Abstract
We used our established experimental model of revision joint replacement to examine the roles of hydroxyapatite coating and bone graft in improving the fixation of revision implants. The revision protocol uses the Søballe micromotion device in a preliminary 8-week period of implant instability for the presence of particulate polyethylene. During this procedure, a sclerotic endosteal bone rim forms, and a dense fibrous membrane is engendered, having macrophages with ingested polyethylene and high levels of inflammatory cytokines. At the time of revision after 8 weeks, the cavity is revised with either a titanium alloy (Ti) or a hydroxyapatite (HA) 6.0 mm plasma-sprayed implant, in the presence or absence of allograft packed into the initial 0.75 mm peri-implant gap. The contralateral limb is subjected to primary surgery with the same implant configuration, and serves as control. 8 implants were included in each of the 8 treatment groups (total 64 implants in 32 dogs). The observation period was 4 weeks after revision. Outcome measures are based on histomorphometry and mechanical pushout properties. The revision setting was always inferior to its primary counterpart. Bone graft improved the revision fixation in all treatment groups, as also did the HA coating. The sole exception was revision-grafted HA implants, which reached the same fixation as primary Ti and HA grafted implants. The revision, which was less active in general, seems to need the dual stimulation of bone graft and HA implant surface, to obtain the same level of fixation associated with primary implants. Our findings suggest that the combination of HA implant and bone graft may be of benefit in the clinical revision implant setting.
Strong anchorage of orthopedic implants is critical to their long-term success. Moreover, clinical studies suggest that the anchorage should be achieved immediately after surgery, since the quality of the initial fixation predicts later failure (Linder 1994, Kärrholm et al. 1997). Therefore, implantation conditions that promote immediate and strong anchorage are desirable. This is particularly important for revision surgery, in which healing is slower and the bone stock less robust.
Implant coatings, such as hydroxyapatite (HA), have been studied experimentally (in the primary setting) and in human clinical trials (in both the primary and revision settings) (Bolander 1992, Søballe et al. 1993a, Kärrholm et al. 1994, Nelissen et al. 1998, Önsten et al. 1998, Thanner et al. 1999, 2000, Toksvig-Larsen et al. 2000). HA has been found to improve the shear strength of implants, and increase the bone in contact with and adjacent to the implant. Under stable conditions, HA can prevent the formation of an intervening fibrous membrane and, under unstable conditions, it has been shown over time to convert a motion-induced fibrous membrane into bone. In clinical trials using radiostereometric analysis (RSA), HA reduces maximum total-point motion for knee and hip replacement implants (Søballe et al. 1993a, Kärrholm et al. 1997, Nelissen et al. 1998). This suggests that HA implants achieve early bony anchorage, with minimal fibrous membrane.
Allograft has also been shown to be successful in securing the anchorage of an implant (Søballe et al. 1992a, Turner et al. 1993, McDonald et al. 1988). Its use in conjunction with HA coatings in the revision setting (in comparison with the primary setting), however, has not been experimentally evaluated under controlled motion and loading conditions.
In this study we evaluated the use of HA and allograft in primary and revision settings. Performance was evaluated as regards mechanical and histomorphometric parameters. We employed our controlled model of primary and revision implantation conditions, using our micromotion device which had been developed for the study of implant anchorage (Bechtold et al. 2001a, b, 2002).
We hypothesized that:
Mechanical and histomorphometric parameters describing implant anchorage are:
Lower with revised implants than with primary ones;
Higher with allograft than with implantation having no allograft (in primary and revision conditions);
Higher with hydroxyapatite than with titanium alloy (in primary and revision conditions).
Material and methods
Controlled motion device
A controlled micromotion device with more than a decade of previous studies (Søballe et al. 1992b, c, 1993b) was used to generate the primary and revision settings, as shown in detail below. This study was approved by our institution’s Animal Care and Use Committee, and was performed in our AAALAC accredited animal care facility.
The controlled motion device consists of a 6-mm diameter cylindrical implant which axially pistons 500 microns in a 0.75 mm circumferential gap, when loaded. This device is inserted into the distal medial femoral condyle of a dog, and is loaded when its knee is flexed. The internal spring restores the piston to its original position when the knee is unloaded. Stable conditions for the implant are established when it initially has no spring, or when previous pistoning is blocked by a stabilizing spacer.
Implant surface and material
Plasma-sprayed HA and plasma-sprayed titanium alloy (Ti) implant coatings were used. The cylindrical implants were nominally 6.0 mm in diameter with an overall length of 10 mm. Ti implants consisted of a solid Ti-6A1-4V alloy core with a coating of Ti-6A1-4V deposited by plasma-spray technique, resulting in a pore size of 200–1000 µm at the substrate and the surface of the coating, respectively (Søballe 1992b, c, 1993). HA-coated implants had a 50–70 µm layer of spray-dried synthetic HA (Ca/P ratio 1.64–1.70) deposited by plasma-spraying technique on a Ti porous-coated implant. X-ray diffraction analysis showed pure hydroxyapatite with no trace of tricalcium phosphate.
