Obesity is associated with lower mortality in individuals with chronic disease.(1) This counterintuitive inverse association—the “obesity paradox”(2)—has been described in patients with cardiovascular disease, diabetes, hip fracture and even Chagas’ disease. If obese individuals with chronic diseases live longer, should we start advising them to gain rather than lose weight?
Though already the basis for calls to change clinical advice,(3) the obesity paradox may be irrelevant for clinical decisions. Here we review a possible characterization of the obesity paradox as an artifact arising from the selection of individuals with chronic disease into the analysis.(4, 5) First, we review how selection may explain paradoxical inverse associations even in randomized clinical trials.
Consider a randomized clinical trial of estrogen and progestin hormone therapy versus placebo in postmenopausal women. The incidence of coronary heart disease in the therapy arm was 50% higher than that in the placebo arm after two years, and 25% higher after eight years. We correctly conclude that therapy increased coronary heart disease incidence. Interestingly, coronary heart disease incidence between years 2 and 8 must have been lower, say 30% lower, in the therapy arm than in the placebo arm. Should we conclude that therapy is beneficial after 5 years of use? Not necessarily.
Since therapy increases the early coronary heart disease risk, the therapy arm is being progressively depleted of women more susceptible to coronary heart disease. After five years, the women remaining on therapy would be the relatively resilient ones who did not develop coronary heart disease even when exposed to therapy. In contrast, women assigned to placebo are not subject to this selective pressure, and after five years, the placebo group would retain a greater proportion of susceptible women than the therapy group. Under this scenario, we would expect a greater incidence of coronary heart disease after five years in the placebo group compared with the therapy group(6), even if therapy did not prevent coronary heart disease in any woman.
We do not say that there is a “hormone therapy paradox” just because the incidence of coronary heart disease in the therapy arm is 25% greater overall and 30% lower after five years. The 30% lower incidence does not imply that therapy becomes beneficial after five years. Rather, receiving hormone therapy becomes a marker of resilience among women who still have no coronary heart disease after 5 years. An analysis of a randomized clinical trial that does not include the first 5 years of follow-up is flawed and should not inform clinical decision making. Therefore, we do not say to postmenopausal women: “Take hormone therapy because, if you happen to survive a few years without heart disease, we will learn that you are resilient to heart disease.”
This same reasoning may apply to the obesity paradox. Consider an observational study of individuals aged 50-60 years newly diagnosed with diabetes. Suppose we find a lower incidence of mortality among those who were obese than among those with normal weight at baseline. Should we conclude that there are individuals who benefit from being obese? Again, not necessarily. Since obesity increases the risk of diabetes, non-obese diabetics are more likely to have characteristics other than obesity (e.g., genes, lifestyle) that caused their diabetes. If these characteristics are also risk factors for mortality, then non-obese diabetics would have, on average, more of these other risk factors than obese diabetics. We might find a greater mortality among non-obese individuals than among obese individuals.
This obesity “paradox” should not change clinical recommendations regarding weight change if, in individuals with diabetes, obesity is just a marker for the absence of more serious risk factors. We would not say to people: “Don’t worry about gaining weight. If you happen to develop diabetes because of your obesity, you will live longer than those who developed diabetes because of genetic predisposition.”
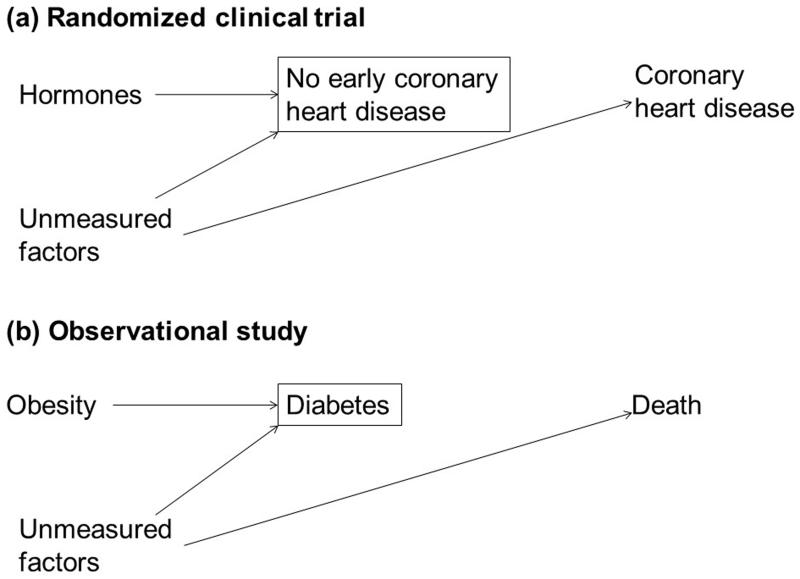
In both examples, selection bias may arise when individuals are selected into the analysis based on a factor (early coronary heart disease in the randomized clinical trial, diabetes in the observational study) that is affected by exposure (hormone therapy, obesity) and shares risk factors with the outcome. The conditions and structure of this selection bias are presented in the Table and Figure.
Table.
