Abstract
Goals:
The aim of this study was to validate the ability of symptom frequency questionnaire to differentiate between irritable bowel syndrome (IBS) patients and healthy subjects.
Background:
A digestive symptom frequency questionnaire (DSFQ) was previously used in a food efficacy trial in a non-IBS population with mild gastrointestinal symptoms.
Study:
We compared 2 well-defined populations: 100 IBS patients fulfilling Rome III criteria (mean age 32 y; range, 18 to 59 y), and 100 sex-matched and age-matched healthy subjects. Frequency of individual digestive symptoms (abdominal pain/discomfort, bloating, flatulence, borborygmi) was assessed using a 5-point Likert scale (from none to everyday of the week) and the IBS severity with the IBS-SSS questionnaire. Health-Related Quality of life (HRQoL) was assessed with the Food and Benefits Assessment (FBA) and Functional Digestive Disorders Quality of Life (FDDQL) questionnaires. The digestive (dis)comfort dimension of these questionnaires was considered as the main dimension for HRQoL.
Results:
The DSFQ discriminated IBS from healthy subjects with a significant difference (P<0.001) between groups (estimated mean difference=5.58; 95% CI, 4.91-6.28). On the basis of the ROC curve (AUC=0.9479), a cutoff value of 5 gives a sensitivity of 92% and a specificity of 84%, with a positive likelihood ratio of 5.75. Composite score of symptoms correlated strongly (P<0.0001) with digestive discomfort measured by FDDQL (−0.816), digestive comfort measured by FBA (−0.789), and the IBS-SSS score (0.762).
Conclusions:
Measurement of digestive symptom frequency by means of the DSFQ can differentiate IBS from healthy subjects, and shows a good correlation with other validated questionnaires (clinical trial #NCT01457378).
Key Words: digestive symptoms, patient-reported outcome, irritable bowel syndrome, quality of life
Digestive symptoms are very common complaints in the general population.1,2 The type, frequency, and intensity of these symptoms largely vary between subjects3–5 and may impact their daily life.2,6 Abdominal pain/discomfort and bloating are often reported as the more troublesome and frequent symptoms, especially in irritable bowel syndrome (IBS).7,8 In the absence of validated biomarkers allowing objective measures of these symptoms, patient-reported outcomes (PRO) are recognized as valuable instruments to measure patients’ perception of symptoms. Such instruments are therefore used in clinical trials to determine the efficacy of food or drugs in alleviating digestive symptoms.9–11 Owing to the interindividual variability in intensity, severity, and fluctuation over the time, appropriate PRO should be used according to the target population.
Measures of digestive symptoms are available for specific functional gastrointestinal (GI) disease, but there is a lack of questionnaires for the general population. Most symptom questionnaires have been developed to be used in IBS and mainly focused on symptom severity. Severity of individual digestive symptoms has been measured in different ways, including 5-point Likert scale (from none to very severe) or 21-, 11-, or 10-point numeric scales, on a daily or weekly basis.12 Overall severity of IBS has been measured with a questionnaire (IBS-SSS) scoring different aspects of the disease (pain, bloating, bowel dysfunction, and quality of life).13 Despite its use in populations of patients with IBS, there are no data in the literature supporting the use of these questionnaires in the general population. Indeed, those PROs may lack sensitivity to detect changes in the general population reporting occasional digestive symptoms or even in the very mild spectrum of IBS.
To measure the efficacy of dietary intervention on digestive symptoms in the general population, a simple PRO was developed. This PRO assessed the frequency over a 1-week period of 4 digestive symptoms (abdominal pain/discomfort, bloating, flatulence, and rumbling stomach/borborygmi) using 5-point Likert scales (from none to everyday of the week).14 This digestive symptom frequency questionnaire (DSFQ) proved to be sensitive to detect significant changes in response to a probiotic compared with control food product in 2 independent interventional studies in the general population reporting mild GI discomfort.14,15
Our current aim was to determine the sensitivity and specificity of this questionnaire to differentiate healthy subjects and IBS patients, to compare it with a validated severity questionnaire in IBS subjects (IBS-SSS), and to correlate the frequency of digestive symptoms with Health-Related Quality of Life (HRQoL).
METHODS
Study Subjects
Main Study
Patients with IBS (n=100; 18 y and above), meeting Rome III criteria (all subtypes),5 and 100 age-matched (18 to 35, 36 to 50, and 51 to 65 y) and sex-matched healthy control (HC) subjects were included in the study. Participants were recruited by public advertisement and from the database of 2 clinical centers in France (Eurofins Optimed, Gières; and RPS France, Caen). Diagnosis of IBS was established using the Rome III questionnaire.5 Patients were required to have a score of >75 on the IBS-SSS at the time of the study (active disease). Patients were excluded if they had clinical signs of alarm (eg, rectorragy, fever, recent weight loss), a known organic disease, including inflammatory bowel disease or cancer, or if they were on antidepressant or analgesic drugs. Healthy subjects were excluded if they had consulted a general practitioner or a gastroenterologist for any functional bowel disorder, had a known organic disease, or received any chronic medical treatment that could influence the GI tract. Healthy subjects completed the Rome III questionnaire to confirm the absence of any functional bowel disorder.
