Abstract
Background
Certain anti-HIV drugs alone or in combination are often associated with liver damages, which are frequently worsened by alcohol consumption. We previously found an endoplasmic reticulum (ER) stress mechanism for the drug- and alcohol-induced hepatic injuries in animal models and in vitro hepatocytes. However, it is unknown whether anti-HIV drugs and alcohol induce similar cellular stress responses and injuries in liver nonparenchymal cells.
Methods
Primary mouse hepatocytes (PMH), kupffer cells (KC), and hepatocellular stellate cells (HSC) were freshly isolated from mouse liver and treated with DMSO, stress-inducing pharmaceutical agents, alcohol alone, or in combination with antiviral ritonavir (RIT), lopinavir (LOP), or efavirenz (EFV). Expression of cellular stress markers, protein colocalization, and cell death were analyzed with immunoblotting, immunocytochemistry, and positive double staining with Sytox green and Hoechst blue, respectively.
Results
Expression of the ER stress markers of BiP, CHOP, and SERCA and the autophagy marker LC3 was significantly changed in PMH in response to combined alcohol, RIT, and LOP, which was companied by increased cell death compared with control. In contrast, although pharmaceutical agents induced ER stress and cell death, no significant ER stress or cell death was found in KC treated with alcohol, RIT, LOP, and EFV singly or in combination. In HSC, alcohol, RIT, LOP, or EFV induced BiP, but not CHOP, SERCA, or cell death compared with vehicle control. Further in PMH, RIT and LOP or in combination with alcohol-induced dose-dependent inhibition of β-actin. Inhibition of β-actin by RIT and LOP was companied with an inhibited nuclear expression of the antioxidant response regulator Nrf2 and reduced GST downstream of Nrf2. Ascorbic acid treatment reduced the alcohol-, RIT-, and LOP-induced cell death.
Conclusions
The data suggest for the first time that sensitivities of hepatocytes and nonparenchymal cells to alcohol and anti-HIV drugs in vitro are different in terms of cellular stress response and cell death injury. Oxidative stress mediated by Nrf2 contributes to the alcohol- and drug-induced toxicity in the hepatocytes.
Keywords: Alcohol, Antivirals, Liver Parenchymal Cells Versus Nonparenchymal Cells, Hepatotoxicity
Approximately 38 million individuals are infected with HIV/AIDS worldwide, and more than 1 million people are living with HIV infection in the United States (CDC, 2012; Doherty et al., 2013; Maartens et al., 2014). The situation demands the development of effective strategies against HIV infection. Antiretroviral agents and nucleic acid-based therapeutics have been developed and shown to be highly effective as prophylactic modalities (Camponeschi et al., 2013; Flexner and Saag, 2013). For instance, HIV protease inhibitors (PIs), a class of antiretroviral agents that inhibit HIV proteinase or protease, have been used in highly active effective antiretroviral therapies (HAART). More and more patients with HIV under lifetime therapies with antivirals have been healthier and live longer. However, there are reports indicating that antivirals singly or in combination increase the risk of comorbidities, including cardiovascular and cerebrovascular disease, pulmonary disease, bone disease, and even cancer, which undermine quality of life for those infected with HIV (Doherty et al., 2013; Gardner et al., 2013; Margolis et al., 2014). In the liver, non-HIV liver disease has emerged as the most common non-AIDS-related cause of death among HIV-infected patients, accounting for 15% of all deaths (Amacher and Chalasani, 2014; Eguchi et al., 2014; Price and Thio, 2010; Sherman et al., 2014). To be worse, nearly half of the HIV-infected patients consume or abuse alcohol, which not only impairs patients’ adherence to HAART but also deteriorates HIV drug-induced hepatotoxicity leading to greater morbidity and mortality (Braithwaite et al., 2007a,b; Hendershot et al., 2009). Even nonhazardous alcohol use of <5 standard drinks once a week can reduce survival of HIV-infected people by 1 year, and daily hazardous use of 5 or more standard drinks per day reduces survival by more than 6 years (Braithwaite et al., 2007a,b; Chander, 2011; Hahn and Samet, 2010; Hendershot et al., 2009; Shuper et al., 2010). Increases in liver injuries such as hepatic fibrosis or cirrhosis are often seen at all levels of alcohol exposure among people with HIV/AIDS (Galvan et al., 2002; Hendershot et al., 2009; Sherman et al., 2014). Currently, there is no consensus on how to manage patients with AIDS suffering from liver disease because mechanisms underlying the hepatotoxicity by combined alcohol and anti-HIV drugs are not well understood.
Recently, mechanisms of the hepatotoxicity during HAART have been hypothesized as follows: (i) direct intrinsic toxicities from individual component anti-HIV drugs, (ii) idiosyncratic hypersensitivity reactions, (iii) aberrant immune activities in patients co-infected with hepatitis C or B viruses, (iv) induction of hepatic metabolic abnormalities, and (v) multiple cellular stress responses (Barve et al., 2010; Ji, 2014; Mastroianni et al., 2014; Sherman et al., 2014; Szabo and Zakhari, 2011). With respect to potential effects of combined alcohol and anti-HIV drugs, the metabolic abnormalities are of interest because alcohol and most anti-HIV drugs are metabolized in the liver by the cytochrome P450 enzyme system, which is bound to interfere with HAART in AIDS patients. In fact, alcohol is reported to affect hepatic CYP activities, especially CYP3A4 that metabolize protease inhibitors (Flexner et al., 2001; Kumar et al., 2012; Sulkowski, 2003). The induction of cellular stress responses is also of interest as there are reports indicating that some antiviral drugs individually or collectively induce overproduction of reactive oxygen species (ROS) resulting in oxidative stress (Apostolova et al., 2013; Cao and Kaufman, 2014), accumulation of unfolded proteins in the endoplasmic reticulum (ER) resulting in ER stress (Ji, 2014), and impaired autophagous response resulting in cell death (Escalante et al., 2013; Gills et al., 2007). All of these cellular stress responses could also be induced by alcohol consumption, which will lead to aggravated liver injuries often seen in the clinic (Szabo and Zakhari, 2011). We have previously reported that combination of alcohol with 2 HIV protease inhibitors, ritonavir and lopinavir, induced synergistic effects on the drug bioavailabilities, ER stress response, and hepatic injuries in mouse models and in primary mouse and human hepatocytes (Ji et al., 2011; Kao et al., 2012). Our findings provide supporting evidence for the latter 2 hypotheses regarding the hepatotoxicity by alcohol and the HIV drugs, which could lead to revealing of potential drug targets for better caring of patients with AIDS under HAART. However, the liver is made up of many cell types including parenchymal hepatocytes and nonparenchymal cells such as kupffer cells (KC; hepatic macrophages), sinusoidal endothelial cells, and stellate cells, contributing to liver normal function with respect to their owner stress responses and survival as well as their influences on hepatocyte stress responses and survival (Cohen and Nagy, 2011; Ishibashi et al., 2009; Malarkey et al., 2005; Malik et al., 2002). Information is limited about toxicities of alcohol and the HIV drugs on nonparenchymal cells. In this study, we compared cellular stress responses in hepatocytes versus kupffer and stellate cells exposed to alcohol and selected anti-HIV drugs.