Conditions necessary for selection bias to appear even when the exposure does not have an effect on the outcome
| Condition | Examples | |
|---|---|---|
| Randomized clinical trial | Observational study | |
| Probability of selection into the analysis must depend on exposure |
Only individuals without coronary heart disease at 5 years were included in the analysis, and hormone therapy is a predictor of coronary heart disease |
Only individuals with diabetes were included in the analysis, and obesity is a predictor of diabetes |
| Association between selection into the analysis and prognostic factors for the outcome |
Early coronary heart disease (selection variable) and late coronary heart disease (the outcome) are associated through shared risk factors |
Diabetes (selection variable) and mortality (outcome variable) are associated through shared risk factors |
Figure.
Simplified diagram representing selection bias due to conditioning on a variable affected by exposure in (a) a randomized clinical trial and (b) an observational follow-up study without confounding. The box around a variable denotes selection on that variable. The magnitude and direction of the bias is hard to predict because the selection bias may be compounded by measurement error and residual confounding, because of complex relations and interactions between chronic disease and obesity(10), because many observational studies measure body weight after it has been affected by chronic disease, and because of potential heterogeneity in the etiology of chronic diseases(5).
A simple way to eliminate the bias, and therefore the paradox, is to ensure the start of exposure and the start of follow-up coincide. This is precisely how randomized clinical trials are typically analyzed: an analysis that selects individuals free of coronary heart disease five years after randomization and then compares the coronary heart disease incidence between arms from year 5 onwards would be unacceptable. This simple rule—ensuring that the start of follow-up and exposure coincide—is often overlooked when analyzing observational studies(7). For example, widespread confusion about the cardiovascular effects of hormone therapy resulted from analyses of observational data that, effectively, ignored the first few years of follow-up by comparing prevalent users versus never users(8).
Admittedly, this rule is hard to apply to exposures like obesity that lack a clear onset. As a result, the obesity-mortality association cannot be readily endowed with a causal interpretation (leaving aside issues concerning the meaning of the effect of obesity)(9). It might be more realistic to accept that observational studies of obesity and mortality cannot answer the question of whether patients with chronic diseases should gain weight to increase survival. Observational studies do tell us that obesity predicts lower mortality in individuals with chronic diseases, but this might simply mean that developing a chronic disease as a consequence of obesity may be less harmful than having the disease through other mechanisms that put one at even higher risk of death. Modification of the current clinical advice regarding weight loss for individuals with chronic disease would therefore not be warranted.
Because the immediate clinical question is about weight change—as opposed to the effect of body weight itself—a natural study design to answer it would be a randomized clinical trial that assigns individuals with chronic disease to weight gain (e.g., via increased caloric intake) versus no weight gain. The follow-up would then start at the same time as the weight change of interest, e.g., at the diagnosis of diabetes. Unfortunately, a follow-up observational study comparing weight gain vs. no weigh gain may have intractable confounding.
In summary, the obesity paradox may be partly explained by selection on a baseline variable (e.g., diabetes) affected by prior exposure (body weight before baseline). Because results from observational studies in which the start of follow-up and exposure do not coincide should be interpreted with care, the obesity paradox provides little evidence that chronic disease patients should gain weight.
Acknowledgments
We thank Sonia Hernández-Díaz for comments to an earlier manuscript version.
Funding: NIH grant R01HL080644 and the Bernard Lown Scholar Program in Cardiovascular Health
Footnotes
Conflict of interest: ML has a non-restricted investigator-initiated grant from AstraZeneca and within the last two years minor research support from Swiss Re. All other authors declare none.
All authors had a role in writing the manuscript.
References
- 1.Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes care. 2013;36(Suppl 2):S276–81. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? Journal of the American College of Cardiology. 2002;39(4):578–84. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 3.Doehner W. Critical appraisal of the obesity paradox in cardiovascular disease: How to manage patients with overweight in heart failure? Heart failure reviews. 2014 doi: 10.1007/s10741-014-9425-z. [DOI] [PubMed] [Google Scholar]
- 4.Banack HR, Kaufman JS. The “obesity paradox” explained. Epidemiology (Cambridge, Mass) 2013;24(3):461–2. doi: 10.1097/EDE.0b013e31828c776c. [DOI] [PubMed] [Google Scholar]
- 5.Lajous M, Bijon A, Fagherazzi G, et al. Body Mass Index, Diabetes, and Mortality in French Women: Explaining Away a “Paradox”. Epidemiology (Cambridge, Mass) 2013 doi: 10.1097/EDE.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernan MA. The hazards of hazard ratios. Epidemiology (Cambridge, Mass) 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernan MA. With great data comes great responsibility: publishing comparative effectiveness research in epidemiology. Epidemiology (Cambridge, Mass) 2011;22(3):290–1. doi: 10.1097/EDE.0b013e3182114039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernán MA, Robins JM. Authors’ Response, Part I: Observational Studies Analyzed Like Randomized Experiments: Best of Both Worlds. Epidemiology (Cambridge, Mass) 2008;19(6):789–92. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernan MA, Taubman SL. Does obesity shorten life? The importance of well-defined interventions to answer causal questions. International journal of obesity (2005) 2008;32(Suppl 3):S8–14. doi: 10.1038/ijo.2008.82. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology (Cambridge, Mass) 2003;14(3):300–6. [PubMed] [Google Scholar]