Abstract
Importance
Colorectal cancer is a major health burden. Screening is recommended in many countries.
Objective
Estimate the effectiveness of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality in a population-based trial.
Design
Randomized controlled trial in individuals aged 50–64 years. Screening was performed in 1999–2000 (55–64 year age-group) and 2001 (50–54 year age-group). End of follow-up: Dec 31st 2011.
Setting
Population of Oslo city and Telemark County, Norway.
Participants
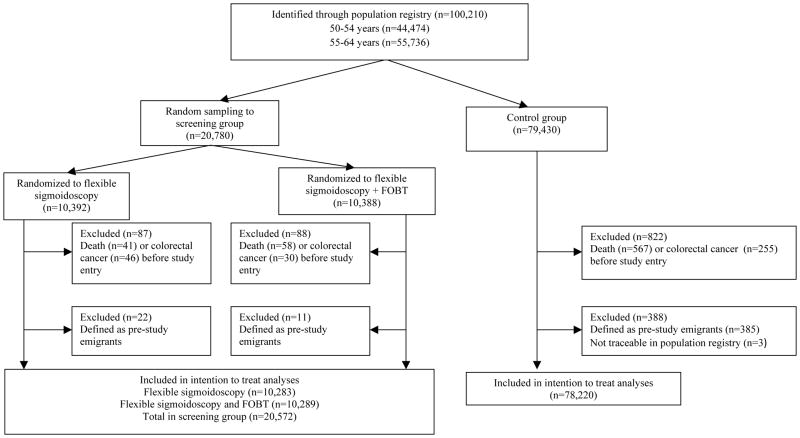
100,210 individuals were identified in the screening areas. 1,415 individuals were excluded due to prior colorectal cancer, emigration, or death. Three individuals could not be traced in the population registry.
Intervention
Individuals randomized to the screening group were invited to screening. Within the screening group, individuals were randomized 1:1 to once-only flexible sigmoidoscopy or combination of once-only flexible sigmoidoscopy and fecal occult blood-testing (FOBT). Individuals with positive screening test (cancer, adenoma, polyp ≥10 mm, or positive FOBT) were offered colonoscopy. The control group received no intervention.
Main outcome measures
Colorectal cancer incidence and mortality.
Results
98,792 individuals were included in the intention to screen analyses; 78,220 in the control group and 20,572 in the screening group (10,283 randomized to flexible sigmoidoscopy and 10,289 to flexible sigmoidoscopy and FOBT). Compliance with screening was 63%. After median 10.9 years, 71 individuals had died from colorectal cancer in the screening group, and 330 in the control group (31.4 vs. 43.1 deaths, absolute rate difference 11.7 (95% CI 3.0–20.4) per 100,000 person-years); hazard ratio [HR] 0.73 (95% confidence interval [CI] 0.56–0.94). Colorectal cancer was diagnosed in 253 individuals in the screening group, and 1,086 in the control group (112.6 vs. 141.0 cases, absolute rate difference: 28.4 (95% CI 12.1–44.7) per 100,000 person-years); HR 0.80 (95% CI 0.70–0.92). Colorectal cancer incidence was reduced in both the 50–54 year age-group (HR 0.68; 95% CI 0.49–0.94) and the 55–64 year age-group (HR 0.83; 95% CI 0.71–0.96). There was no difference between the flexible sigmoidoscopy only and the flexible sigmoidoscopy/FOBT screening groups.
Conclusion and relevance
In Norway, once-only flexible sigmoidoscopy screening or flexible sigmoidoscopy and FOBT reduced colorectal cancer incidence and mortality on a population level compared with no screening. Screening was effective both in the 50–54 and the 55–64 year age-group.
Trial registration
ClinicalTrials identifier NTC00119912, http://clinicaltrials.gov
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide.1 Most CRC cases develop from adenomas.2 Removal of adenomas by colonoscopy or flexible sigmoidoscopy has therefore been endorsed as a primary prevention tool for CRC.3,4
Recently, results from three large randomized trials comparing flexible sigmoidoscopy screening with no screening showed reductions in CRC incidence (by 18–23%) and CRC mortality (by 22–31%).5–7 However, these trials had some limitations. First, they only included individuals aged 55 years and older, while US guidelines recommend starting screening at age 50.3 Second, the trials were not population-based and included only volunteers, which might have resulted in estimates that do not reflect the effectiveness of national screening programs with similar compliance. Last, prior trials were partly conducted in settings in which CRC screening outside the trial was available to the no-screening group, which may result in attenuated effect estimates. The ideal scenario to overcome these problems is a population-based randomized trial with no screening outside the trial in individuals aged 50 years and older.
The Norwegian Colorectal Cancer Prevention Trial (NORCCAP) is such a randomized trial. Eligible individuals aged 50 to 64 years were randomized directly from the Norwegian Population Register to screening with flexible sigmoidoscopy or no screening (care as usual in Norway during the trial period). Preliminary trial findings showed no reduction in CRC incidence or mortality after seven years of follow-up.8 Here, we report incidence and mortality of colorectal cancer after 11 years of follow-up.
Methods
In November 1998, all men and women aged 55–64 years living in the City of Oslo or Telemark County, Norway, were identified through the Norwegian Population Register. Equal numbers of men and women were randomly sampled from the birth cohorts of 1935–1945 and invited by mail for screening (screening group). Remaining individuals in the screening areas constituted the control group; controls were never contacted and were not offered any screening. Individuals in the screening group were further randomized (1:1) to receive an invitation for once only flexible sigmoidoscopy, or a combination of once only flexible sigmoidoscopy and immunological fecal occult blood testing (FOBT) (FlexSure OBT, Beckman-Coulter, Palo Alto, CA, USA). An independent body performed both randomization procedures using computerized algorithms. At the end of year 2000, the study funding bodies (Norwegian Government and Norwegian Cancer Society) decided to extend the study to also include all individuals aged 50–54 years in the same geographic areas to obtain more information about the ideal age to start screening. No power calculations were employed for this extension of the trial. The randomization, invitation and screening procedures were similar to the 55–64 year age group. Due to higher birth rates in the 50–54 year age group (born 1946–1950, after World War II), the ratios between the screening and control group were 1:3 in the 55–64 year age group and 1:5.4 in the 50–54 year age group. The screening interventions took place in 1999 and 2000 for the 55–64 year age group, and in 2001 for the 50–54 year age group. During the course of the trial, there was no CRC screening program in Norway, and there has been virtually no screening colonoscopies outside the trial.9 Thus, all cases of CRC in the control group were identified by work-up of gastrointestinal symptoms.