MATERIALS AND METHODS
Liver Cell Isolation and Culturing
Primary mouse hepatocytes (PMH) were isolated from 6-month-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor ME) and provided by the Cell Culture Core (USC Research Center for Liver Disease). The cell culturing and media were described previously (Ji et al., 2011; Kao et al., 2012). Treatments were administered directly to the medium at the following concentrations: 10 μl/ ml DMSO (Sigma, St. Louis, MO) as a vehicle control, 7.5 μg/ml of tunicamycin (Sigma), 6 μg/ml of thapsigargin (Sigma), 25 μg/ml of chloroquine (Sigma), 7.5 μg/ml of 95% ethanol, 22.5 μg/ml of ritonavir and lopinavir (Moravek Biochemicals, Inc., Brea, CA), 22.5 μg/ml of ritonavir and lopinavir with 7.5 μL of 95% ethanol, and 15 μg/ml of efavirenz (Moravek Biochemicals, Inc.). Concentrations used in drug dose–response studies ranged from 0 to 50 μg/ml and in alcohol dose–response studies ranged from 0 to 800 mM. In some experiments, the primary hepatocytes were treated with L-ascorbic acid (1 mM of final concentration). The treatments lasted for 12 to 24 hours. Part of the cells treated for 24 hours were double-stained with Sytox green and Hoechst blue and visualized on a fluorescence microscope. The cell death rate (combination of necrosis and apoptosis) was counted as previously described (Ji et al., 2011). Other parts of the treated cells were detached using 0.05% trypsin-EDTA (Gibco/Life Technologies, Grand Island, NY), centrifuged at 3,000×g, washed with phosphate buffered saline (PBS), and used for protein and RNA extractions and molecular analyses.
Primary hepatocellular stellate cells (HSC) were isolated by the Non-Parenchymal Liver Cell Core (Southern California Research Center for Alcoholic Liver and Pancreatic Diseases) as previously described (Zhu et al., 2012). Briefly, HSC were isolated from the mouse livers by in situ enzymatic dissociation and density gradient centrifugation. The purity of isolated HSC was examined by phasecontrast microscopy and ultraviolet-excited fluorescence microscopy, and the viability (>85%) of the HSC was based on trypan blue exclusion. The freshly isolated cells were allowed to attach and maintained in DMEM/F12 medium (Gibco/Life Technologies) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin/streptomycin (Sigma) and 5% CO2 atmosphere at 37 °C for 2 days before treatments. Treatments and drug doses were identical to the PMH.
Primary KC were isolated from the mice by in situ sequential digestion of the liver with pronase and collagenase and subsequent ultracentrifugation in arabinogalactan gradient as previously described (Xiong et al., 2008). The method of selective adherence to plastic was used to purify KC up to >95% (Blomhoff and Berg, 1990). The viability (>95%) of the adherent KC was determined by the trypan blue exclusion test. The freshly isolated cells were incubated with DMEM containing 5% FBS overnight before treatments. Treatments and drug doses were identical to the PMH.
Protein and RNA Extraction and Analysis
Methods for protein extraction and immunoblotting and for mRNA extraction and RT-PCR analysis were reported previously (Ji et al., 2011). PCR primers for Nrf2 are TAGATGACCATGAGTCGCTTGC and GCCAAACTTGCTCCATGTCC. PCR primers for 18s rRNA as an internal control are GTAACCCGTTGAACCCCATT and CCATCCAATCGGTAGTAGCG. For some analyses, nuclear and cytoplasmic protein separations were performed through differential centrifugation. Briefly, after detaching the cells and washing 3 times with PBS, the primary hepatocytes were incubated in hypotonic lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT pH 7.9) for 5 minutes. This was followed by homogenization with a pestle to break the cytoplasm of the swollen cells. The suspension was then transferred to a 0.25 M sucrose solution and centrifuged at 1,430×g for 5 minutes to separate the nuclear fragments from the cytoplasmic. The supernatant was kept as the cytoplasmic proteins, while the pellet was resuspended in a 0.88 M sucrose solution and centrifuged at 3,000×g to separate further the nuclear proteins. The pellet obtained after centrifugation was then immersed in 150μl RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and centrifuged at 20,000×g at 4°C for 1 hour. The supernatant was removed postcentrifugation and further concentrated using Pierce PES concentrators with a 3K molecular weight cutoff (Thermo Scientific, Rockford, IL). Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) was conducted to measure the total cytoplasmic or nuclear protein levels before the immunoblotting. Antibodies against actin and microtubule-associated protein 1 light chain-3B (LC3B) were from Cell Signaling (Boston, MA). Antibodies against BiP (glucose response protein 78 or GRP78), GST (glutathione S-transferase), Keap1 (Kelch-like ECH-associated protein 1), PDI (protein disulfide isomerase), SERCA (sarco-endoplasmic reticulum calcuim 2+ ATPase), and UGT1A (UDP-glucuronosyltransferase 1A) were from Santa Cruz Biotechnology. Antibodies against Nrf2 (the nuclear factor erythroid 2-related factor 2), vinculin (load control for whole-cell proteins), and TBP (TATA-binding protein, loading control for nuclear proteins) were from Abcam (Cambridge, MA). Antibodies against GAPDH (glyceraldehyde 3-phosphate dehydrogenase) were from Millipore (Billerica, MA). The intensity of protein bands on the immunoblots was quantified with the U.S. NIH software, ImageJ, after the blots of protein samples were scanned into TIF files.
