backward method linear multiple regression analysis to predict the increase in FFA during exercise with an inclusion criteria of p < 0.05 and an exclusion criteria of p > 0.10. Analysis of residuals was performed. Significance was set at p < 0.05 (2-tailed). SPSS version 17 was used to perform all statistical evaluations (SPSS, Chicago, Illinois).
Table 1.
Baseline Characteristics
| p Value |
||||||
|---|---|---|---|---|---|---|
| CHF/DM (n = 18) |
CHF/IR (n = 31) |
CHF/No-IR (n = 47) |
IR vs. No-IR | IR vs. DM | No IR vs. DM | |
| Age, yrs | 56.6 ± 9.9 | 51.6 ± 14.0 | 52.1 ± 12.0 | 0.871 | 0.186 | 0.125 |
| Male | 15 (83) | 20 (65) | 30 (65) | 0.950 | 0.160 | 0.168 |
| BMI, kg/m2 | 31.3 ± 3.8 | 33.2 ± 6.5 | 25.6 ± 3.9 | <0.001* | 0.540* | <0.001* |
| Beta-blocker | 16 (89) | 28 (90) | 44 (96) | 0.560 | 0.624 | 0.189 |
| CHF etiology | 0.740 | 0.142 | 0.291 | |||
| Ischemic | 9 (50) | 9 (29) | 16 (33) | |||
| Nonischemic | 9 (50) | 22 (71) | 31 (67) | |||
| LVEF, % | 19.8 ± 8.7 | 25.8 ± 10.6 | 24.4 ± 10.8 | 0.620* | 0.042* | 0.103* |
| pVO2, ml/kg/min | 14.4 ± 4.6 | 15.6 ± 3.8 | 18.2 ± 5.7 | 0.001* | 0.152* | 0.004* |
| pVO2, l/min | 1.37 ± 0.51 | 1.58 ± 0.57 | 1.36 ± 0.52 | 0.089* | 0.255* | 0.958* |
| RER, beats/min | 1.04 ± 0.11 | 1.06 ± 0.10 | 1.04 ± 0.11 | 0.972 | 0.708* | 0.972 |
| Resting HR | 75 ± 12 | 73 ± 12 | 69 ± 14 | 0.148 | 0.667 | 0.113 |
| Peak HR | 116 ± 20 | 120 ± 21 | 126 ± 24 | 0.311 | 0.400* | 0.056* |
Values are mean ± SD or n (%). For comparison of quantitative variables between groups, we used the Student t test except as noted. For comparison of categorical variables between groups, we used the chi-square test, with the Fisher exact test when appropriate.
Mann-Whitney U test used for the comparison.
BMI = body mass index; CHF = congestive heart failure; DM = diabetes mellitus; HR = heart rate; IR = insulin resistance; LVEF = left ventricular ejection fraction; pVO2 = peak oxygen consumption; RER = respiratory exchange ratio.
Table 2.