Ancillary Study
In addition, a larger cohort of subjects (521 women, age 18 to 60 y), who had participated in 2 previous independent randomized controlled trials using the same questionnaires, were analyzed to assess the stability of the questionnaire14,15; these subjects recruited from the general population reported minor GI discomfort, defined by the presence of mild digestive symptoms (ie, abdominal discomfort/pain, bloating, flatulence/passage of gas, borborygmi/rumbling stomach) in the past month, but did not fulfill the criteria of functional digestive disorders.
The study protocol was approved by the Ethics Committee Sud Est III (Lyon, France). All participants gave written informed consent before inclusion in the study. The study is registered at Clinicaltrials.gov: NCT 01457378.
Study Design and Procedures
Main Study
A multicenter, observational, prospective study was conducted in France with a single visit for all participants. During the visit, all participants [patients with IBS (n=100) and 100 age-matched and sex-matched HC subjects] were instructed to fill out 4 questionnaires: the DSFQ (frequency of digestive symptom), IBS-SSS (severity of IBS symptoms), Food and Benefits Assessment (FBA) questionnaire (HRQoL), and Functional Digestive Disorders Quality of Life (FDDQL) questionnaire (HRQoL). The recall period was 7 days for DSFQ questionnaire, 10 days for the IBS-SSS, and 2 weeks for both FDDQL and FBA questionnaires.
Ancillary Study: Stability of the Questionnaire
Retrospective study reanalyzing data of participants in 2 previous studies who had filled out the questionnaire 2 times 1 week apart during baseline before intervention.
Frequency of Digestive Symptoms (DSFQ)
The frequency of 4 digestive symptoms (abdominal pain/discomfort, bloating, flatulence/passage of gas, and borborygmi/rumbling stomach) was evaluated using the DSFQ. The frequency of each digestive symptom over a 1-week period was assessed with a 5-point Likert scale [never (0); 1 time per week (1); 2 to 3 days per week (2); 4 to 6 days per week (3); everyday during the week (4)]. A composite score was calculated corresponding to the sum of the scores of the 4 individual symptoms, leading to a score ranging from 0 (never=0 for the 4 symptoms) to 16 (everyday of the week=4 for the 4 symptoms).14
IBS Symptom Severity Scale (IBS-SSS)
IBS severity was assessed using the validated IBS-SSS questionnaire.13 This symptom questionnaire contains 5 questions scoring abdominal pain (2 questions), abdominal distension/bloating, satisfaction with bowel habit, and impact of IBS on patients’ life. All 5 questions contribute equally to a score from 0 to 500. This questionnaire established 3 IBS severity classes: mild (score 75 to 174), moderate (score 175 to 299), or severe (300 to 500). Scores <75 indicate normal bowel function or IBS subjects in inactive period.
HRQoL (FBA and FDDQL)
HRQoL of subjects was assessed by self-administration of the FBA16 and the FDDQL17 questionnaires.
The FBA questionnaire assessed the impact of diet and nutrition on HRQoL. The FBA questionnaire comprises 41 items scoring 7 dimensions (snacking, vitality, well-being, physical appearance, esthetics, digestive comfort, and disease prevention). The scores range from 0 (worst) to 100 (best possible).
The FDDQL questionnaire assesses the impact of digestive complaints on HRQoL. This measure comprises 43 items scoring 8 dimensions (daily activities, anxiety, diet, sleep, health perception, digestive discomfort, coping with disease, and impact of stress). An algorithm provides a complete score for each dimension ranging from 0 (worst) to 100 (best possible).
Statistical Analyses
The sample size of this study had to capture appropriately the variability induced by the pattern of the population (IBS and HC subject), and to allow the exploration of all the range of the questionnaires. This consideration taken into account and the exploratory nature of the study led to state the sample size to 200 completed subjects (100 IBS subjects and 100 HC subjects). With a single evaluation visit, no dropouts were expected, and 200 subjects had to be included.
For this study, all the analyses were performed on the Full Analysis Set population that corresponds to all the subjects screened and included in the study (ie, all the eligible subjects).