The study entry date for individuals in the screening group was the date of the screening examination as proposed in their invitation letter. For the control group, each individual was assigned an entry date evenly distributed throughout the screening period (January 1st 1999 to December 31st 2000 for the 55–64 year age group; January 1st 2001 to December 31st 2001 for the 50–54 year age group). The only exclusion criterion was prior history of CRC. We did not have any information on family history of CRC at the time of the random sampling. Details of the study design, baseline findings, and short-term follow-up have been published previously.8,10,11 All participants who attended the screening examination provided written, informed consent. The study was approved by the Ethics Committee of South-East Norway and the Norwegian Data Inspectorate.
All screening examinations were performed at three dedicated centers, two in Telemark and one in Oslo. Bowel cleansing was restricted to a 240 milliliter Sorbitol enema administered on attendance at the screening center. All examinations were performed using standard colonoscopes (140cm Olympus colonoscopes, Olympus Europa GmbH, Hamburg, Germany) with the exception of a small screening center in Telemark, where a disposable endoscopy sheath was used (Vision Sciences 60 cm disposable Endosheath, Vision Sciences, Natick, MA, USA). During flexible sigmoidoscopy, all visible lesions were biopsied and subjected to histopathological evaluation. The screening participant brought the FOBT to the screening center and the test was analyzed on-site prior to flexible sigmoidoscopy. There was no option to be screened with FOBT only. Persons with a positive screening test, defined as any polyp ≥10 mm (irrespective of histology), any adenoma, CRC, or positive FOBT, were referred for colonoscopy at the screening centers. During colonoscopy, all lesions were removed. Designated pathologists examined all specimens. Post-polypectomy surveillance recommendations followed Norwegian guidelines.12
The primary study endpoints were CRC incidence and mortality. We also analyzed incidence and mortality from CRC located distal and proximal to the descending-sigmoid junction, stage-specific incidence, and all-cause mortality. All residents in Norway are assigned a unique personal identification number, and all included individuals were followed through linkage to public registries. Date of diagnosis, stage, and localization of colorectal cancer was obtained from the Cancer Registry of Norway (near complete registration of all cancers in Norway).13 Colorectal cancer was defined as adenocarcinoma of the colon or rectum and classified as localized (Dukes A or B) or advanced (Dukes C or D). Cases were also included if they were reported to the Cancer Registry as clinically diagnosed CRC without confirmatory histology (14 cases). Date and cause of death was obtained from the Cause of Death Registry. Socioeconomic data of all individuals was obtained from Statistics Norway.
Statistics
The power calculation was conducted for the 55–64 year age group and for the two screening groups combined versus the control group. 8 Assuming 70% compliance, and 30% intention-to-treat reduction in CRC incidence after 5 years with 90% power, and a significance level of 5%, 14,000 individuals had to be included in the screening group and 42,000 in the control group. Half of the individuals in the screening group were offered flexible sigmoidoscopy screening only, and the other half screening with flexible sigmoidoscopy and FOBT (the effectiveness comparison of the two different screening groups was a secondary analysis).
Our primary analytic approach followed the intention-to-screen principle. Each individual was followed from entry date until diagnosis of colorectal cancer, death, emigration, or December 31st 2011, whichever occurred first. We computed age-standardized rates, with the screening group as the standard, to adjust for a slightly higher mean age in the screening group than in the control group (56.9 vs. 56.1 years). We also estimated age-adjusted hazard ratios (HR) with 95% confidence intervals (95% CI) for the screening group versus the control group by fitting Cox models adjusted for age. We performed two sensitivity analyses: The ratio between the control group and screening group was higher in Oslo (6.2:1) than in Telemark (1.5:1) due to higher population numbers in Oslo. The age-adjusted CRC incidence and mortality rates, however, were comparable in the control groups in both areas. Consequently, in a sensitivity analysis when we included screening center in the Cox model, the results were unchanged. We also performed analyses with follow-up restricted to 11 years in both screening and control group to take into account the slightly different follow-up time (which was due to age differences), with comparable results.
To test for heterogeneity, we included product (“interaction”) terms (between sex and study group and between age-group and study group) in the Cox model. We computed age-standardized cumulative probability of CRC incidence and mortality. Further, we calculated the yearly age-standardized risk ratios after randomization in the screening group and screening compliers relative to the control group. The number needed to invite for screening to prevent one CRC case or death within 10 years was calculated as the inverse of the age-standardized risk difference at 10 years (all individuals were followed for at least 10 years). We estimated the incremental cost-effectiveness ratios (ICER) expressed in terms of prevented CRC-cases and CRC-deaths over ten years by comparing no screening to screening of the two age-groups (50–54 versus 55–64) separately and overall. Costs of screening, treatment, and follow-up were based on UK data with NICE’s recommended discount rate of 3.5%.14,15 All numbers were adjusted to 2013 level using the UK Consumer Price Index and reported in US$16. (See Supplementary appendix).
As a secondary analytic approach, we estimated a per protocol effect that measures the effect of the intervention adjusted for noncompliance (see supplementary appendix for details).17,18 We used instrumental variable estimation with randomization group as the instrument to estimate the per protocol 10-year risk differences of CRC mortality and incidence for screening versus no screening via two-stage least-squares estimation. We focused on the 10-year risk difference because all individuals were followed for at least 10 years. All analyses were conducted with STATA 13.0 software (StataCorp, College Station, Texas, USA).
Results
Of 100,210 randomized individuals, 1,415 (1.4%) were excluded due to diagnosis of CRC, death, or emigration before study entry (Figure 1) and three persons could not be traced through the population register. Thus, our analyses include 78,220 individuals in the control group and 20,572 individuals in the screening group. A total of 10,283 individuals were randomized to flexible sigmoidoscopy screening only and 10,289 individuals were randomized to be screened with flexible sigmoidoscopy and FOBT. Baseline characteristics are displayed in Table 1. End of follow-up was Dec 31st 2011.