Change in Circulating Substrate Levels as a Function of IR
| p Value |
||||||
|---|---|---|---|---|---|---|
| Diabetic (n = 18) |
CHF/IR (n = 31) |
CHF/No-IR (n = 47) |
IR vs. No-IR | IR vs. DM | No-IR vs. DM | |
| Glucose, mg/dl | ||||||
| Rest | 188.56 ± 71.30 | 113.03 ± 18.00 | 97.63 ± 11.16 | <0.001 | <0.001 | <0.001 |
| Peak exercise | 183.31 ± 68.28 | 115.26 ± 17.14 | 108.43 ± 15.0 | 0.270 | <0.001 | <0.001 |
| ΔGlucose | −0.69 ± 13.61 | 2.23 ± 11.13 | 10.87 ± 10.99 | <0.001 | 0.770 | 0.005 |
| FFA, mmol/l | ||||||
| Rest | 0.56 ± 0.25 | 0.65 ± 0.29 | 0.60 ± 0.25 | 0.275 | 0.312 | 0.506 |
| Peak exercise | 0.61 ± 0.21 | 0.66 ± 0.28 | 0.75 ± 0.33 | 0.694 | 0.519† | 0.136 |
| ΔFFA | 0.043 ± 0.14 | −0.008 ± 0.16 | 0.14 ± 0.27 | 0.030 | 0.081 | 0.064† |
| Insulin, μU/ml | ||||||
| Rest | 51.83 ± 69.4 | 28.19 ± 26.02 | 6.13 ± 1.55 | <0.001 | 0.626 | <0.001 |
| Peak exercise | 52.74 ± 68.97 | 24.57 ± 13.06 | 8.10 ± 4.71 | <0.001 | 0.297 | <0.001 |
| ΔInsulin | 0.91 ± 28.37 | −3.79 ± 20.26 | 1.91 ± 4.35 | 0.041 | 0.749 | 0.288 |
| NE, nM* | ||||||
| Rest | 4.75 ± 4.90 | 3.56 ± 2.57 | 3.07 ± 1.72 | 0.516 | 0.882 | 0.507 |
| Peak exercise | 6.55 ± 5.34 | 4.71 ± 2.64 | 5.61 ± 3.39 | 0.301 | 0.790 | 1.000 |
| ΔNE | 1.79 ± 1.77 | 1.15 ± 1.26 | 2.53 ± 2.09 | 0.004 | 0.328† | 0.428 |
| CRI | 6.06 ± 2.75 | 7.12 ± 2.57 | 8.49 ± 3.38 | 0.175† | 0.405† | 0.098† |
Values are mean ± SD.
NE levels were measured only in the first 55 patients: 6 CHF/DM, 20 CHF/IR, and 29 CHF/no IR. For all comparisons between groups, we used the Mann-Whitney U test, except as noted.
We used the Student t test because the variables followed a normal distribution.
CRI = Chronotropic Responsiveness Index; FFA = free fatty acids; NE = norepinephrine; other abbreviations as in Table 1.
Figure 1. Mean Glucose Levels Change in Response to Exercise.
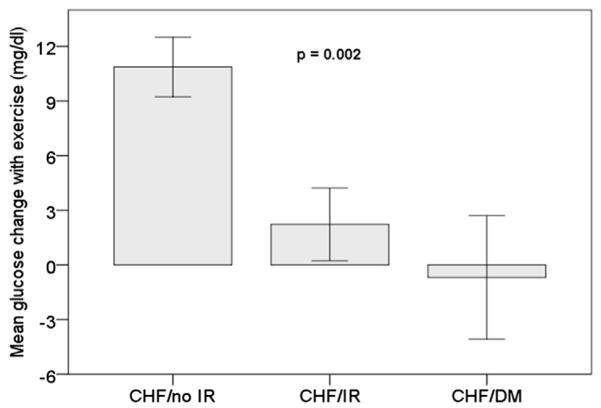
During exercise, CHF/No-IR subjects increased their plasma glucose level, whereas CHF/IR subjects did not. Of note, although glucose levels increased in 87% of CHF/No-IR subjects, glucose levels actually decreased in 31% of CHF/IR subjects. CHF = congestive heart failure; DM = diabetes mellitus; IR = insulin resistance.
Figure 2. Mean FFA Levels Change in Response to Exercise.
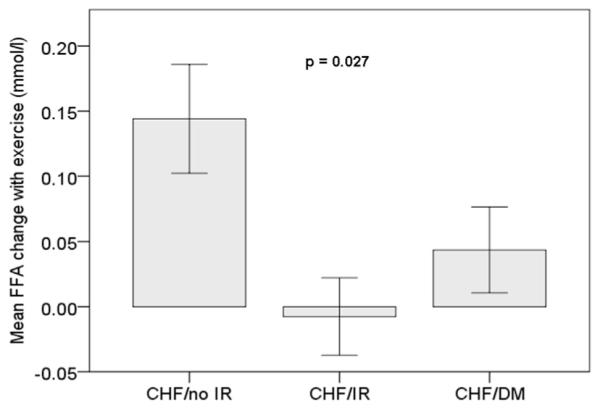
During exercise CHF/No-IR subjects increased their circulating FFA (0.14 ± 0.27 mmol/l; p = 0.027), but not in CHF-IR subjects. FFA = free fatty acids; other abbreviations as in Figure 1.