To compare the composite score between IBS and HC subjects, the quantitative composite score was analyzed using a variance analysis with subject status (IBS vs. HC) and the stratification covariates (age and sex) as explicative factors. Regardless of the significance, all variables were kept in the model. Adjusted means values of composite score were computed. The underlying assumption of the normality of residuals was checked according to Skewness and Kurtosis indicators as to which values should be in range of −1.5 to 1.5.
To determine with more accuracy whether the composite score is a good tool for discriminating IBS from HC subjects, a receiver operating characteristic (ROC) built to assess the prediction of the subject status according to the composite score was computed. The value of the area under the ROC was calculated, and this ROC curve enabled to choose a reliable threshold to transform the composite score into a binary variable. The threshold was chosen upstream of other analyses to guarantee the integrity of the decision. This threshold of definition of binary composite score variable was chosen in favor of the discrimination of the rate of “true” IBS subjects, thus basing it on the best sensitivity. On the basis of this threshold, the sensitivity and specificity were calculated according to (i) and (ii).
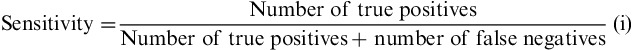 |
 |
Then according to (iii) and (iv) likelihood ratios (LR) were calculated. LRs summarize the information contained in both sensitivity and specificity, a highlight on how likely a given test result is in people with the condition, compared with how likely it is in people without the condition.
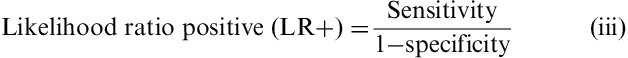 |
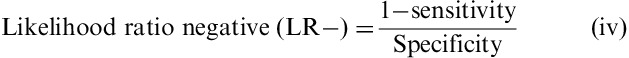 |
The higher the positive LR, the more likely a composite score ≥5 predicted an IBS subject as IBS; the lower the negative LR, the more likely a composite score <5 predicted a healthy subject as healthy; a LR close to 1 means that the composite score is not predictive of the subjects' status (IBS or HC).
To study the relationship between the composite score and IBS-SSS, a logistic regression was performed with the binary composite score as a response variable and with IBS-SSS modalities and age/sex stratification as explicative variables. Regardless of the significance, all variables were kept in the model. To study the relationship between the composite score and digestive (dis)comfort of HRQoL questionnaires, correlations were computed and assessed with the Spearman rank correlation coefficient.
Effect sizes were used to better understand the relationship between the frequency of the digestive symptoms (through the composite score) and the variation of HRQoL questionnaire dimensions (all dimensions of FBA and FDDQL questionnaires), without the subjects’ status dimension. Four modalities of the composite score were defined according to the quartiles, with the first quartile as the reference modality.
To evaluate possible relationships between the digestive symptoms and HRQoL questionnaires, a Canonical Correlation Analysis was performed. Results are presented with a relevance network computing similarity values between each variable, representing the composite score of the digestive symptoms and HRQoL questionnaires. The closer the similarity value is to 1 (or −1), the better the association is (positive for +1 and negative for −1). The closer this similarity value is to 0, the worse the association is. The relevance network with similarity values >0.5 was considered as a relevant association.
Reproducibility of the questionnaire (stability over time), in the ancillary study, was measured by the intraclass correlation coefficient (ICC) between 2 consecutive assessments 1 week apart during the baseline period of 2 randomized controlled trials. During this period, subjects are considered in stable healthy status.
Statistical analyses were performed using SAS Software (SAS Institute Inc.) version 9.2 and or R software version 2.15.2.
RESULTS
Demographics
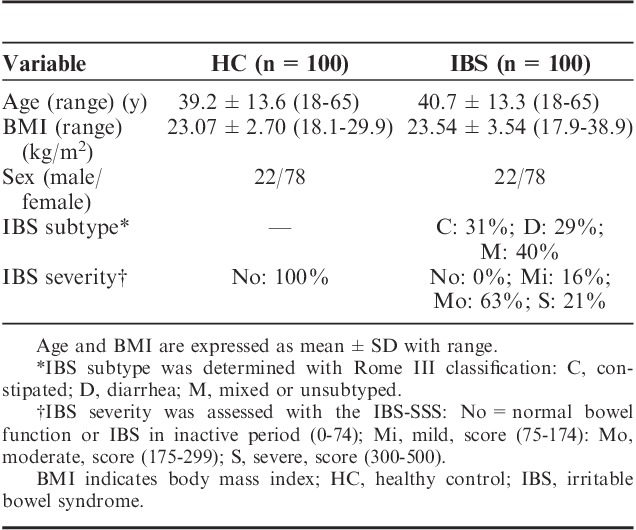
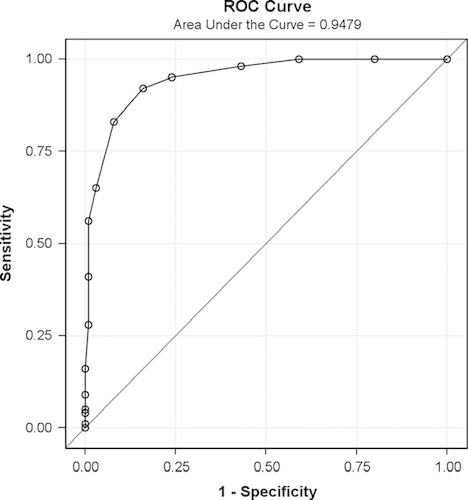
IBS patients and HC subjects were comparable for age and BMI (Table 1). The population was female predominant (78%). The severity of IBS was mainly moderate (63%) and IBS subtypes were well represented in the sample. All HC subjects reported normal bowel as measured by IBS-SSS (scores <75).