Figure 1. Flowchart. Norwegian Colorectal Cancer Prevention trial (NORCCAP).
Individuals aged 55–64 and 50–54 years were invited to screening in 1999–2000 and in 2001, respectively.
The screening examination date originally proposed in the invitation letter was considered the date of study entry for the screening group. A randomly allocated date (Jan 1st 1999 – Dec 31st 2000 for the 55–64 year age-group and Jan 1st – Dec 31st 2001 for the 50–54 year age-group) was considered the date of study entry for the control group.
Table 1. Baseline characteristics of the study participants.
Results are given as numbers (percentages) if not stated otherwise.
| Control group (n=78,220) | Screening group (n=20,572) | |
|---|---|---|
| Age (mean, SD) | 56.1 (3.8) | 56.9 (3.8) |
| Sex | ||
| Men | 38,922 (49.8) | 10,269 (51.1) |
| Women | 39,298 (50.2) | 10,303 (49.9) |
| Age group | ||
| 50–54 years | 37,131 (47.5) | 6,920 (33.6) |
| 55–64 years | 41,089 (52.5) | 13,652 (66.4) |
| Area of residence | ||
| Telemark | 15,176 (19.4) | 10,314 (50.1) |
| Oslo | 63,044 (80.6) | 10,258 (49.9) |
SD: standard deviation
Of 20,572 individuals invited to screening, 12,955 (63%) attended the screening examination. Compliance was 60.9 % in the combined screening group and 65.1% in those invited for flexible sigmoidoscopy screening only (p<0.001). Screening findings and key endoscopy figures are displayed in table 2. There were no complications after flexible sigmoidoscopy. A total of 2,816 colonoscopies were performed in 2,520 individuals (19.5% of those who attended screening). Perforation occurred during colonoscopy in six individuals, and four individuals were admitted to hospital for post-polypectomy bleeding following snare polypectomy. Two patients had complications after surgery. No screening-associated deaths occurred.
Table 2. Findings at screening and endoscopy key figures.
Results in numbers (percentages) which include findings at flexible sigmoidoscopy and colonoscopy of screen-positive individuals in screening-compliers.
| Flexible sigmoidoscopy (n=6,692) | Flexible sigmoidoscopy and FOBT (n=6,263) | P-value | |
|---|---|---|---|
| Any adenomaa | 1,156 (17.3) | 1,054 (16.9) | 0.5 |
| Advanced adenomaa | 304 (4.6) | 278 (4.5) | 0.8 |
| Colorectal cancer | 21 (0.3) | 20 (0.3) | 1.0 |
| Referred for colonoscopy | 1,303 (19.5) | 1,336 (21.3) | 0.008 |
| Attended colonoscopy examination | 1,249 (18.7) | 1,271 (20.3) | 0.02 |
| Cecum intubationb | 1,130 (90.5) | 1,140 (89.7) | 0.3 |
| Number of colonscopies | 1403 | 1413 | 0.2 |
| Complications with flexible sigmoidoscopy | 0 | 0 | - |
| Complications with colonoscopyc | 5 | 5 | 0.8 |
| Recommended surveillance | 670 (10.1) | 598 (9.6) | 0.4 |
Not included individuals with screen-detected colorectal cancer. Advanced adenoma: Adenoma with size 10 mm or lagrer, with villous histology or with high-grade dysplasia.
Of thos who attended the colonoscopy examination.
Perforations (n=6) and admittance to hospital for post-polypectomy bleeding (n=4).
FOBT: Fecal occult blood test
Colorectal cancer incidence
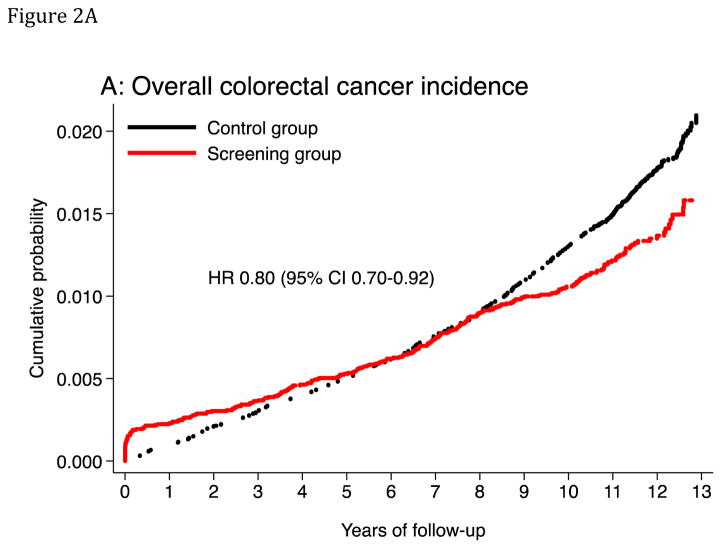
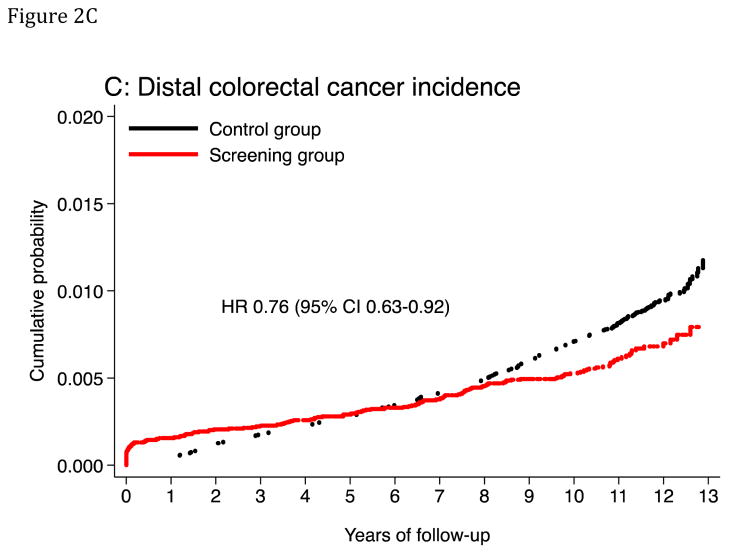
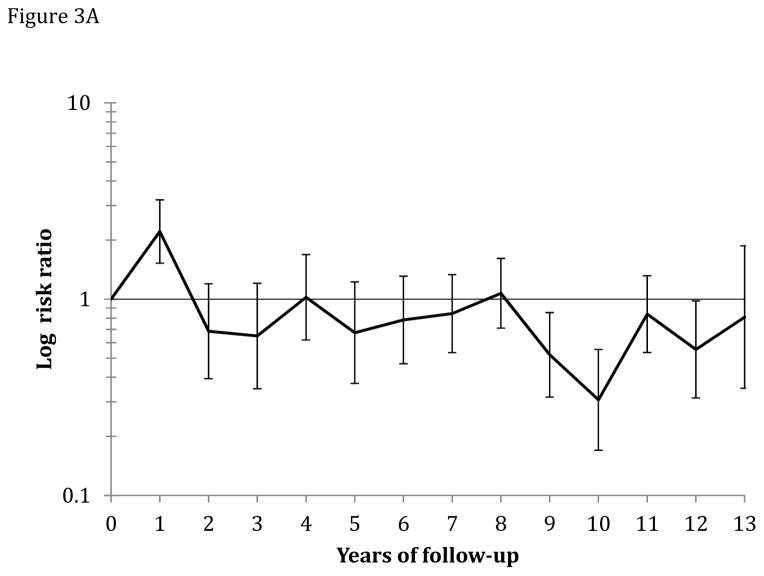