Figure 3. Relationship Between FFA and pVO2.
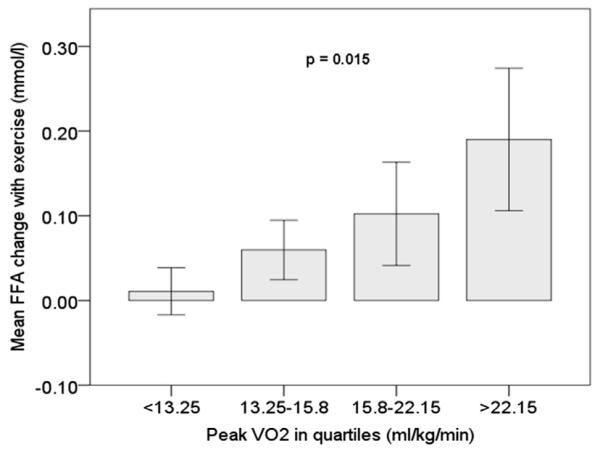
Circulating FFA increased more during exercise in CHF patients with higher pVO2. The p value was calculated using the Jonckheere-Terpstra test for trend. pVO2 = peak oxygen consumption; other abbreviations as in Figures 1 and 2.
Results
Ninety-six subjects age 52.5 ± 12.5 years were enrolled. The etiology of CHF was ischemic cardiomyopathy in 33 (34%) patients. The mean LVEF was 23.5 ± 10.5%, and pVO2 was 16.7 ± 5.2 ml/kg/min. Eighteen (17%) patients had been previously diagnosed with diabetes and were receiving insulin and/or oral anti-hyperglycemic agents. In addition to the 18 diabetic patients (CHF/DM), 31 CHF subjects had IR (CHF/IR), and 47 CHF subjects did not have IR (CHF/No-IR). There was no difference in the etiology of CHF between CHF/IR, CHF/DM, and CHF/No-IR subjects. CHF/IR subjects had a higher body mass index (BMI) compared with CHF/No-IR subjects. Only 7 patients were not receiving a beta-blocker (2 CHF/No-IR and 5 CHF/IR). Type and dose of beta-blocker were similar in the CHF groups (Table 1).
Resting levels of circulating fuel substrate
Resting levels of glucose and insulin were significantly lower in CHF/No-IR compared with CHF/IR and CHF/DM; NE levels were lower as well in CHF/No-IR, but this did not reach statistical significance (Table 2). There was no difference in resting FFA levels between the groups.
Response to exercise
CHF/IR and CHF/DM patients did not at all increase glucose or insulin in response to exercise (p > 0.05) and had the smallest increase in NE (p = 0.001 in CHF/IR and p = 0.03 in CHF/DM). By contrast, CHF/No-IR increased glucose (p < 0.001), insulin (p = 0.002), and NE (p < 0.001) in response to exercise.
Of note, although glucose levels increased in 87% of CHF/No-IR subjects, glucose levels actually decreased in 31% of CHF/IR and CHF/DM subjects (Fig. 1).
Similarly, FFA increased in CHF/No-IR subjects (0.14 ± 0.27 mmol/l; p = 0.027), but not in CHF/IR and CH/DM subjects (Fig. 2).
Relationship of pVO2, IR, CRI, and changes in circulating fuel substrate in CHF subjects
CHF/IR and CHF/DM subjects had lower pVO2 compared with CHF/No-IR subjects (Table 1), and pVO2 and HOMA Index were inversely correlated (r= −0.254; p=0.026). Age, sex, LVEF, and etiology of cardiomyopathy did not differ between CHF/IR, CHF/DM, and CHF/No-IR subjects.
NE was lower and CRI was higher in CHF/No-IR subjects. Furthermore, CRI was correlated with delta FFA (r = 0.41, p < 0.005), and FFA increased more during exercise in those with higher pVO2 (Fig. 3).