Immunocytochemistry and Fluorescence Microscopy
PMH were seeded onto microscope cover slips and treated with the agents mentioned above. After 12 hours of treatment, the cells were fixed in 5% buffered neutral formalin for 20 minutes. The coverslips were blocked with 5% normal goat serum in PBS + 0.1% Triton X-100 for 1 hour. Coverslips were incubated with the anti-Nrf2 antibodies for 1 hour and then probed with a rhodamine TRITC fluorescent antibody (Jackson Immunoresearch, West Grove PA) for another hour. Filamentous actin double staining was performed using Alexa Fluor 488 conjugated phalloidin (Life Technologies, Grand Island, NY). Nuclear counterstaining was performed using Hoechst blue, and the coverslip was mounted onto a glass slide and visualized on a Nikon Eclipse TE300 inverted fluorescence microscope (Nikon Inc., Melville, NY). A negative control with only rhodamine TRITC antibody added without a primary was performed to control for autofluorescence. Cells with colocalized nuclear positive staining were counted across 3 slides at 20× magnification and expressed as a percentage.
Statistical Analysis
Values are expressed as means ± SEM unless otherwise indicated. Statistical analyses were performed using the Student’s t-test or analysis of variance for comparison of multiple groups. p < 0.05 or less was considered significant.
RESULTS
Differential Effects of Alcohol and Anti-HIV Drugs on Parenchymal Versus Nonparenchymal Cells
To know effects of alcohol and anti-HIV drugs on major liver cell types, we examined cellular ER stress and autophagous responses as well as cell death injury. Compared with vehicle control and positive ER stress-inducing agent tunicamycin, alcohol, ritonavir (HIV protease inhibitor), or lopinavir (HIV protease inhibitor) alone induced mild increase in expression of selected ER stress markers including BiP and CHOP in the primary hepatocytes (Fig. 1A). However, alcohol in combination with the 2 anti-HIV drugs induced BIP and CHOP significantly in the hepatocytes, which were companied by a significant reduction of SERCA. In contrast, although tunicamycin induced ER stress in KC, no significant changes in BiP, CHOP, or SERCA were found in the KC treated with alcohol and/or the anti-HIV protease inhibitors (Fig. 1B). In the HSC, alcohol and the 2 protease inhibitors increased BiP expression and slight inhibition of SERCA (Fig. 1C,D). CHOP was increased by tunicamycin but not by alcohol or the 2 protease inhibitors. Similarly, efavirenz that is a non-nucleoside reverse transcriptase inhibitor (NNRTI) also induced BIP, but not CHOP in the HSC.
Fig. 1.
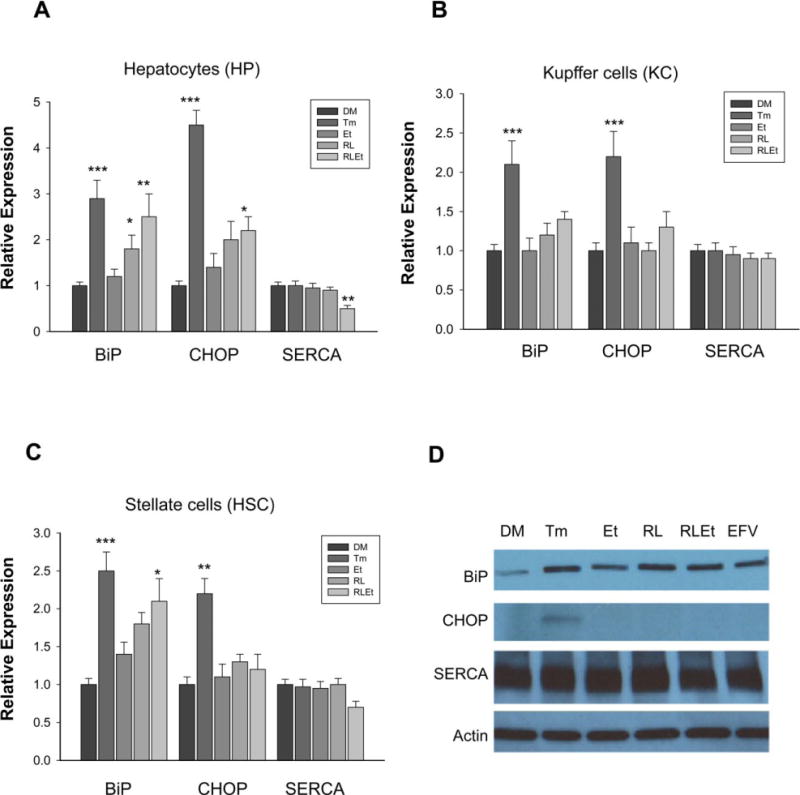
Effects of alcohol and antivirals on endoplasmic reticulum (ER) stress response in primary mouse liver parenchymal versus nonparenchymal cells. The cells were treated with DMSO (DM), tunicamycin (Tm), alcohol (Et), ritonavir and lopinavir (RL), Et plus RL (RLEt), or efavirenz (EFV). Proteins were analyzed with immunoblotting. (A) Protein expression of chaperone BiP (glucose-regulated protein 78 or GRP78), CHOP (transcription factor C/ EBP homologous protein), and SERCA (sarco/ER calcium ATPase) in the hepatocytes; (B) protein expression in kupffer cells; (C) protein expression in hepatic stellate cells; representative immunoblots of stellate cell proteins were shown in (D); *p < 0.05, **p < 0.01, ***p < 0.005 compared with DMSO. n = 3 to 5.
ER stress response and autophagous response are often co-presented upon cellular stress (Ding et al., 2007; Ogata et al., 2006). To know that, we examined expression of the autophagy marker protein LC3 in the liver cell types (Fig. 2). In the hepatocytes, induction of LC3 by alcohol alone was detected at concentrations around 85 mM and the induction was in a dose-dependent manner at concentrations ranging from 0 to 800 mM (Fig. 2A). Further, LC3II was increased in response to chloroquine (an autophagy-inducing agent), thapsigargin (the SERCA inhibitor), or the HIV drugs (Fig. 2B). LC3II was increased markedly in response to the combination of ritonavir, lopinavir, and alcohol (at 100 mM). Mild increase of LC3II was detected in the drug-treated stellate cells treated with ritonavir and lopinavir or alcohol plus the 2 drugs. No change was observed in KC in any of the alcohol or drug treatments.
Fig. 2.