The surface roughness (Ra) of Ti- and HA-coated implants was determined using a roughness meter (Perthen, Hannover, Germany) with a stylus tip radius of 3 µm. Ra was 41 µm on HA and 47 µm on Ti implants. The profile depth was also measured and shown to be 445 µm for HA and 496 µm for Ti implants.
All implants were sterilized by gamma irradiation (35 kGy of Co-60 for 14 h, Risø National Laboratory, Roskilde, Denmark).
Allograft
Allograft was prepared under sterile conditions from pooled canine humeral heads immediately after they had been sacrificed for other studies. All cartilage was removed from the articulating surface, and a bone mill (fine setting, 3M, St. Paul, MN) made fine chips. The minced allograft was pooled and mixed before being divided into 0.25 g portions, which were individually frozen at −20 °C. Allograft was thawed for 1 hour before use. It was tightly packed into the peri-implant gap with a specially-designed tool having a thin curved blade. For consistency, all graft compaction was performed by a single surgeon.
Surgical procedure
The dogs were placed under general anesthesia, sterile technique was used, and perioperative antibiotics given (Keflex: 1 g i.v. pre-operatively, 1 g intraoperatively, and 1 g every 8 hours for 1 day). Following medial arthrotomy, the weight-bearing portion of the medial femoral condyle was identified during flexion. A guide wire was inserted in a 2.1-mm hole, spatially oriented under fluoroscopic control, to pass through the weight-bearing articulating surface and remain in the central portion of the condyle. A cannulated hand-drill created a deep cavity (6 mm in diameter by 10 mm in length) and a superficial cavity (7.5 mm in diameter by 20 mm in length), with the most superficial 4 mm being tapped. The PMMA implant and polyethylene cap were screwed onto the distal portion of the threaded piston and manually adjusted to the minimal protrusion that would cause full cylinder displacement during loading. Implant displacement and proper functioning were verified intraoperatively with a custom spring gauge. Soft tissues were closed routinely, and anteroposterior and lateral radiographs were taken immediately. After a recuperation period of about 3 days, the dogs were housed 2 per cage, and allowed unlimited cage activity in 24 square-foot runs. Their hind-limb function was assessed and noted daily, to ensure they were regularly loading their implants. They were also allowed 2 hours a day of free exercise.
Protocol for loosening implants–revision setting
Our controlled protocol for loosening implants, used to establish a revision setting is described in detail elsewhere (Bechtold et al. 2001a, b). Briefly, this protocol creates tissue and environment conditions that are representative of loosened human implants (dense fibrous tissue, macrophages with intracellular polyethylene (PE), sclerotic endosteal bone rim (SEBR), an increase in inflammatory cytokines and a decrease in anabolic cytokines).
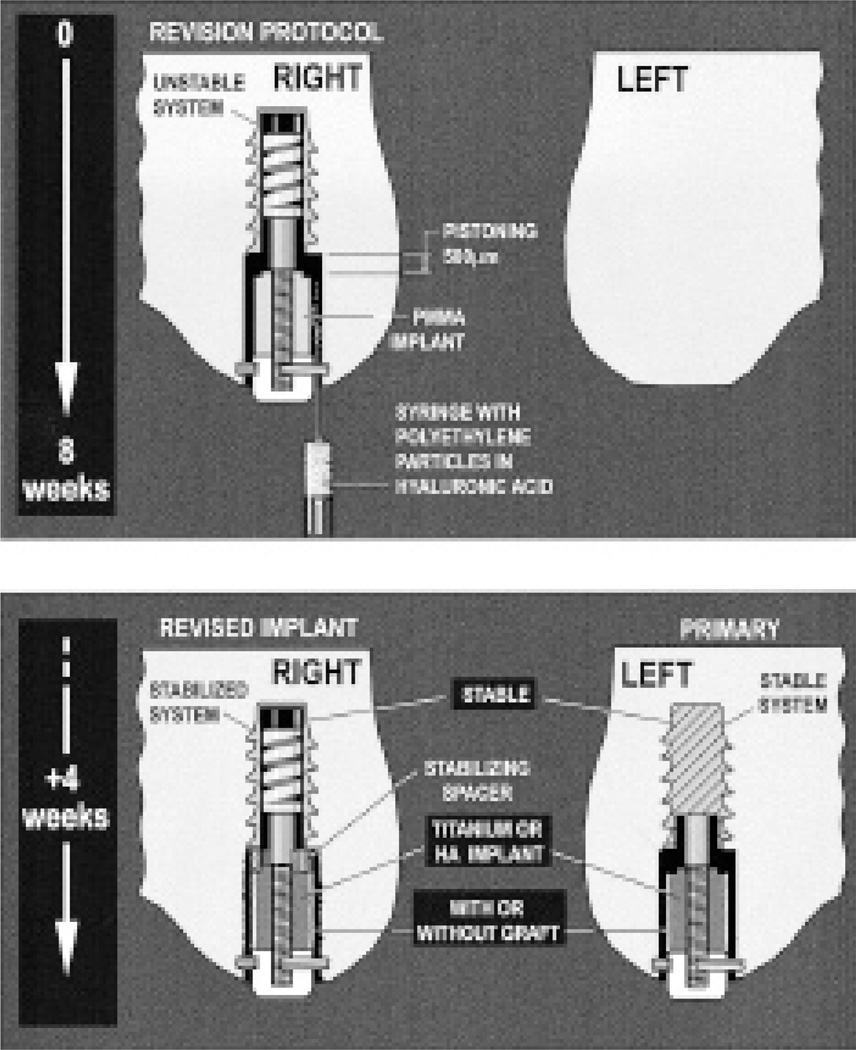
The primary and revision settings are engendered by first subjecting one knee to an established loosening protocol. This consists of an 8-week period with an unstable (pistoning) polymethylmethacrylate (PMMA) implant pistoning 500 microns in the presence of particulate PE (Figure 1). The molded PMMA implant represents a loose cement mantle.