TABLE 1.
Demographics in Healthy Control (n=100) and IBS Patients (n=100)
Discrimination of IBS Patients From Healthy Subjects
As expected, IBS patients had higher composite and individual digestive symptoms scores than HC subjects (Table 2). Flatulence was the more frequent symptom in both groups. IBS-C exhibited the highest composite score among IBS subtypes. The composite score significantly discriminated between IBS and healthy subjects (P<0.001 significance between group and estimated mean difference=5.58; 95% CI, 4.91-6.28), with no impact of sex or age. Receiver-operating curve analysis comparing all IBS patients with HC subjects (Fig. 1) gave a ROC score of 0.95. The choice of the cutoff value was based on 3 points on the curve corresponding to the range 4 to 6 of the composite score. Among these 3 points, the sensitivity varied from 95% to 83% and specificity varied from 76% to 92%. A cutoff of >5 was chosen to obtain the best sensitivity for discriminating IBS patients fulfilling Rome III criteria from subjects in the general population, with 92% sensitivity and 84% specificity, and a positive LR of 5.75 and a negative LR of 0.17.
TABLE 2.
Composite Score of Digestive Symptoms, and Individual Digestive Symptoms Scores in Healthy Controls (n=100) and IBS Patients (n=100)
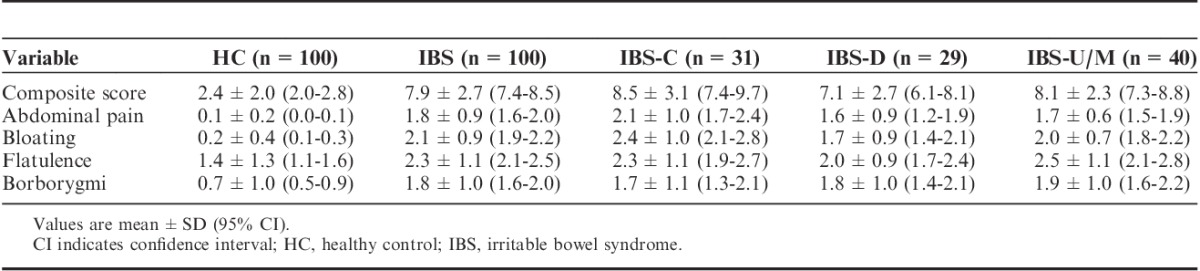
FIGURE 1.
Receiver-operating curve (ROC) for distinguishing IBS (n=100) from HC (n=100) using composite score of digestive symptoms. Sensitivity versus 1−specificity is given for different cutoff values. The area under the curve was 0.95. HC indicates healthy control; IBS, irritable bowel syndrome.
Receiver-operating curves comparing all IBS patients with HC subjects were also performed using the digestive (dis)comfort dimension scores of FBA and FDDQL questionnaires. ROC scores were also excellent for both questionnaires: 0.98 and 0.95 for FDDQL and FBA, respectively. The cutoff values of 72.22 and 69.44 were retained for FDQQL and FBA, respectively. The associated sensitivity and specificity were: 98% and 94% for FDDQL; 91% and 87% for FBA.