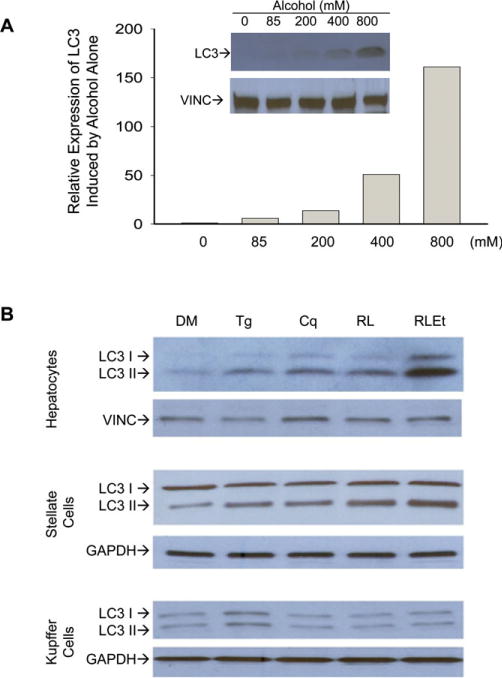
Effects of alcohol and antivirals on autophagous response in primary mouse liver parenchymal versus nonparenchymal cells. (A) Dose-dependent induction of LC3 in response to alcohol exposure. LC3 protein levels in primary mouse hepatocytes were quantified after being normalized with a loading control, vinculin (VINC); LC3, microtubule-associated protein 1 light chain-3B used as autophagy marker; (B) LC3 expression in liver cell types. The cells were treated with DMSO (DM), thapsigargin (Tg), chloroquine (Cq), ritonavir + lopinavir (RL), or RL + alcohol (RLEt). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Little to no cell death of the primary hepatocytes indicated by positive Sytox green staining was seen at any time point in the treatment groups of vehicle control, alcohol, or anti-HIV protease inhibitors alone. However, corresponding to the cellular stress responses, the cytoplasm and nuclei of the hepatocytes were distorted and irregularly shaped, and significantly increased cell death was observed in the hepatocytes in response to alcohol combined with the anti-HIV protease inhibitor drugs (Fig. 3A, B). In contrast, very little cell death was seen in any of the treatments in the kupffer or stellate cells, and morphology between control and experimental groups was nearly identical (Fig. 3A,B). Efavirenz alone induced significant cell death in PMH (Fig. 3C,D).
Fig. 3.
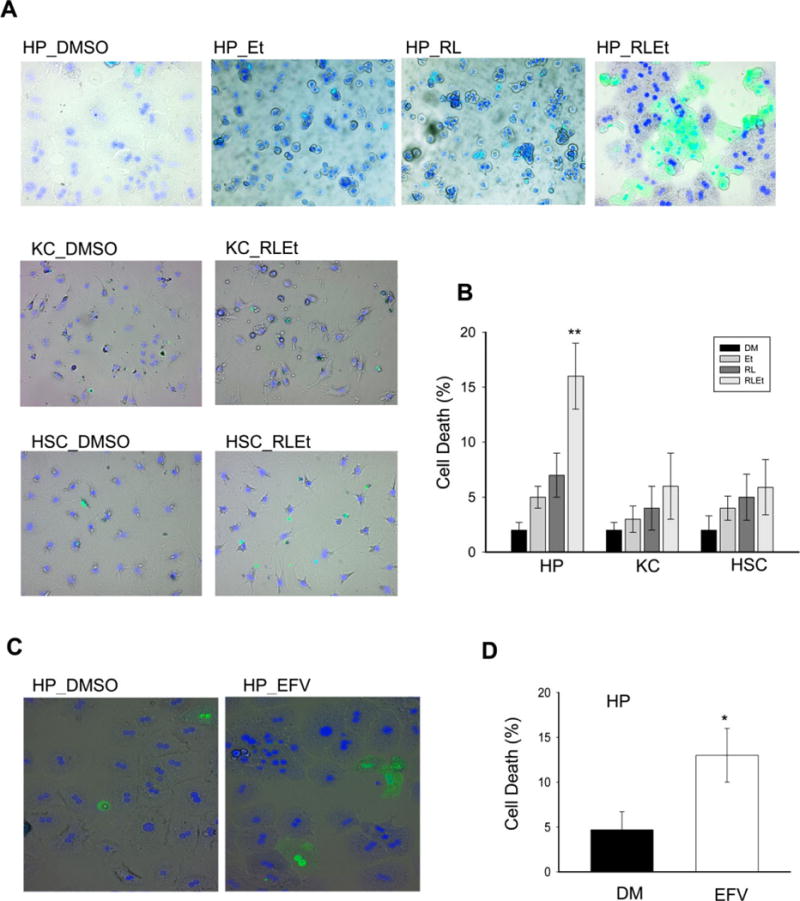
Cell deaths in primary mouse liver parenchymal versus nonparenchymal cells treated with alcohol and/or antivirals. (A) Cell morphology after double staining of Sytox green and Hoechst blue. HP, primary mouse hepatocytes; KC, primary kupffer cells; HSC, primary hepatic stellate cells; DM, DMSO; Et, alcohol; RL, ritonavir and lopinavir; RLEt, Et plus RL; (B) relative cell death rate (%); (C) cell morphology after treated with efavirenz (EFV) and stained with Sytox green and Hoechst blue; (D) relative cell death rate (%); *p < 0.05, **p < 0.01 compared with DMSO. n = 3 to 5.
Inhibition of β-actin by Combined Alcohol and Anti-HIV Drugs in the Hepatocytes
As no apparent effects by alcohol and the anti-HIV drugs were observed in the KC or stellate cells, further experiments and measurements were focused on the primary hepatocytes. In seeking potential mechanistic targets for the alcohol- and drug-induced hepatic stress and injury, we analyzed expression of additional proteins using immunoblotting. Interestingly, the protein of the housekeeping β-actin was reduced in the cells treated with the 2 anti-HIV protease inhibitors, which was nearly depleted in the presence of combination of alcohol and the drugs (Fig. 4A). The loss of β-actin was specific to the HIV protease inhibitors as it appeared normal in the hepatocytes treated with the NNRTI, efavirenz. Vinculin, a loading control for the whole-cell proteins, was consistently stable under the same experimental conditions. In subsequent experiments of dose–response, the reduction of the actin protein started at a concentration of 10 μM and maximized at 22.5 μM, whereas vinculin was evenly expressed across all concentrations (Fig. 4B). The specific loss of actin protein by the 2 protease inhibitors was not observed in the hepatocytes treated with different doses of efavirenz or with the ER stress-inducing agent, tunicamycin (Fig. 4B,C). In addition, alcohol enhanced the inhibition of the actin protein by the protease inhibitor drugs (Fig. 4C,D).
Fig. 4.