Figure 1.
Schematic diagram showing the protocol for loosening implants
Polyethylene particles were injected around and in the gap surrounding the implant at the time of insertion. The particles consisted of 85% of high-density polyethylene, having a mean diameter of 2.09 (0.2–11) µm, and 15% of an ultra-high molecular weight polyethylene resin, having a mean diameter of 30 (10–50) µm on a sphere; Hoechst Celanese Corporation, Houston, TX). They were obtained from industrial sources, and were not retrieved from human implantations or produced during wear simulations. The number of particles injected around each implant was 5 × 107, in a volume of 200 mm3 (Goodman 1994, Lalor et al. 1999). They were sterilized and suspended via manual mixing in 0.27% sterile synthetic hyaluronic acid (LifeCore Biomedical, Minneapolis, MN) before injection. The volume of hyaluronic acid in the syringe was chosen so that the implant gap would be completely filled. Any minimal spillover was removed with sponges. The surgical site was not further lavaged or irrigated.
After the 8-week period establishing the revision setting, the knee was revised by removing the PMMA implant, scraping and lavaging the revision cavity, and then reinserting randomly a Ti or HA implant. During the same setting, a primary implant was inserted in the contralateral knee. In this way, the primary and revision implants were paired, and underwent the same surface/graft treatment combination.
Treatment and control groups
32 skeletally mature dogs were included (20–25 kg, 12–14 months) in this study. They were randomly allocated to receive either a HA or Ti plasma-sprayed implant, and to have either grafted or ungrafted gaps (n = 8 per group; total 64 implants). The right knee received a revision implant and the left knee the control primary implant. Treatment groups had HA-coated implants, with or without an allograft. Control groups had Ti implants, with or without an allograft. The implants in all groups were stable and loaded.
Outcome measures
Anteroposterior and lateral radiographs were taken after sacrifice. The knees were surgically exposed, and joint fluid and bacterial cultures were taken from the joint. The distal femur was removed and frozen at −20 °C. The implant and surrounding bone was cut into two pieces perpendicular to the implant, using a water-cooled diamond band saw (EXAKT, Norderstadt, Germany). The distal medial condyle was aligned transversely to the long axis of the implant, through a predrilled alignment cannula on the implant. One transverse block was used for pushout testing and was stored frozen at −20 °C. The other block was used for histomorphometry, and its preparation is described below.
Quantitative histomorphometry
The specimens were fixed in 70% ethanol, dehydrated in graded ethanol (70–99%), embedded in methylmethacrylate, and ground and polished (EXACT-Micro Grinding System) to a thickness of 50 µm. The ground sections were stained with basic fuchsin and counterstained with light green. To verify that the polyethylene particles were retained in the peri-implant space, polarized microscopy was used.