Relationship Between Composite Score of Digestive Symptoms and IBS-SSS and Digestive (Dis)Comfort of HRQoL Questionnaires
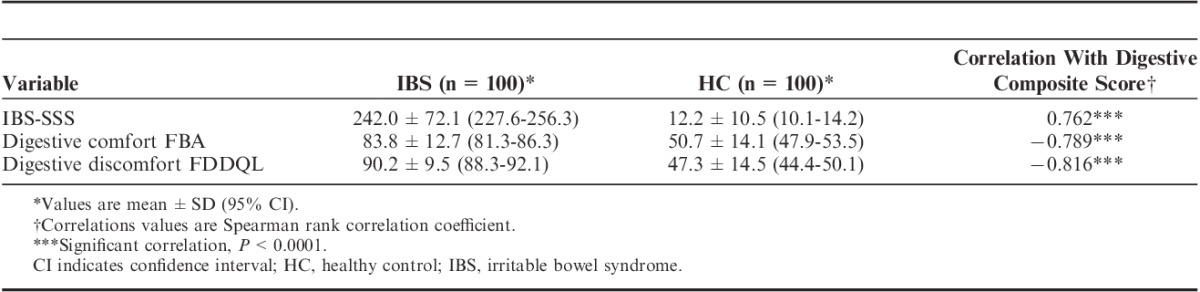
IBS patients had significantly lower score of both digestive comfort dimension of FBA questionnaire and digestive discomfort FDDQL questionnaires than healthy subjects (Table 3). A significant correlation (Spearman coefficient=0.762, P<0.001) between the composite score of the DSFQ and IBS-SSS score was observed (Table 3). The composite score of the DSFQ negatively correlated (P<0.001) with these 2 HRQoL dimensions (Table 3).
TABLE 3.
Correlations Between Composite Score of Digestive Symptoms Frequency and Other Measures
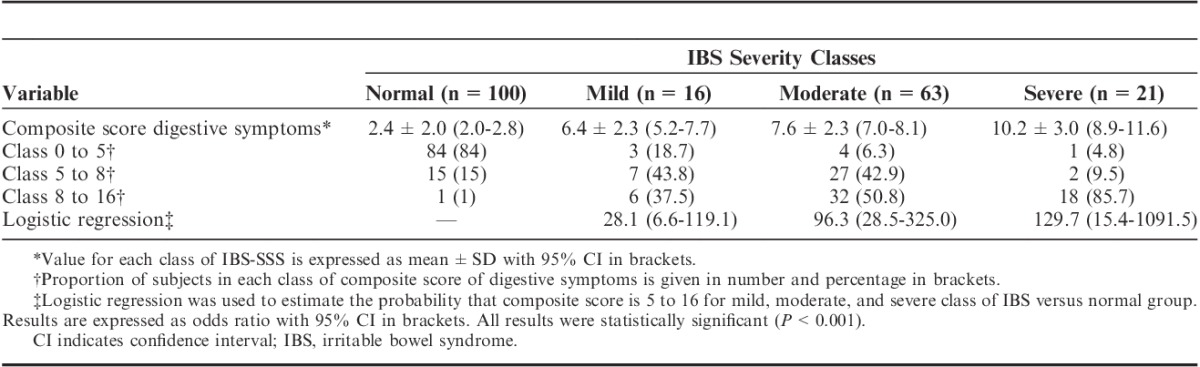
Logistic regression modeling of the probability that the composite score of digestive symptoms frequency is superior to the cutoff of 5 according to IBS-SSS modalities showed that IBS classes (normal, mild, moderate, severe) were statistically significantly (P<0.001) with a gradient of odd ratios with the severity of IBS (Table 4).
TABLE 4.
Relationship Between Composite Score of Digestive Symptoms Frequency and Severity of IBS
Relationship Between Digestive Symptoms and HRQoL Questionnaires
The scores for all the dimensions of both questionnaires were lower in IBS patients than in healthy subjects (Table 5). Effect size calculation was used to investigate the relationship between the increase in composite score of digestive symptoms and worsening of HRQoL corresponding to decreased scores of dimensions of FBA and FDDQL questionnaires (Figs. 2, 3). Effect size value increased with composite score for all dimensions of FBA and FDDQL, except for FBA disease prevention and FDDQL impact of stress and health perception. For FBA questionnaire, higher gradient was observed for digestive comfort with effect size ranging from −1.23 to −3.52, whereas the effect size for other dimensions was mainly <0.8. For FDDQL, most of the dimensions (anxiety, coping with disease, daily activities, diet, digestive discomfort, sleep) showed a relevant gradient, with an effect size >0.8 for the 2 groups with higher composite score. The digestive discomfort dimension showed the larger effect size ranging from −0.82 to −4.21.
TABLE 5.