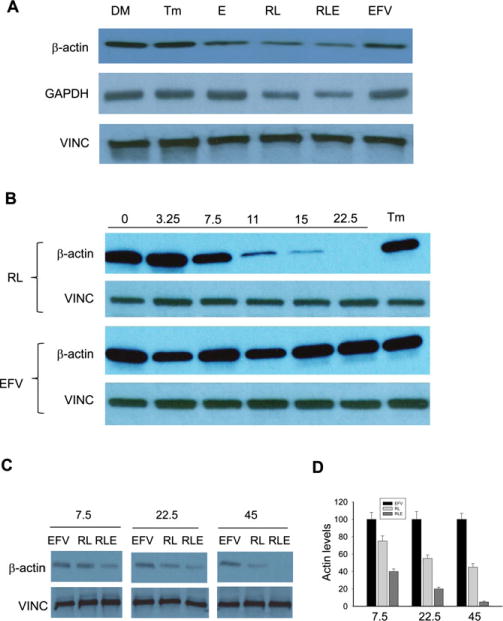
Inhibition of β-actin and GAPDH by alcohol and anti-HIV drugs. (A) Immunoblotting of proteins from primary hepatocytes treated with DMSO (DM), tunicamycin (Tm), alcohol (E), ritonavir and lopinavir (RL), E plus RL (RLE), or efavirenz (EFV); (B) dose-dependent expression of β-actin in response to ritonavir and lopinavir. Note: neither β-actin nor vinculin (VINC) was changed in response to EFV; the numbers on top of the blots are drug concentrations (μg/ml); (C) effects of alcohol on β-actin inhibition by RL or EFV; (D) relative expression of β-actin.
Inhibition of Nrf2 and Impaired Antioxidant Response by Alcohol and Anti-HIV Drugs
Actin has been associated with Nrf2 antioxidant response signaling through influencing the nuclear translocation of Nrf2 (Itoh et al., 1999). To know whether the inhibition of β-actin by the protease inhibitors and alcohol affect the actin–Nrf2 signaling, we separated nuclear and cytosolic proteins of the primary hepatocytes and measured cytoplasmic actin and Nrf2 versus nuclear actin and Nrf2. TATA-binding protein TBP was used as a nuclear control to further confirm nuclear and cytoplasmic separations. TBP bands appeared in the nuclear fractions of all treatment groups and were absent from all cytoplasmic fractions (Fig. 5A). The band for Nrf2 protein was much less intense in the nuclear fraction of the protease inhibitors treated groups. Keap1, which is also associated with Nrf2 (Kobayashi et al., 2004; Turpaev, 2013), was not changed in nuclear or cytoplasmic fractions from any of the treated cells. UGT1A and GST are 2 downstream proteins regulated by Nrf2. UGT1A had even bands across all treatments in the nuclear fraction and no bands in the cytoplasmic (Fig. 5A). GST bands had reduced intensity in the protease inhibitor–treated groups in the cytoplasmic fractions, and no bands in the nuclear fractions. No consistent effects by efavirenz were detected on cytoplasmic and nuclear expression of Nrf2 or the actin between the treatments (Fig. 5A). At transcriptional level, Nrf2 mRNA was increased in response to alcohol, inhibited in response to the protease inhibitors or combination of protease inhibitors and alcohol (Fig. 5B). Significant changes in Nrf2 mRNA were not detected in the hepatocytes in response to efavirenz or alcohol plus efavirenz.
Fig. 5.
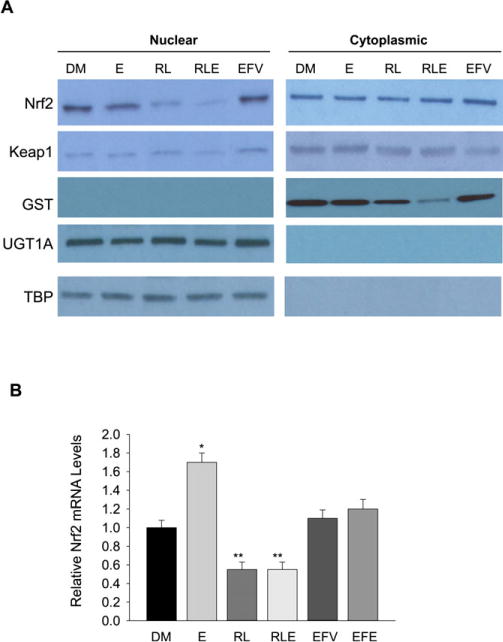
Effects of alcohol and anti-HIV drugs on cellular antioxidant response. (A) Immunoblotting of nuclear versus cytoplasmic proteins from primary mouse hepatocytes treated with DMSO (DM), alcohol (E), ritonavir and lopinavir (RL), E plus RL (RLE), or efavirenz (EFV); Nrf2, the nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like ECH-associated protein 1; GST, glutathione S-transferase; UGT1A, UDP-glucuronosyltransferase 1A; TBP, TATA-binding protein; (B) relative expression of Nrf2 mRNA in the hepatocytes in response to alcohol and anti-HIV drugs. *p < 0.05, **p < 0.01 compared with DMSO. n = 3.
To confirm the impairment of Nrf2 nuclear transport, immunocytochemistry was performed on the PMH. The nuclei of the hepatocytes were free of autofluorescence, and without DAPI counterstaining showed as empty areas. In ritonavir- and lopinavir-treated groups, the red nuclear Nrf2 stained with rhodamine was significantly reduced and completely disappeared in most areas compared with DMSO control. Green staining actin filaments were generally long and fibrous in DMSO group, but shorter and more disjointed in the protease inhibitor-treated groups (Fig. 6). The red Nrf2 rhodamine-positive staining appeared in the nuclei of DMSO control, colocalized to a purple when counterstained with DAPI (Fig. 6). Quantification of the nuclear colocalization of Nrf2 revealed over 90% of cells in DMSO-treated group express Nrf2-positive staining, while <10% of the protease inhibitor-treated cells show Nrf2 nuclear expression (Fig. 7A), confirming the inhibitory effects on actin–Nrf2 translocation by the anti-HIV drugs. In addition, antioxidant treatment with L-ascorbic acid significantly reduced alcohol- and the drug-induced cell death (Fig. 7B).
Fig. 6.