In order to evaluate what factors were associated with an increase in FFA levels during exercise in CHF, we performed backward multivariate linear regression analysis using the following variables: HOMA Index, CRI, age, pVO2, and BMI. In this analysis, only CRI remained significant in the model (Beta = 0.027; p = 0.039).
Discussion
We measured plasma levels of FFA, glucose, NE, and insulin at rest and following maximal exercise in stable outpatients with CHF. Our principal findings are as follows:
First, IR was present in 50% of the CHF patients, and its severity was inversely correlated with pVO2.
Second, CHF/IR and CHF/DM subjects had higher resting levels of glucose, insulin, and NE.
Third, in response to exercise, different patterns of change in circulating hormones and fuel substrate occurred; the stress response in CHF/No-IR was characterized by increases in NE, insulin, and glucose, as well as FFA levels. CHF/IR and CHF/DM patients did not have increased glucose, insulin, or FFA and had the lowest increase in NE. Importantly, all subjects provided similar effort and maximal effort as evidenced by nearly identical respiratory exchange ratios averaging 1.04 during metabolic testing. At this similar stage of metabolic effort, changes in circulating substrate were statistically significantly different between groups. This remained the case after excluding diabetic patients.
Lastly, an increase of circulating FFA was associated with better exercise capacity and better CRI, which may indicate that FFA release is important for exercise capacity and possibly mediated by preserved sympathetic activation.
Many studies have described an association of DM and CHF: in the Framingham Heart Study, diabetes mellitus was an independent risk factor for the development of CHF, with a 2.4-fold risk increase in men and a 5-fold risk increase in women (12). In the UKPDS (UK Prospective Diabetes Study), for every 1% increase in glycosylated hemoglobin (HbA1c), the risk of developing CHF rose by 16% (13). Although this potentially causal relationship of DM and CHF is now well described, evidence is mounting that heart failure itself can cause IR. Swan et al. (14) demonstrated that CHF is associated with marked IR, and the adverse association of IR and exercise capacity has also been established (15).
Our data on the prevalence of IR and DM, as well as their association with diminished exercise capacity, are concordant with the previously cited studies (12–15). We tried to further characterize this metabolic phenotype and/or the root cause of IR in CHF. Our study, although cross sectional in nature, is the first to explore changes in both resting and exercise-induced levels of circulating fuel substrate, and simultaneously investigate the relationship between fuel substrate availability, IR, and adrenergic activation. Adrenergic activation is extremely well studied in CHF, and our observations regarding higher resting NE levels, diminishing NE release in response to exercise, and reduced beta receptor sensitivity (CRI) in patients with more advanced CHF (i.e., lower pVO2) were not only expected (9,16), but underscore a well-established paradigm of compensatory, but ultimately detrimental, neurohormonal activation in CHF (17). Clearly, the ability of the end-stage CHF patients to release NE is diminished, and our data seem to suggest a similar phenomenon for glucose and FFA.
Resting levels of glucose were higher, apparently resulting in an “exhausted” mechanism for glucose release during exercise (much like what is seen with NE and possibly linked to it). We believe that in this setting, FFAs are mobilized to serve as a complimentary fuel; thus, exercise-induced increases in FFA are seen. This increase in FFA is probably mediated by NE, and the fact that CRI and not the HOMA Index predicted the exercise-induced increase in FFA after correcting for age, pVO2, and BMI supports this. Although this compensatory mechanism is activated, it is outweighed by the loss of glucose utilization, and consequently, exercise capacity decreases. The inverse relationship of HOMA Index (or other measures of IR) and exercise capacity shown in our study and previously by others (15) are consistent with a dominant role of glucose utilization in determining exercise capacity.
We observed nominally higher resting FFA levels in CHF/IR versus CHF/No-IR subjects, possibly by increased lipolysis due to rising catecholamine levels, a phenomenon some have referred to as the “hyperadrenergic free fatty acid load in CHF” (18). Regrettably, we were unable to demonstrate a statistically significant difference in FFA resting levels in the nondiabetic CHF patients with and without IR. Interestingly, diabetic patients with CHF had the lowest levels of FFA, indicating either a completely different pathophysiology or even higher reliance on FFA. It is equally speculative, but conceivable, that the very high insulin levels in this group suppressed lipolysis at rest.