Scores of the Dimensions of FBA and FDDQL Questionnaires in HC Subjects and IBS Patients
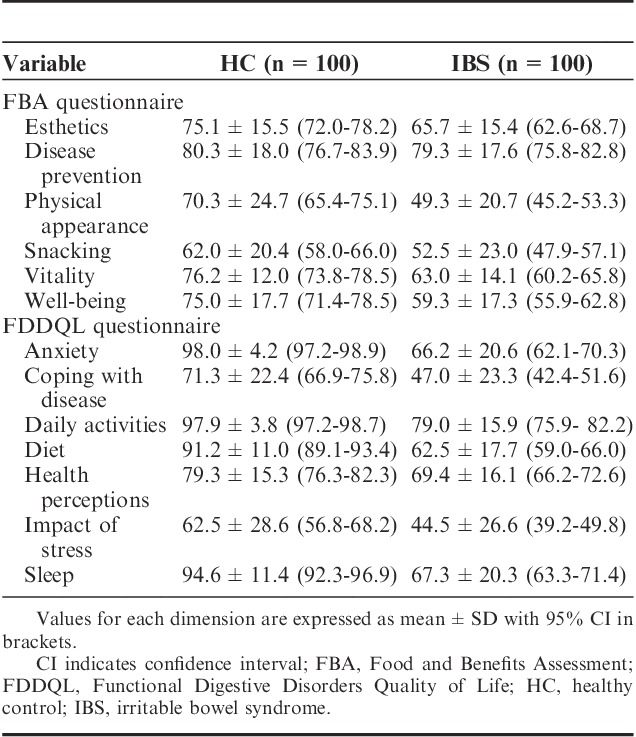
FIGURE 2.

Effect size of FBA questionnaire dimensions according to composite score of digestive symptoms (FAS population, n=200). Results are expressed as effect size for each class (2 to 5; 5 to 8; 8 to 16) for each dimension of the FBA questionnaire. The reference group used for effect size estimation is the group with the lowest composite score scores (0 to 2). Effect sizes of 0.5 and 0.8 are typically considered moderate and large between-group differences, respectively. FAS indicates Full Analysis Set; FBA, Food and Benefits Assessment.
FIGURE 3.

Effect size of FDDQL questionnaire dimensions according to composite score of digestive symptoms (FAS population, n=200). Results are expressed as effect size for each class (2 to 5; 5 to 8; 8 to 16) for each dimension of the FBA questionnaire. The reference group used for effect size estimation is the group with the lowest composite score scores (0 to 2). Effect sizes of 0.5 and 0.8 are typically considered moderate and large between-group differences, respectively. FAS indicates Full Analysis Set; FBA, Food and Benefits Assessment; FDDQL, Functional Digestive Disorders Quality of Life.
Canonical Correlation Analysis was performed to assess the network of associations between individual digestive symptom and HRQoL dimensions. Digestive comfort of FBA and digestive discomfort of FDDQL were the only 2 dimensions associated with the 4 digestive symptoms. Higher associations were observed for abdominal pain and bloating for FBA digestive comfort (−0.75 for both symptoms) and FDDQL digestive discomfort (−0.87 and −0.90, respectively). These 2 symptoms were also associated with 4 other dimensions of FDDQL (daily activities, sleep, anxiety, and diet). PCA also showed that abdominal pain and bloating were highly correlated.
Stability of the Measure
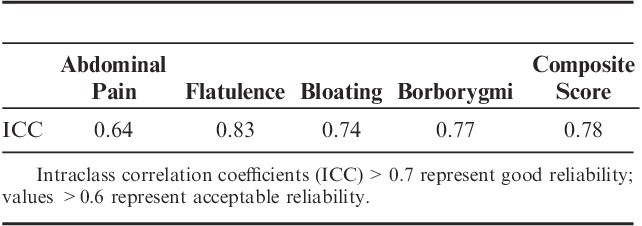
The reproducibility of the measure was tested with baseline data corresponding to readministration of the questionnaire 1 week apart (Table 6). The ICCs for individual digestive symptoms and for composite score range from 0.64 to 0.83, suggesting good stability.
TABLE 6.
Stability of the Frequency Digestive Symptom Questionnaire
DISCUSSION
Our data show that the sensitivity and specificity of the composite score of the 4 digestive symptoms measured by the DSFQ are good, as shown by the ROC curve analysis, in discriminating 2 well-defined groups of healthy subjects and IBS patients. This composite score correlated well with different validated PROs, including a severity questionnaire in IBS (IBS-SSS),13 and with the digestive (dis)comfort of 2 HRQoL questionnaires (FDDQL and FBA).16,17 These data support the validity and robustness of this symptom questionnaire.
Development of new valid and reliable PROs measuring digestive symptoms in clinical trials is an important area of research. For example, no fully validated PRO, meeting the requirement of drug agencies, is available to date for IBS drug trials, and partially validated PRO, such as 11-point numeric rating scale for abdominal pain,18 is therefore judged as acceptable study outcome by both FDA and EMA.10,11 Most of these research studies have focused on IBS or other types of functional GI disorders, whereas few initiatives have been taken in the general population without functional GI disorders. This population is the target for claims on the reduction of digestive discomfort for many foods (eg, fiber, probiotics) both in Europe and the United States. However, more studies are recommended in this general healthy population to provide adequate scientific evidence to support such claims.19 The development of simple and reliable PRO for measuring digestive symptoms in this population should be useful in this perspective.