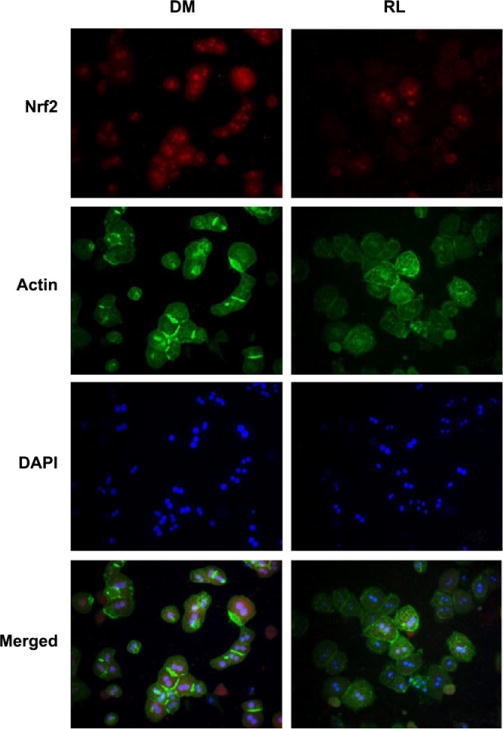
Immunocytochemistry reveals localization of Nrf2 in the nuclei of primary hepatocytes. Top row, Nrf2 stained with immunofluoresent rhodamine TRITC secondary antibody. Second row, actin stained with Alexa Fluor 488-conjugated phalloidin. Third row, nuclei stained with DAPI. Fourth row, merged image showing colocalization of Nrf2 and DAPI in nuclei as purple. DM, cells treated with DMSO; RL, cells treated with ritonavir and lopinavir.
Fig. 7.
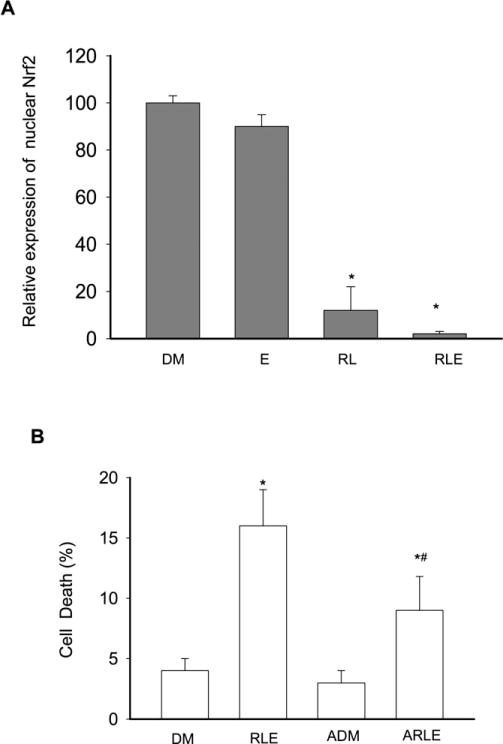
Relative expression of nuclear Nrf2 and cell death rate in primary mouse hepatocytes treated with alcohol, anti-HIV drugs, or antioxidants. DM, DMSO; E, alcohol; RL, ritonavir and lopinavir; RLE, E plus RL; ADM, DMSO plus ascorbic acid; ARLE, ascorbic acid plus RLE. (A) Positive cells with nuclear Nrf2; (B) cell death rate in the presence or absence of ascorbic acid. *p < 0.05 compared with DMSO; #, p < 0.05 compared with ascorbic acid treatment. n = 3.
DISCUSSION
HIV protease inhibitors and nuclease inhibitors are clinically prescribed to patients with AIDS as part of HAART, in which ritonavir is frequently prescribed to enhance the effects of other anti-HIV drugs. However, side effects of some drugs boosted by ritonavir are often reported (Margolis et al., 2014; Sherman et al., 2014), which can be worsen by alcohol intake (Braithwaite et al., 2007a,b; Galvan et al., 2002; Hendershot et al., 2009; Kao et al., 2012). Based on previous discoveries on the HIV drug- and alcohol-induced ER stress and hepatic injuries made from in vivo mouse models and from in vitro primary mouse and human hepatocytes, we compared for the first time the toxicity effects of alcohol and anti-HIV drugs on parenchymal and nonparenchymal cell types. Our results from these in vitro tests suggest that, unlike the hepatocytes with which cellular stresses such as ER stress and autophagy can be induced, nonparenchymal stellate or kupffer cells do not seem involved significantly in alcohol- and anti-HIV drug-mediated cytotoxicity. Little cell death in the stellate or kupffer cells was seen in the Sytox green staining, and there were no significant differences between controls and combined alcohol- and drug-treated groups in Western blots of ER stress and autophagy markers. During the experiments, they retained their quiescent stellate shaped cytoplasm, with numerous visible lipid vesicles (data not shown). The morphology was indistinguishable between nonparenchymal cells treated with vehicle control, the HIV protease inhibitors, or the NNRTI efavirenz. These results might suggest that nonparenchymal cells per se do not primarily contribute to the alcohol- and antiviral-caused side effects observed in vivo. However, our results from this study do not rule out 2 other possibilities. One is that important hepatic functions such as CYP450 expression, metabolite formation, and reaction velocities went down during or after the primary cell isolation. The functional decrease might be more intense in nonparenchymal cells than the hepatocytes, which make the formers not responsive. Another possibility is that nonparenchymal cells in the quiescent state are resistant or insensitive to the in vitro alcohol and HIV drug exposures. It is known that nonparenchymal cells undergo DNA synthesis and cell division later than the hepatocytes, and only upon liver injury, they become activated and start to produce cytokines such as tumor necrosis factor-α, interleukin (IL)-6, IL-1, and transforming growth factor-β, which initialize reconstruction and reformation of matrix proteins, exerting either positive or negative influences on hepatocyte proliferation and damage repairing (Cohen and Nagy, 2011; Malik et al., 2002). Such interactions between hepatocytes and nonparenchymal cells may be needed for the toxicity effects of alcohol and the anti-HIV drugs on the nonparenchymal cells. This possibility could be verified by comparing liver cell populations isolated from treated animals or by developing 3-dimensional culturing systems with different types of liver cells in the future.