Median follow-up was 11.2 years in the screening group and 10.9 years in the control group. The age-standardized CRC incidence rate (per 100,000 person-years) was 112.6 in the screening group and 141.0 in the control group, absolute rate difference: 28.4 (95% CI 12.1–44.7), and the HR was 0.80 (95% CI 0.70–0.92) (Table 3; Figure 2). The corresponding HR was 0.68 (95% CI 0.49–0.94) in the 50–54 year age group, and 0.83 (95% CI 0.71–0.96) in the 55–64 year age group (P-value for heterogeneity: 0.3). The HR was 0.73 (95% CI 0.60–0.89) in men and 0.87 (95% CI 0.72–1.06) in women (P-value for heterogeneity: 0.3). The HR was 0.76 (95% CI 0.63–0.92) for distal CRC and 0.90 (95% CI 0.73–1.10) for proximal CRC. For flexible sigmoidosxopy screening only, the HR was 0.72 (95% CI 0.59–0.87), and for flexible sigmoidoscopy and FOBT, HR was 0.88 (95% CI 0.74–1.05) (P-value for heterogeneity: 0.1). Screen-detected CRC was more often diagnosed at an earlier stage than non screen-detected CRC (Table 4). The relative risk of CRC was lower each year after screening in the screening group compared to the control group except for the first year after randomization due to screen-detected cancers (Figure 3 and 4A and B). The number needed to invite for screening to prevent one CRC case over 10 years was 498 (ICER $58,448; see supplementary appendix).
Table 3.
Colorectal cancer incidence and mortality in the screening and control group.
| Screening group | Control group | Hazard ratio (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| n | Cases/100,000 py | n | Cases/100,000 py | |||
| Colorectal cancer incidence | 253 | 112.6 | 1086 | 141.0. | 0.80 (0.70–0.92) | 0.001 |
| Person-years of observation | 221,429 | 828,207 | ||||
| Location | ||||||
| Distal | 137 | 60.9 | 621 | 80.1 | 0.76 (0.63–0.92) | 0.004 |
| Proximal | 112 | 49.8 | 424 | 55.5 | 0.90 (0.73–1.10) | 0.3 |
| Sex | ||||||
| Men | 128 | 115.6 | 586 | 157.6 | 0.73 (0.60–0.89) | 0.002 |
| Women | 125 | 109.6 | 500 | 125.5 | 0.87 (0.72–1.06) | 0.2 |
| Age group | ||||||
| 50–54 years | 40 | 57.2 | 315 | 84.3 | 0.68 (0.49–0.94) | 0.02 |
| 55–64 years | 213 | 140.6 | 771 | 169.6 | 0.83 (0.71–0.96) | 0.02 |
| Screening modality | ||||||
| Flexible sigmoidoscopy | 114 | 101.9 | 1086 | 141.0 | 0.72 (0.59–0.87) | 0.001 |
| Flexible sigmoidoscopy + FOBT | 139 | 123.3 | 0.88 (0.74–1.05) | 0.2 | ||
| Colorectal cancer mortality | 71 | 31.4 | 330 | 43.1 | 0.73 (0.56–0.94) | 0.02 |
| Person-years of observation | 222,677 | 832,003 | ||||
| Location | ||||||
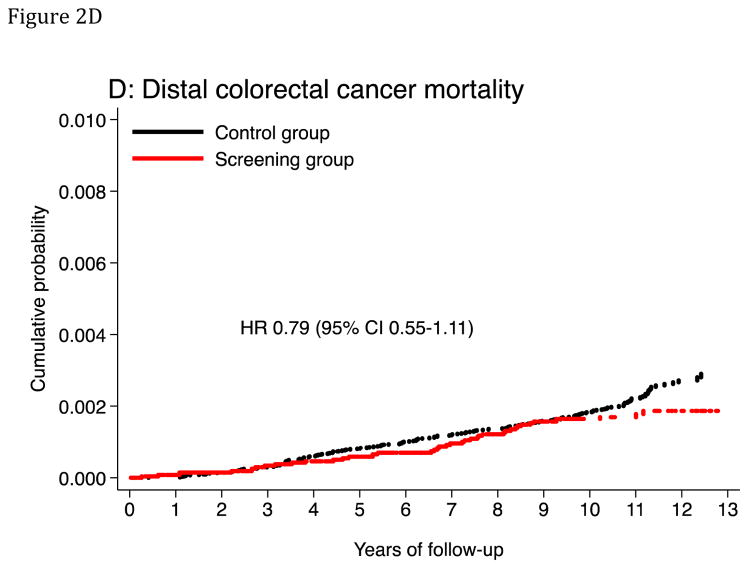
| Distal | 39 | 17.2 | 168 | 21.8 | 0.79 (0.55–1.11) | 0.2 |
| Proximal | 30 | 13.4 | 139 | 18.3 | 0.73 (0.49–1.09) | 0.1 |
| Sex | ||||||
| Men | 32 | 28.6 | 182 | 49.1 | 0.58 (0.40–0.85) | 0.005 |
| Women | 39 | 34.2 | 148 | 37.4 | 0.91 (0.64–1.30) | 0.6 |
| Age group | ||||||
| 50–54 years | 12 | 17.1 | 87 | 23.2 | 0.74 (0.40–1.35) | 0.3 |
| 55–64 years | 59 | 38.7 | 243 | 53.1 | 0.73 (0.55–0.97) | 0.03 |
| Screening modality | ||||||
| Flexible sigmoidoscopy | 41 | 36.4 | 330 | 43.1 | 0.84 (0.61–1.17) | 0.3 |
| Flexible sigmoidoscopy + FOBT | 30 | 26.5 | 0.62 (0.42–0.90) | 0.01 | ||
| All-cause mortality | 2,183 | 969.0 | 7,762 | 994.6 | 0.97 (0.93–1.02) | 0.3 |
py: person-years of observation
FOBT: Fecal occult blood test.