This symptom questionnaire distinguished a well-characterized group of IBS patients from healthy subjects as demonstrated by the high ROC score (0.95). Using a cutoff of >5, the sensitivity of this instrument to identify IBS is very high (92%), with a very good specificity (84%). These results are comparable to those obtained with the digestive discomfort dimension of the FDDQL questionnaire (sensitivity: 98%; specificity: 91%), which was specifically developed in patients with functional bowel disorders including IBS.17 With a similar level of sensitivity and specificity, the symptom frequency questionnaire is simpler, quicker, and easier to apply than FDQL. The reliability of this new instrument was good, with an ICC>0.70.20 The individual symptoms also showed a good (>0.7 for flatulence, bloating, and borborygmi) or acceptable reliability (>0.6 for abdominal pain). The Gastrointestinal Symptom Rating Scale, one of the most established, reliable, and responsive disease-specific PROs in functional bowel disorders, has been shown to exhibit lower low test-retest reliability ranging from 0.42 to 0.69 according to dimension.21,22
The symptom questionnaire was also tested against validated IBS severity questionnaire and digestive (dis)comfort dimension of HRQoL questionnaire. The composite score of the DSFQ significantly correlated with these 3 validated PROs. This supports the idea that symptom frequency is moving in the same direction of other meaningful measures. The increase in the severity of IBS as measured by IBS-SSS is clearly associated with an increase in the composite score that seems independent from the IBS subtype. Regarding the 2 HRQoL questionnaires, larger effect sizes (up to 3 to 4) were observed for digestive discomfort (FDDQL) and digestive comfort (FBA). Analysis of the network of associations between HRQoL dimensions and the symptoms showed that the 4 symptoms assessed with the composite score were all associated with these 2 dimensions measuring digestive comfort concept. Other HRQoL dimensions were less associated with composite score, anxiety, daily activities, diet dimensions of FDDQL, and, in a lesser extent, vitality and well-being dimensions of the FBA being the more impacted by changes in composite score. In addition, this symptom frequency questionnaire was also shown to be associated with changes (improvement and worsening) in GI well-being in a general population of subjects reporting mild digestive symptoms.23
Another important aspect to consider when evaluating a digestive symptom questionnaire is its ability to detect changes in efficacy clinical trials. This instrument was previously used in 2 independent double-blind controlled clinical trials in women reporting mild digestive symptoms testing the ability of a probiotic food to improve digestive comfort.14,15 Significant decreases in both trials were observed supporting the ability of this PRO to detect changes between treatment groups. However, this PRO was never tested in an IBS trial yet. Interestingly, in these 2 clinical trials, participants with mild digestive symptoms but not fulfilling the criteria of IBS had baseline composite scores (mean score around 7) within the range of mild-to-moderate IBS observed in the present study. This may suggest a continuum in digestive symptoms from normal digestive sensations to severe digestive complaints. Such continuum has been described in human physiology such as blood pressure.24 This opens the possibility to investigate continuum in terms of frequency of digestive symptoms between different types of apparently different populations reporting mild-to-moderate digestive symptoms. This could help in better characterizing study population in future clinical trials and to select homogenous group of participants regarding symptoms frequency. Such a simple and easy to administer instrument could be a useful tool in large population survey, looking at the prevalence of digestive symptoms in different sets of population (general population, patients with functional GI disorders).
A potential limitation of our questionnaire is that it captures only 1 dimension, that is, frequency of the digestive symptoms, and in the more severe spectrum of IBS, the intensity, the bothersomeness, and the interference of the symptoms with daily activities may be important.25 This may mask differences between patient groups; however, the present data showed the ability of this PRO to detect differences between IBS patients with different degrees of disease severity. Another point to consider when using this PRO is the contribution of each symptom to the composite score that may vary between subjects. This should be of importance according to the research question addressed, that is, looking at overall symptoms pattern or at specific individual symptoms. Further investigations are required to better determine how this questionnaire could be used in IBS clinical trials. Frequency might be a better indicator of digestive symptoms in the general healthy population compared with severity or intensity. Owing to the low severity/intensity of digestive symptoms in this nondisease population, PROs measuring this aspect of the symptoms are likely to not be enough sensitive to capture changes because of floor effect.
In conclusion, the findings of this study support the validity of the DSFQ assessing the frequency of 4 digestive symptoms (abdominal pain, bloating, flatulence, and borborygmi/rumbling stomach). This instrument is a simple quantitative questionnaire test by which clinical responses to therapeutic trials or variation of functional digestive symptom over time may be evaluated in clinical settings. The questionnaire may be a particularly useful tool for the evaluation of symptoms in the general population, and in patients with mild-to-moderate complaints not fulfilling the criteria of functional GI disorders, and in this context the questionnaire covers a heretofore unmet need.