Given the paucity of data in the fuel-availability arena, a recent study by Melenovsky et al. (4) is noteworthy. In an experimental setup similar to ours, the authors identified circulating pyruvate—a product of glycolysis—as closely associated with pVO2. Although we did not measure pyruvate, our data on increased resting levels of insulin, glucose, and FFA are concordant with their findings. Interestingly, these authors observed very high resting FFA levels and a sharp decrease following exercise in diabetic patients, an observation concordant with our hypothesis on higher reliance of FFA in diabetic patients. With regard to other findings, some of them discordant, it is of note that Melenovsky et al. (4) in their intriguing study compared diabetic and nondiabetic CHF patients with an average HOMA Index of 4, whereas we separated nondiabetics according to HOMA Index <2.5. Ultimately, it is important to emphasize that circulating substrates such as FFA and glucose are not necessarily indicative of tissue utilization, and neither Melenovsky et al. nor we examined the production of FFA or glucose by the liver or its consumption by heart and skeletal muscle (19,20). Future studies should build on the current evidence and simultaneously study fuel availability and utilization.
We believe our data support the following sequence of events: CHF ensues, catecholamine levels rise, catecholamines stimulate gluconeogenesis and lipolysis, glucose and FFA levels rise, glucose and FFA stores are depleted, impaired exercise tolerance ensues, and the less efficient fuel FFA is utilized to compensate (CHF/No-IR phenotype). Eventually, FFA and glucose stores are depleted, and circulating substrate fails to increase during demand situations (CHF/IR or CHF/DM phenotypes).
Although such a hypothesis cannot solely explain the complex syndrome of CHF and is somewhat speculative based on our data alone, at least 3 prior studies conducted in different settings support this concept. Nikolaidis et al. (21) studied the changes in resting plasma levels of NE, FFA, glucose, and insulin in 12 dogs during the evolution from a normal state to advanced CHF. With the induction of heart failure by pacing, NE and FFAs progressively increased and IR worsened. Of note, these metabolic changes preceded changes in cardiac output and left ventricular dilation. Examining the role of FFA as a fuel in exercise in humans, Tuunanen et al. (5) attempted to facilitate glucose uptake by suppressing FFA production during exercise using acipimox, a lipolysis inhibitor. Suppression of FFA resulted in decreased cardiac work and efficiency in CHF patients. Although this result was entirely unanticipated, it supported findings by Augustus et al. (22), previously made in a lipase-deficient mouse model, where deprivation of lipase-derived FFA resulted in cardiac dysfunction. We are the first, to our knowledge, to demonstrate an association of better exercise capacity (pVO2) and higher exercise-induced FFA increases in CHF. Our finding is concordant with the aforementioned observations by Nikolaidis et al. (21) in dogs, Augustus et al. (22) in mice, and by Tuunanen et al. (5) in humans, and further underscores the importance of FFA availability in CHF.
The overall goal of studies in fuel metabolism in CHF is to develop new therapeutic approaches. How does the current data compliment and/or expand existing knowledge in this regard? Our main contribution is that we have identified FFA availability as a potentially critical element of exercise intolerance in advanced CHF. Furthermore, we have begun to elucidate mechanisms underlying FFA release in CHF and identify adrenergic activation as a possible root cause for IR in CHF. The latter finding may be of particular interest, given recent efforts to treat CHF with parasympathetic stimulation, which may counteract adrenergically mediated IR development via a mechanism different from beta blockade (23).