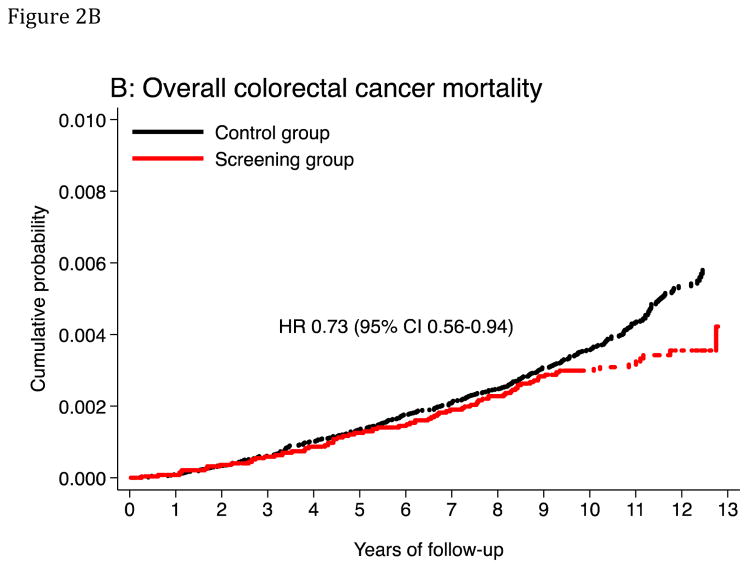
Figure 2. Cumulative probability of colorectal cancer incidence and mortality.
The panels show cumulative probability for colorectal cancer incidence (panels A and C) and mortality (panels B and D), for the colorectum overall and for the distal colon. HR: Hazard ratio. CI: Confidence interval.
At-risk table, panel A and C
| At risk | |||||||
|---|---|---|---|---|---|---|---|
| Year | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Screening | 20572 | 20141 | 19731 | 19306 | 18808 | 18298 | 5285 |
| Control | 78220 | 76648 | 75059 | 73415 | 71598 | 69508 | 17277 |
At-risk table, panel B and D
| At risk | |||||||
|---|---|---|---|---|---|---|---|
| Year | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Screening | 20572 | 20204 | 19816 | 19411 | 18945 | 18448 | 5656 |
| Control | 78220 | 76777 | 75272 | 73722 | 72044 | 70127 | 17517 |
Table 4. Colorectal cancer stages.
Number of localized and advanced colorectal cancers (age-standardized per 1,000 individuals) by randomization group status and by mode of detection.
| Screening group | Control group | ||||
|---|---|---|---|---|---|
|
| |||||
| Compliers n=12,955 | Noncompliers n=7,617 | Total n=20,572 | n=78,220 | ||
| Stage | Screen detected | Post-screen detected | |||
| Localized | 29 (2.2) | 48 (3.6) | 40 (5.4) | 117 (5.7) | 470 (6.7) |
| Advanced | 10 (0.8) | 49 (3.7) | 62 (8.3) | 120 (5.8) | 562 (7.8) |
| Unclassified | 2 (0.1) | 4 (0.3) | 9 (1.2) | 16 (0.8) | 54 (0.8) |
| Total | 41 (3.1) | 101 (7.6) | 111 (15.0) | 253 (12.3) | 1,086 (15.3) |
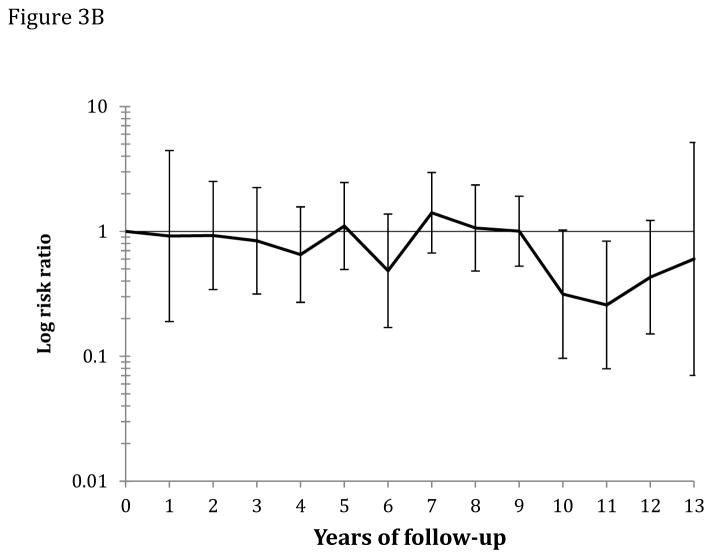
Figure 3.
Figure 3A: Yearly risk ratio (with error bars) of CRC incidence for the screening group relative to the control group.
Figure 3B: Yearly risk ratio (with error bars) of CRC mortality for the screening group relative to the control group.