Footnotes
Supported in full by Danone Research.
F.A. is a member of the scientific committee of Instituto Danone (Barcelona, Spain) and member of an advisory board for Danone Research (Paris, France). F.G. is a member of the scientific committee of Instituto Danone (Barcelona, Spain). D. Guyonnet and J.T. are employees of Danone Research. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.van Kerkhoven LA, Eikendal T, Laheij RJ, et al. Gastrointestinal symptoms are still common in a general Western population. Neth J Med. 2008;66:18–22. [PubMed] [Google Scholar]
- 2.Tielemans MM, Focks JJ, van Rossum LGM, et al. Gastrointestinal symptoms are still prevalent and negatively impact health-related quality of life: a large cross-sectional population based study in The Netherlands. PloS One. 2013;8:e69876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton KW, O’Donnell LJD, Braddon FE, et al. Symptoms of irritable bowel syndrome in a British urban community: consulters and nonconsulters. Gastroenterology. 1992;102:1962–1967. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Li Z, Andruzzi E, et al. United-states householder survey of functional gastrointestinal disorders: prevalence, sociodemography and health impact. Dig Dis Sci. 1993;38:1569–1580. [DOI] [PubMed] [Google Scholar]
- 5.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [DOI] [PubMed] [Google Scholar]
- 6.Frexinos J, Denis P, Allemand H, et al. Descriptive study of digestive functional symptoms in the French general population. Gastroenterol Clin Biol. 1998;22:785–791. [PubMed] [Google Scholar]
- 7.Azpiroz F, Malagelada JR. Abdominal bloating. Gastroenterology. 2005;129:1060–1078. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel BMR, Bolus R, Harris LA, et al. Characterizing abdominal pain in IBS: guidance for study inclusion criteria, outcome measurement and clinical practice. Aliment Pharmacol Ther. 2010;2:1192–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EFSA on dietetic products, nutrition and allergies (NDA); guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 2011;9:1984. [Google Scholar]
- 10.US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. 2012Rockville Maryland: US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). [Google Scholar]
- 11.European Medicines Agency. Guideline on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome. Draft. CPMP/EWP/785/97 Rev. 1. 2013London: European Medicines Agency. [Google Scholar]
- 12.Spiegel BM, Bolus R, Agarwal N, et al. Measuring symptoms in the irritable bowel syndrome: development of a framework for clinical trials. Aliment Pharmacol Ther. 2010;32:1275–1291. [DOI] [PubMed] [Google Scholar]
- 13.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 14.Guyonnet D, Schlumberger A, Mhamdi L, et al. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in a global population of women. A randomised, double-blind, parallel, controlled study. Br J Nutr. 2009;22:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Marteau P, Guyonnet D, Lafaye de Micheaux P, et al. A randomized, double-blind, controlled study and pooled analysis of two identical trials of fermented milk containing probiotic Bifidobacterium lactis CNCM I-2494 in healthy women reporting minor digestive symptoms. Neurogastroenterol Motil. 2013;25:331–e252. [DOI] [PubMed] [Google Scholar]
- 16.Guyonnet D, Chassany O, Picard C, et al. Perceived subject outcomes and impact on health-related quality of life associated with diet using the new Food Benefits Assessment (FBA) questionnaire: development and psychometric validation. Public Health Nutr. 2008;11:1163–1172. [DOI] [PubMed] [Google Scholar]
- 17.Chassany O, Marquis P, Scherrer B, et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther. 2009;30:1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salminen S, van Loveren H. Probiotics and prebiotics: health claim substantiation. Microb Ecol Health Dis. 2012;23:40–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayars PM, Machin D. Quality of Life: Assessment, Analysis and Interpretation. 2000:2nd edChichester, West Sussex: John Wiley & Sons Ltd. [Google Scholar]
- 21.Revicki DA, Wood M, Wiklund I, et al. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75–82. [DOI] [PubMed] [Google Scholar]
- 22.Talley NJ, Fullerton S, Junghard O, et al. Quality of life in patients with endoscopy-negative heartburn: reliability and sensitivity of disease-specific instruments. Am J Gastroenterol. 2001;96:1998–2004. [DOI] [PubMed] [Google Scholar]
- 23.Guyonnet D, Naliboff B, Rondeau P, et al. Gastrointestinal well-being in subjects reporting mild gastrointestinal discomfort: characteristics and properties of a global assessment measure. Br J Nutr. 2013;110:1263–1271. [DOI] [PubMed] [Google Scholar]
- 24.Pickering GW. The concept of essential hypertension. Ann Intern Med. 1955;43:1153–1160. [DOI] [PubMed] [Google Scholar]
- 25.Mönnikes HJ. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:S98–S101. [DOI] [PubMed] [Google Scholar]