Study limitations
Our study is limited mainly by its cross-sectional nature and the lack of a healthy control group, but our data clearly demonstrate different patterns in circulating fuel substrates at rest and in response to exercise between CHF/IR, CHF/DM, and CHF/No-IR subjects. These differences in metabolic phenotype have not previously been reported. That said, only tracer studies can elucidate whether, for example, FFA production by the liver and adipose tissue and/or clearance by muscle is altered in CHF. Our data should prove helpful to design such studies, which would add fundamental insights into the pathophysiology of CHF. Similarly, although our results may suggest a progressive worsening of IR with detrimental effects on functional capacity, we cannot exclude that physical inactivity and increased BMI resulted in IR rather than vice versa. In this context, it is of note that Sokos et al. (24) recently demonstrated a remarkable improvement of LVEF and pVO2 after 5 weeks of chronic administration of glucagon-like peptide, a naturally occurring peptide with both insulinomimetic and insulinotropic actions (24). Here, restoration of insulin effects preceded improvement in functional parameters. We believe that, conversely, and similar to the observation made by Nikolaidis et al. (21) in dogs, the development of IR in CHF precedes a decrease in exercise capacity. Longitudinal studies are needed to prove this assertion.
Conclusions
IR was present in 50% of the CHF patients, and its severity was inversely correlated with pVO2. Patients with CHF/IR and CHF/DM had higher resting levels of glucose, insulin, and NE. These patients did not increase glucose, insulin, or FFA and had a lower increase in NE with response to exercise.
Acknowledgments
This study was supported by National Institutes of Health grants R01HL45095 (to Dr. Jorde) and 1K23HL103795-01A1 (to Dr. Uriel).
Abbreviations and Acronyms
- BMI
body mass index
- CHF
congestive heart failure
- CRI
Chronotropic Responsiveness Index
- DM
diabetes mellitus
- FFA
free fatty acids
- HOMA
Homeostasis Model Assessment
- IR
insulin resistance
- LVEF
left ventricular ejection fraction
- NE
norepinephrine
- pVO2
peak oxygen consumption
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–55. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Davila-Roman VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension. 2003;41:83–7. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 3.Paolisso G, Gambardella A, Galzerano D, et al. Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism. 1994;43:174–9. doi: 10.1016/0026-0495(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 4.Melenovsky V, Kotrc M, Polak J, et al. Availability of energetic substrates and exercise performance in heart failure with or without diabetes. Eur J Heart Fail. 2012;14:754–63. doi: 10.1093/eurjhf/hfs080. [DOI] [PubMed] [Google Scholar]
- 5.Tuunanen H, Engblom E, Naum A, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–7. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 6.Schneider JG, von Eynatten M, Parhofer KG, et al. Atorvastatin improves diabetic dyslipidemia and increases lipoprotein lipase activity in vivo. Atherosclerosis. 2004;175:325–31. doi: 10.1016/j.atherosclerosis.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab. 2003;284:E331–9. doi: 10.1152/ajpendo.00298.2002. [DOI] [PubMed] [Google Scholar]
- 8.Naughton J, Sevellus G, Balke B. Physiologic responses of normal and pathological subjects to a modified work capacity test. J Sports Med Phys Fitness. 1963;44:201–7. [PubMed] [Google Scholar]
- 9.Jorde UP, Vittorio TJ, Kasper ME, et al. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: time to pace? Eur J Heart Fail. 2008;10:96–101. doi: 10.1016/j.ejheart.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart study. Diabetes Care. 2002;25:1177–84. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 11.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: The Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swan JW, Anker SD, Walton C, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–32. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 15.AlZadjali MA, Godfrey V, Khan F, et al. Insulin resistance is highly prevalent and is associated with reduced exercise tolerance in nondiabetic patients with heart failure. J Am Coll Cardiol. 2009;53:747–53. doi: 10.1016/j.jacc.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 16.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of postsynaptic beta-adrenergic desensitization. Circulation. 1989;80:314–23. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 17.Feldman DS, Elton TS, Sun B, Martin MM, Ziolo MT. Mechanisms of disease: detrimental adrenergic signaling in acute decompensated heart failure. Nat Clin Pract Cardiovasc Med. 2008;5:208–18. doi: 10.1038/ncpcardio1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–46. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271–83. doi: 10.2337/diabetes.51.2007.s271. [DOI] [PubMed] [Google Scholar]
- 20.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 21.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Augustus AS, Buchanan J, Park TS, et al. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–23. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- 23.De Ferrari GM, Crijns HJ, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–55. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 24.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]