Consistent with previous results (Ji et al., 2011; Kao et al., 2012), cellular stress responses continue to be observed in the primary hepatocytes treated with alcohol and the anti-HIV drugs in this study. Alcohol boosts the bioavailability of the anti-HIV protease inhibitor drugs possibly through competing for the same intracellular metabolic pathways (e.g., CYP3A4). High on-site levels of drugs may account for the synergistic effects on drug-targeted molecules (Kao et al., 2012). Besides, this study revealed a few new insights into the mechanisms behind the side effects. First, in the PMH, although both the HIV PIs and NNRTI (i.e., efavirenz) caused cell death, the morphology of the dead cells as well as expression of the stress responsive markers suggest that HIV PI and NNRTI induce the cell injury through different pathways. The former induces hepatic cell death mainly through an ER stress mechanism as already proposed previously (Ji et al., 2011; Zha et al., 2012). The latter might induce cell death through mitochondrial damages, which is consistent with the published evidence demonstrating that cells without a Rho mitochondrial receptor show significantly less cytotoxic reactivity to efavirenz (Apostolova et al., 2013; Escalante et al., 2013). In addition, the inhibition of GAPDH (Fig. 4A), alongside deformed cytoplasm in HIV PI-treated cells, might also suggest that the cell death result from a disruption of cellular metabolic pathways. This is because GAPDH plays a role in the final steps of glycolysis, and its depletion could indicate a disruption of the glycolytic pathway which leads to alterations of glucose metabolism and cell death (Lee et al., 2005). However, the mechanisms by which inhibited GAPDH causes cell death are not clear. Previous work by others indicated that GAPDH induces apoptosis when it was translocated into the nucleus of the cell by the E3 ubiquitin ligase, SIAH1 (7 in absentia 1 transporter; Hara et al., 2006). SIAH1 is normally a very transient protein, with an intracellular half-life of 5 minutes (Hara et al., 2005, 2006). GAPDH stabilizes this protein when bound to it and is translocated through the nuclear membrane in this bound complex. GAPDH cannot innately cross through the nuclear membrane otherwise. This hypothesis was curiously tested during this study using the nuclear/cytoplasmic separation to probe for nuclear expression of GAPDH. Unexpectedly, no GAPDH was detected in the nuclear fractions of any treatment groups, so it is conceivable to rule out the possibility that the hepatocellular cytotoxicity of ritonavir/lopinavir HIV cocktail is mediated by GAPDH-induced apoptosis. The role of the inhibition of GAPDH in the hepatotoxicity awaits further investigations.
Ruling out the possibility of GAPDH-inducing apoptosis, the disappearance of another normally stable cytoplasmic protein, β-actin, suggests a disruption of cytoplasmic processes in the HIV PI-treated hepatocytes, possibly through heightened oxidative stress that involves Nrf2. Nrf2 is a transcription factor that promotes transcription of many genes required for defense against ROS in eukaryotic cells. Nrf2 is normally sequestered in the cytoplasm by a composite of Keap1 and actin, where it is degraded by Cullin 3 via ubiquitination (Itoh et al., 1999; Kobayashi et al., 2004). Under oxidative stress conditions, the central cysteine residues in Keap1 are disrupted and Nrf2 is released and then translocated through the actin cytoskeleton to the nucleus (Sekhar et al., 2010; Yamamoto et al., 2008), where it activates downstream-targeted (ARE) genes including GST, UGT1A, and NQO1 (NADH quinone oxidoreductase 1) (Hayes et al., 2000; Venugopal and Jaiswal, 1996; Yueh and Tukey, 2007). As Keap1 constitutively shuttles between the nuclear and the cytoplasm under basal conditions (Sun et al., 2011) and was not changed in either cytoplasmic or nuclear fractions in any of the treatment groups, the Nrf2–Keap1–actin pathway might be disrupted by alcohol combined with the protease inhibitor drugs through both inhibition of Nrf2 expression or translocation Nrf2 proteins. The mRNA of Nrf2 was reduced by the drugs and alcohol, which moderately reduced Nrf2 protein content. More significantly, the loss of actin in both cytoplasmic and nuclear fractions may suggest that actin structure is disrupted in the cytoplasm, resulting in an inability to transport Nrf2. This is supported by the remarkable reduction of nuclear Nrf2 protein level in the HIV protease inhibitor-treated groups. The disruption of Nrf2–actin was further confirmed with cellular immunocytochemistry, from which Nrf2 was widely expressed in over 90% of all nuclei except when treated with the HIV protease inhibitors, where the expression falls under 10%. Given the results of the downstream effectors from Nrf2, it seems likely that the cytotoxic effects are caused by a loss of activity in the GST pathway, as there is no difference in band intensity in the UGT1A immunoblots between the drug-treated and control groups. Thus, the lowered antioxidant activities due to suppressed GST resulted in enhanced oxidative stress, ER stress, and cell death, which could be reduced partially by the application of antioxidants. In addition, the NNRTI, efavirenz, had no effects on the expression of Nrf2, which not only indicates a different mechanism of cytotoxicity by this drug (Apostolova et al., 2013; Escalante et al., 2013) but also indicates a quite specific Nrf2 inhibition by the HIV protease inhibitors.
In conclusion, the results of this study suggest an oxidative stress-mediated ER stress mechanism for alcohol- and anti-HIV protease inhibitor-induced cytotoxicity in the primary hepatocytes. The Nrf2–actin signaling might be an attractive drug target for preventing alcohol- and antiviral-caused side effects in the liver of patients with AIDS.
Acknowledgments
This study was supported by US NIH/NIAAA grant: R01AA018612 (to CJ). The authors thank the USC Research Center for Liver Disease (P30DK48522 and AA014428), and Southern California Research Center for Alcoholic Liver and Pancreatic Diseases (P50AA011999) for technical assistance in liver histopathology.