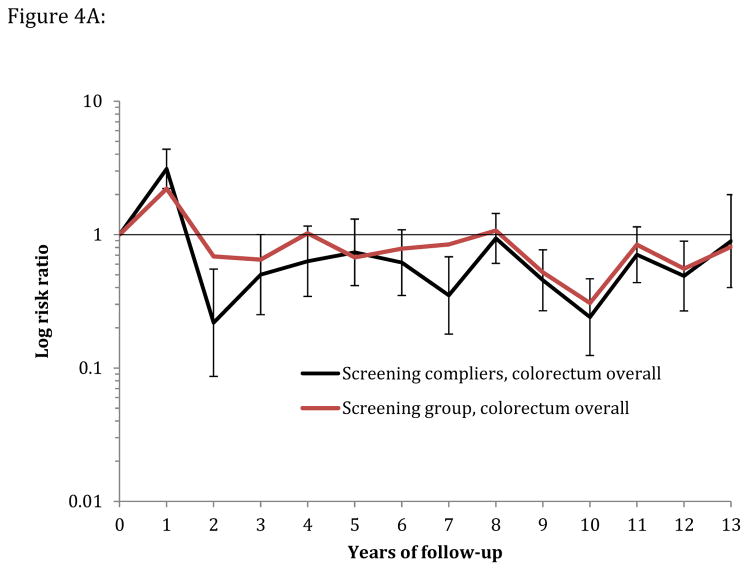
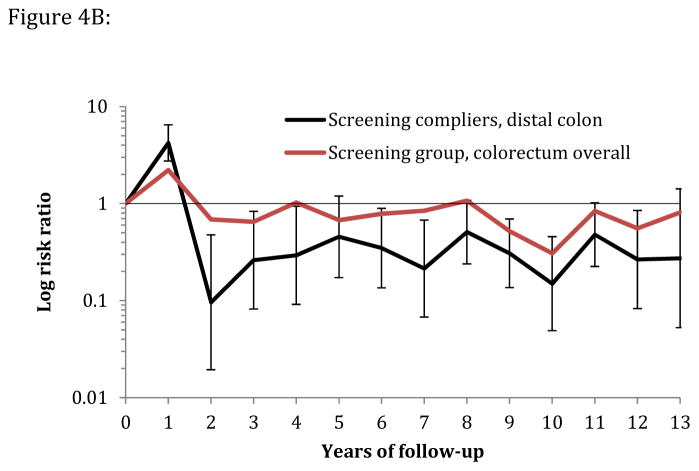
Figure 4.
Figure 4A: Yearly risk ratio (with error bars) for overall colorectal cancer incidence in screening compliers (n=12,955) and the screening group (compliers and noncompliers, n=20,572) relative to the control group (n=78,220).
Figure 4B: Yearly risk ratio (with error bars) for distal colorectal cancer incidence in screening compliers (n=12,955) and for overall colorectal cancer incidence in the screening group (compliers and noncompliers, n=20,572) relative to the control group (n=78,220).
Colorectal cancer mortality
The age-standardized CRC mortality rate (per 100,000 person-years) was 31.4 in the screening group and 43.1 in the control group, absolute rate difference 11.7 (95% CI 3.0–20.4), HR was 0.73 (95% CI 0.56–0.94) (Table 3). The corresponding HR was 0.74 (95% CI 0.40–1.35) in the 50–54 year age group and 0.73 (95% CI 0.55–0.97) in the 55–64 year age group. The HR was 0.58 (95% CI 0.40–0.85) in men and 0.91 (95% CI 0.64–1.30) in women (P-value for heterogeneity: 0.1). The HR was 0.79 (95% CI 0.55–1.11) for distal CRC mortality and 0.73 (95% CI 0.49–1.09) for proximal CRC mortality (Table 3). For flexible sigmoidoscopy screening only, HR was 0.84 (95% CI 0.61–1.17), and for flexible sigmoidoscopy and FOBT, the HR was 0.62 (95% CI 0.42–0.90) (P-value for heterogeneity: 0.2). A visual inspection of Figures 2B and 3 suggests that CRC mortality started to diverge in the two groups after 9 years of follow-up. The number needed to invite for screening to prevent one CRC death over 10 years was 1,547 (ICER $226,002; see supplementary appendix).
There were no differences in all-cause mortality between the screening and control group HR 0.97 (95% CI 0.93–1.02).
Compliance-adjusted analysis (per protocol effect)
The intention to screen 10-year risk absolute difference (risk in screening group minus risk in control group) was −0.22% (95% CI −0.38% to −0.06%) for CRC and −0.06% (95% CI −0.14% to 0.03%) for CRC-death in the entire study population. After adjustment for noncompliance, the corresponding 10-year risk differences were −0.42% (95% CI −0.69% to −0.15%) and −0.10% (95% CI −0.25% to 0.05%), respectively. See Appendix for details.
Discussion
In this study, flexible sigmoidoscopy screening reduced CRC incidence by 20% and CRC mortality by 27%. Younger individuals aged 50–54 years seem to benefit at least as much from the screening intervention as older individuals aged 55–64 years.
Three other large randomized trials of flexible sigmoidoscopy screening with comparable length of follow-up have been published. In the trials from the UK (Flexi Scope trial) and Italy (SCORE), offering once only flexible sigmoidoscopy examination to people aged 55–64 years, CRC incidence was reduced by 23% and 18% and CRC mortality by 31% and 22%, respectively.5,7 In the PLCO trial from the US, including individuals aged 55–74 years offering flexible sigmoidoscopy screening at two occasions, CRC incidence was reduced by 21% and CRC mortality by 26%.6
Our results are in accordance with those reported from the previous trials and extend them in three important ways. First, unlike the other trials, our estimates were not affected by screening contamination in the control group. Second, our population-based design with random sampling directly from the population registry allows estimation of the effectiveness of a national screening program with similar compliance. The study populations of the other trials consisted of volunteers. Therefore, their findings may not be generalizable to their national populations if those included in the trial had a different risk of CRC diagnosis or death than the background population. Indeed, in the Italian SCORE trial, the CRC mortality rate was 46% lower in the control group than in the background population.7 Third, our estimates show, for the first time, the effectiveness of screening in individuals aged 50–54 years.
The ideal age to start screening for CRC has not been firmly established, and national screening recommendations vary accordingly.19 Until now, no study has reported on effectiveness of flexible sigmoidoscopy screening under the age of 55. We show that individuals aged 50–54 benefit as much from flexible sigmoidoscopy screening as those older than 55 years with respect to CRC incidence.
The four flexible sigmoidoscopy trials had important differences in the threshold for referral to colonoscopy. In the present study, we adapted a low referral threshold, meaning that any adenoma (irrespective of size) qualified for colonoscopy. The PLCO trial referred all individuals with any detected lesion or polyp to follow-up, while only people with advanced or multiple adenomas were offered colonoscopy in the UK trial. The Italian trial adopted the recommendation from the UK trial, but in addition referred individuals with adenomas 6–9 mm in size. These differences led to widely varying colonoscopy rates (19.5% in NORCCAP; 5.0% in UK; 7.8% in Italy; 21.9% in US trial, respectively).6,20,21 Despite these differences, reported reductions in CRC incidence were similar (18–23%).5–7 This observation may infer that the least extensive referral approach could be sufficient, implying that only individuals with advanced adenomas at flexible sigmoidoscopy should be referred to colonoscopy.
Adaption of this recommendation would have great impact on costs of flexible sigmoidoscopy screening programs, but would also reduce the number of advanced adenomas detected in the proximal colon. We have previously reported that the number of colonoscopies would have been reduced by 66% if only participants in the NORCCAP trial with advanced adenomas were referred to colonoscopy. But, as a consequence, 38% of patients with proximal advanced adenomas would not have been detected.22 Other trials have confirmed that about half of proximal advanced adenomas did not have a synchronous distal lesion, and would thus have been missed due to normal findings at flexible sigmoidoscopy.23,24 In our trial, we found a 10% reduction in proximal CRC incidence, which is in accordance with the PLCO trial in which a 14% reduction in proximal CRC incidence was found with a similar colonoscopy referral rate. The optimum threshold for colonoscopy referral in a screening program should be weighted against costs and available endoscopy resources.
Adding one-time FOBT did not lead to additional screen-detected cancers or the detection of more advanced adenomas. This is in keeping with previous results.25 To reduce CRC mortality, FOBT has to be repeated.26–28 In fact, the combined screening approach led to lower compliance in our trial and could thus have a negative impact on a screening program. No reduction in all-cause mortality was observed. This was not unexpected, as only 4% of all deaths in the NORCCAP population were due to CRC and the trial was not powered to detect any difference in all-cause mortality.
Even if underpowered for subgroups analyses, our results may suggest a stronger effect of the screening intervention in men than in women. CRC incidence and mortality was reduced by 27% and 42% in men and 13% and 9% in women, respectively. A larger benefit for men was also evident in the PLCO and the UK trials.5,6 Possible explanations, supported by previous studies, may be that more women than men have proximal advanced adenomas without distal lesions which would have triggered a full colonoscopy, and more women than men have proximal sessile serrated lesions which may be more difficult to detect.29–31
In this paper we use observed data for prevented CRC incidence and CRC-deaths as a measure for clinical cost-effectiveness in a ten-year perspective. Hence, our findings are not comparable to most other cost-effectiveness analyses which are model-based and apply a lifetime perspective.32 Our estimate of the costs per prevented CRC shows that screening in the 50–54 year age group is at least as cost-effective as in the 55–64 year age groups, despite lower baseline risk of CRC (Supplementary appendix).
Estimating the per-protocol effect under full adherence is important to quantify the maximum benefit of the screening intervention that may be achieved in a screening program. Our results show that, in case of full compliance, the absolute reduction in 10-year CRC risk would be twice as high as in the intention-to-treat analysis (−0.42% versus −0.22%) if the compliers were approximately representative of the general population. The CRC incidence rate in non-compliers was equal to that in the control group (eFigure 1), which supports the generalizability of these estimates to the entire population. Figure 4 A and B approximately quantifies the effectiveness of a flexible sigmoidoscopy screening program on CRC incidence under perfect compliance.
A possible limitation of our trial is that we based mortality estimates on public registries and did not include a death review committee or expert coder to re-review death certificates. However, using a death review committee did not significantly alter the number of deaths attributable to CRC in two previous CRC screening trials.7,33 Even if more CRC deaths were found in the UK flexible sigmoidoscopy trial by an expert coder than were obtained from public registries, the added yield was similar in both the screening and control group and therefore did not change the effect estimates of the screening intervention.5
Conclusion
Compared with no screening, once only flexible sigmoidoscopy screening or flexible sigmoidoscopy with faecal occult blood testing reduced CRC incidence and mortality in a population-based trial in Norway. Screening effects were similar in 50–54 and 55–64 year old individuals.
Supplementary Material
Acknowledgments
Funding/Support: The NORCCAP trial was funded by research grants from the Norwegian Government and the Norwegian Cancer Society. Work with the present manuscript was funded by research grants from the Norwegian Cancer Society, the Research Council of Norway, The South-East Regional Health Authority of Norway, The Fulbright Foundation, Sorlandet Hospital Kristiansand, and the National Institutes of Health grant PO1 CA134294.
Footnotes
Conflict of interest disclosure: Michael Bretthauer is member of the European scientific advisory board of Exact Sciences and has received equipment for testing in scientific studies from Olympus, Fujinon, Falk Phgroup and CCS Healthcare. Holme, Løberg, Skovlund, Schneede, Aas, Hoff, Eide, Tveit, Kalager, Hernán report no conflicts of interest.
Role of the funders: The funders were not involved in design and conduct of the study, collection, management, analysis and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
NORCCAP collaborators were: Per Efskind MD, Nedre Glommen Physicians, Fredrikstad, Norway; Ghous Gondal MD PhD, Østfold Hospital, Fredrikstad, Norway; Gert Huppertz-Hauss MD, Telemark Hospital, Skien, Norway; Bjørn Hofstad MD PhD, Oslo University Hospital, Oslo, Norway; Solveig Thorp Holmsen MD MPH, Oslo University Hospital, Oslo, Norway; Inger Kristin Larsen PhD, Cancer Registry of Norway, Oslo, Norway; Volker Moritz MD, Telemark Hospital, Skien, Norway; Sverre Nyhus MD, Vestfold Hospital, Tonsberg, Norway; Espen Thiis-Evensen MD PhD, Oslo University Hospital, Oslo, Norway; Tom Grotmol MD Professor, Cancer Registry of Norway, Oslo, Norway. The NORCCAP steering committee comprises Geir Hoff MD Professor, Cancer Registry of Norway, Oslo and Telemark Hospital, Skien, Norway; Eva Skovlund Professor, School of Pharmacy, University of Oslo, Oslo, Norway; Morten Vatn MD Professor, Oslo University Hospital, Oslo, Norway; Tor Iversen Professor, University of Oslo, Oslo, Norway; Jørn Schneede MD Professor, Umeå University, Umeå, Sweden; Michael Bretthauer MD Professor, Oslo University Hospital and University of Oslo, Oslo, Norway; Kjell Magne Tveit MD Professor, Oslo University Hospital, Oslo, Norway; Jon Lekven MD Professor, University of Bergen, Bergen, Norway and Tor Jac Eide MD Professor, University of Oslo and Oslo University Hospital, Oslo, Norway. Dedicated nurses and staff at the screening centers made this trial possible.