References
- Amacher DE, Chalasani N. Drug-induced hepatic steatosis. Semin Liver Dis. 2014;34:205–214. doi: 10.1055/s-0034-1375960. [DOI] [PubMed] [Google Scholar]
- Apostolova N, Gomez-Sucerquia LJ, Alegre F, Funes HA, Victor VM, Barrachina MD, Blas-Garcia A, Esplugues JV. ER stress in human hepatic cells treated with efavirenz: mitochondria again. J Hepatol. 2013;59:780–789. doi: 10.1016/j.jhep.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Barve S, Kapoor R, Moghe A, Ramirez JA, Eaton JW, Gobejishvili L, Joshi-Barve S, McClain CJ. Focus on the liver: alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. Alcohol Res Health. 2010;33:229–236. [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R, Berg T. Isolation and cultivation of rat liver stellate cells. Methods Enzymol. 1990;190:58–71. doi: 10.1016/0076-6879(90)90009-p. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Conigliaro J, Roberts MS, Shechter S, Schaefer A, McGinnis K, Rodriguez MC, Rabeneck L, Bryant K, Justice AC. Estimating the impact of alcohol consumption on survival for HIV+ individuals. AIDS Care. 2007b;19:459–466. doi: 10.1080/09540120601095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, Gibert C, Rodriguez-Barradas M, Mole L, Justice AC. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007a;21:1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camponeschi G, Fast J, Gauval M, Guerra K, Moore M, Ravinutala S, Ripin D, Shepel V. An overview of the antiretroviral market. Curr Opin HIV AIDS. 2013;8:535–543. doi: 10.1097/COH.0000000000000012. [DOI] [PubMed] [Google Scholar]
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supplemental Report. 2012;17:1–26. Available at: < http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed November 7, 2014. [Google Scholar]
- Chander G. Addressing alcohol use in HIV-infected persons. Top Antivir Med. 2011;19:143–147. [PMC free article] [PubMed] [Google Scholar]
- Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis. 2011;12:3–9. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Gao W, Hou YF, Melan MA, Chen X, Stolz DB, Shao ZM, Yin XM. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J Biol Chem. 2007;282:4702–4710. doi: 10.1074/jbc.M609267200. [DOI] [PubMed] [Google Scholar]
- Doherty M, Ford N, Vitoria M, Weiler G, Hirnschall G. The 2013 WHO guidelines for antiretroviral therapy: evidence-based recommendations to face new epidemic realities. Curr Opin HIV AIDS. 2013;8:528–534. doi: 10.1097/COH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Takatsuki M, Kuroki T. Liver transplantation for patients with human immunodeficiency virus and hepatitis C virus co-infection: update in 2013. J Hepatobiliary Pancreat Sci. 2014;21:263–268. doi: 10.1002/jhbp.31. [DOI] [PubMed] [Google Scholar]
- Escalante AM, McGrath RT, Karolak MR, Dorr RT, Lynch RM, Landowski TH. Preventing the autophagic survival response by inhibition of calpain enhances the cytotoxic activity of bortezomib in vitro and in vivo. Cancer Chemother Pharmacol. 2013;71:1567–1576. doi: 10.1007/s00280-013-2156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner C, Saag M. The antiretroviral drug pipeline: prospects and implications for future treatment research. Curr Opin HIV AIDS. 2013;8:572–578. doi: 10.1097/COH.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Flexner CW, Cargill VA, Sinclair J, Kresina TF, Cheever L. Alcohol use can result in enhanced drug metabolism in HIV pharmacotherapy. AIDS Patient Care STDS. 2001;15:57–58. doi: 10.1089/108729101300003636. [DOI] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gardner K, Hall PA, Chinnery PF, Payne BA. HIV treatment and associated mitochondrial pathology: review of 25 years of in vitro, animal, and human studies. Toxicol Pathol. 2013;42:811–822. doi: 10.1177/0192623313503519. [DOI] [PubMed] [Google Scholar]
- Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, Hollander MC, Kawabata S, Tsokos M, Figg WD, Steeg PS, Dennis PA. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- Hahn JA, Samet JH. Alcohol and HIV disease progression: weighing the evidence. Curr HIV/AIDS Rep. 2010;7:226–233. doi: 10.1007/s11904-010-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection bypharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52:180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Nakamura M, Komori A, Migita K, Shimoda S. Liver architecture, cell function, and disease. Semin Immunopathol. 2009;31:399–409. doi: 10.1007/s00281-009-0155-6. [DOI] [PubMed] [Google Scholar]
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C. New insights into the pathogenesis of alcohol-induced ER stress and liver diseases. Int J Hepatol. 2014;2014:513787. doi: 10.1155/2014/513787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao E, Shinohara M, Feng M, Lau MY, Ji C. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology. 2012;56:594–604. doi: 10.1002/hep.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Jin M, Ande A, Sinha N, Silverstein PS, Kumar A. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin Drug Metab Toxicol. 2012;8:1363–1375. doi: 10.1517/17425255.2012.714366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GA, Rao MN, Grunfeld C. The effects of HIV protease inhibitors on carbohydrate and lipid metabolism. Curr HIV/AIDS Rep. 2005;2:39–50. doi: 10.1007/s11904-996-0008-z. [DOI] [PubMed] [Google Scholar]
- Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- Malarkey DE, Johnson K, Ryan L, Boorman G, Maronpot RR. New insights into functional aspects of liver morphology. Toxicol Pathol. 2005;33:27–34. doi: 10.1080/01926230590881826. [DOI] [PubMed] [Google Scholar]
- Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425–431. doi: 10.1016/s1084952102001301. [DOI] [PubMed] [Google Scholar]
- Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni CM, Lichtner M, Mascia C, Zuccala P, Vullo V. Molecular mechanisms of liver fibrosis in HIV/HCV coinfection. Int J Mol Sci. 2014;15:9184–9208. doi: 10.3390/ijms15069184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar KR, Rachakonda G, Freeman ML. Cysteine-based regulation of the CUL3 adaptor protein Keap1. Toxicol Appl Pharmacol. 2010;244:21–26. doi: 10.1016/j.taap.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman KE, Thomas D, Chung RT. Human immunodeficiency virus and liver disease forum 2012. Hepatology. 2014;59:307–317. doi: 10.1002/hep.26638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS – a systematic review. Alcohol Alcohol. 2010;45:159–166. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS. Hepatotoxicity associated with antiretroviral therapy containing HIV-1 protease inhibitors. Semin Liver Dis. 2003;23:183–194. doi: 10.1055/s-2003-39949. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wu T, Zhao F, Lau A, Birch CM, Zhang DD. KPNA6 (Importin {alpha}7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol Cell Biol. 2011;31:1800–1811. doi: 10.1128/MCB.05036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Zakhari S. Mechanisms of alcohol-mediated hepatotoxicity in human-immunodeficiency-virus-infected patients. World J Gastroenterol. 2011;17:2500–2506. doi: 10.3748/wjg.v17.i20.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpaev KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry (Mosc) 2013;78:111–126. doi: 10.1134/S0006297913020016. [DOI] [PubMed] [Google Scholar]
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, She H, Zhang AS, Wang J, Mkrtchyan H, Dynnyk A, Gordeuk VR, French SW, Enns CA, Tsukamoto H. Hepatic macrophage iron aggravates experimental alcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G512–G521. doi: 10.1152/ajpgi.90327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Tukey RH. Nrf2-Keap1 signaling pathway regulates human UGT1A1 expression in vitro and in transgenic UGT1 mice. J Biol Chem. 2007;282:8749–8758. doi: 10.1074/jbc.M610790200. [DOI] [PubMed] [Google Scholar]
- Zha W, Zha BS, Zhou F, Zhou H, Wang G. The cellular pharmacokinetics of HIV protease inhibitors: current knowledge and future perspectives. Curr Drug Metab. 2012;13:1174–1183. doi: 10.2174/138920012802850119. [DOI] [PubMed] [Google Scholar]
- Zhu NL, Asahina K, Wang J, Ueno A, Lazaro R, Miyaoka Y, Miyajima A, Tsukamoto H. Hepatic stellate cell-derived delta-like homolog 1 (DLK1) protein in liver regeneration. J Biol Chem. 2012;287:10355–10367. doi: 10.1074/jbc.M111.312751. [DOI] [PMC free article] [PubMed] [Google Scholar]