The NORCCAP data safety and monitoring committee comprises Hans-Olov Adami MD Professor, Harvard School of Public Health, Boston, USA, and Karolinska Institutet, Stockholm, Sweden; Sandra J Lee Sc.D. Assistant Professor, Harvard Medical School and Dana-Farber Cancer Institute, Boston, USA, and Douglas K Rex MD Professor, Indiana University School of Medicine, Indianapolis, USA.
Author contributions: Dr Holme and Dr Løberg had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hoff, Bretthauer, Eide, Skovlund, Schneede, Tveit
Acquisition of data: Hoff, Bretthauer, Løberg, Aas
Analysis and interpretation of data: All authors
Drafting of the manuscript: Holme
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Holme, Løberg, Kalager, Hernán
Obtained funding: Hoff, Kalager, Løberg, Holme, Bretthauer, Hernán
Study supervision: Hoff, NORCCAP Data and Safety Monitoring Board.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. [Accessed March 11th 2014]. http://globocan.iarc.fr/Pages/online.aspx. [Google Scholar]
- 2.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 3.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.West NJ, Boustiere C, Fischbach W, et al. Colorectal cancer screening in Europe: differences in approach; similar barriers to overcome. Int J Colorectal Dis. 2009;24(7):731–740. doi: 10.1007/s00384-009-0690-6. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 6.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 8.Hoff G, Grotmol T, Skovlund E, et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Registry of Norway. [Accessed May 8th, 2014];Gastronet. Resultater 2010 identifiserbart pr senter. 2010 http://kreftregisteret.no/Global/Gastronet%20p%c3%a5logging/Resultater/Centre%20identifiable%20Gastronet%20Colo%202010%20GH060211%20Update%20GH240511.pdf.
- 10.Bretthauer M, Gondal G, Larsen K, et al. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colorectal Cancer Prevention) Scand J Gastroenterol. 2002;37(5):568–573. doi: 10.1080/00365520252903125. [DOI] [PubMed] [Google Scholar]
- 11.Gondal G, Grotmol T, Hofstad B, et al. The Norwegian Colorectal Cancer Prevention (NORCCAP) screening study: baseline findings and implementations for clinical work-up in age groups 50–64 years. Scand J Gastroenterol. 2003;38(6):635–642. doi: 10.1080/00365520310003002. [DOI] [PubMed] [Google Scholar]
- 12.Hoff G, Sauar J, Hofstad B, et al. The Norwegian guidelines for surveillance after polypectomy: 10-year intervals. Scand J Gastroenterol. 1996;31(9):834–836. doi: 10.3109/00365529609051989. [DOI] [PubMed] [Google Scholar]
- 13.Larsen IK, Smastuen M, Johannesen TB, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Tappenden P, Eggington S, Nixon R, et al. Colorectal cancer screening options appraisal: cost-effectiveness, cost-utility and resource impact of alternative screening options for colorectal cancer. Report to the English Bowel Cancer Screening Working Group; September 2004; [Accessed June 2nd, 2014]. Available at: http://www.cancerscreening.nhs.uk/bowel/scharr.pdf. [Google Scholar]
- 15.National Institute for Health and Clinical Excellence. [Accessed June 2nd, 2014]; Available at: http://www.nice.org.uk/media/68D/29/The_guidelines_manual_2009_-_Chapter_7_Assessing_cost_effectiveness.pdf.
- 16. [Accessed June 9th, 2014];Consumer Price Index UK. http://www.rateinflation.com/consumer-price-index/uk-historical-cpi.
- 17.Toh S, Hernan MA. Causal inference from longitudinal studies with baseline randomization. The international journal of biostatistics. 2008;4(1):Article 22. doi: 10.2202/1557-4679.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology. 2006;17(4):360–372. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 19.Zavoral M, Suchanek S, Zavada F, et al. Colorectal cancer screening in Europe. World J Gastroenterol. 2009;15(47):5907–5915. doi: 10.3748/wjg.15.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segnan N, Senore C, Andreoni B, et al. Baseline findings of the Italian multicenter randomized controlled trial of “once-only sigmoidoscopy”--SCORE. J Natl Cancer Inst. 2002;94(23):1763–1772. doi: 10.1093/jnci/94.23.1763. [DOI] [PubMed] [Google Scholar]
- 21.Atkin WS, Cook CF, Cuzick J, et al. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359(9314):1291–1300. doi: 10.1016/S0140-6736(02)08268-5. [DOI] [PubMed] [Google Scholar]
- 22.Gondal G, Grotmol T, Hofstad B, et al. Grading of distal colorectal adenomas as predictors for proximal colonic neoplasia and choice of endoscope in population screening: experience from the Norwegian Colorectal Cancer Prevention study (NORCCAP) Gut. 2003;52(3):398–403. doi: 10.1136/gut.52.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343(3):169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman DA, Weiss DG. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345(8):555–560. doi: 10.1056/NEJMoa010328. [DOI] [PubMed] [Google Scholar]
- 26.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 27.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 28.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 29.Forsberg AM, Kjellstrom L, Agreus L, et al. Prevalence of colonic neoplasia and advanced lesions in the normal population: a prospective population-based colonoscopy study. Scand J Gastroenterol. 2012;47(2):184–190. doi: 10.3109/00365521.2011.647062. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352(20):2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 31.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63(8):681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 32.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev. 2011;33(1):88–100. doi: 10.1093/epirev/mxr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ederer F, Geisser MS, Mongin SJ, et al. Colorectal cancer deaths as determined by expert committee and from death certificate: a comparison. The Minnesota Study. J Clin Epidemiol. 1999;52(5):447–452. doi: 10.1016/s0895-4356(99)00016-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.