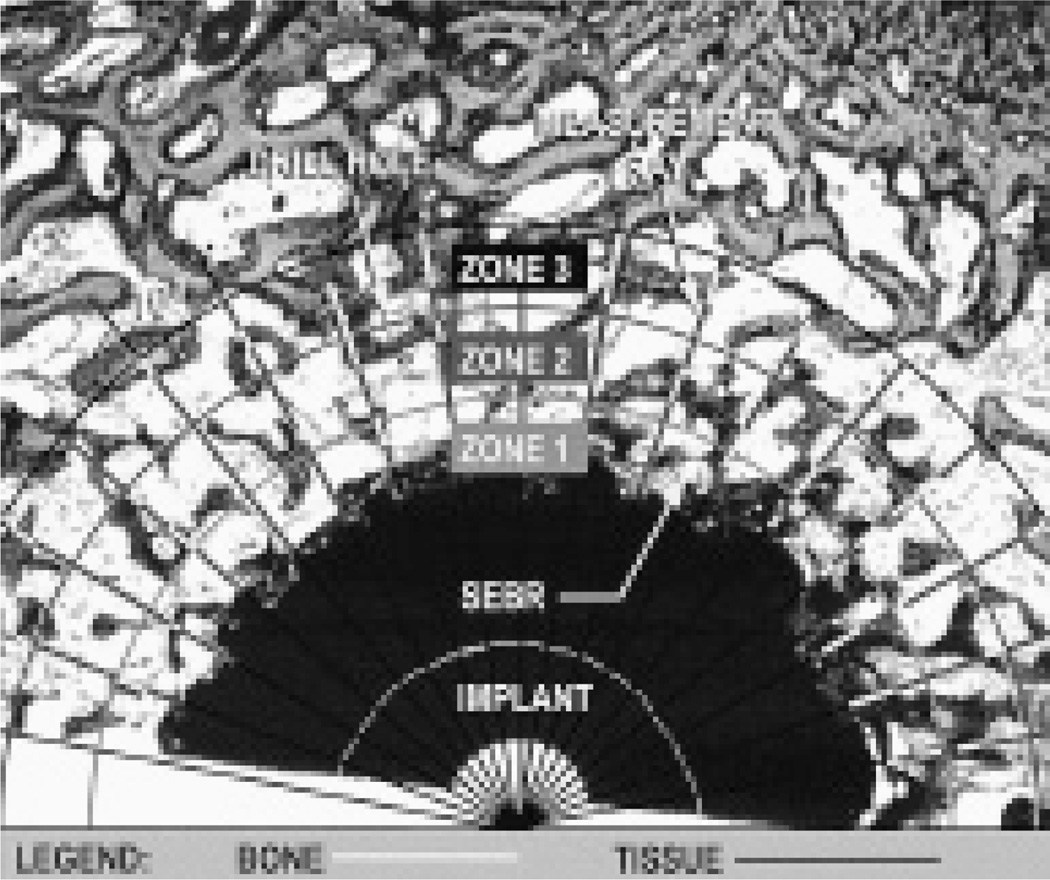
The procedure was performed blindly and in a random order with a microscope feeding an image to a computer via a video camera using two techniques (a method derived from the linear intercept technique, and a standard digitizing method). Histomorphometric measurements were partitioned into 2 zones: zone 1 was defined as extending from the implant to 50% of the drill gap; zone 2 as extending from 50% of the gap to 95% of the original drill gap.
Linear intercept method (Figure 2)
Figure 2.
Linear intercept ray method of estimating bone area and bone in contact with an implant for zones in the region of the original gap.
Rays originating at the center of the implant were tracked every 10 degrees at 2× magnification. Transitions between bone and fibrous tissue along the ray were identified. This method was used to estimate, for each zone, the percentage bone area (percentage of measured bone compared with total length of ray).
Digitizing method
This analysis was done at a BioQuant Workstation (R&M Biometrics, Nashville, TN). The analysis quantified the amount of bone in two zones in the gap according to the same parameters as the ray method, by tracing features of interest on the video screen. It was also used to quantify the percentage of bone in contact with the implant. BioQuant software translated these tracings into the histomorphometric parameters.
Mechanical parameters, interfacial shear strength, stiffness and energy
The distal transverse section was kept frozen at −20 °C, and was thawed before pushout testing. The pushout tests were done with a universal test machine (Instron Ltd.). The specimens were placed on a metal platform with a central circular opening supporting the bone to within 500 µm of the interface. A metal rod was placed in the upper holding device for the axial pushout test of the implant from the surrounding tissues. A displacement rate of 5 mm/min was used for all tests and load-deformation curves were obtained. Ultimate shear strength, and apparent shear stiffness were estimated from the load-displacement curves (Søballe et al. 1992b, 1993b).
Statistics
Paired t-tests, using pooled variance criteria, were employed in the within-dog comparisons for the primary setting as compared to the revision setting, following consultation with a biostatistician. Unpaired t-tests, using separate variance criteria, were employed for group comparisons between the various treatment groups (Ti or HA implants with or without a bone graft). A normal distribution was determined with a Q-Q plot of the data.
Results
All bacterial cultures were negative. Histomorphometric findings are shown in Table 1 and Figure 3, and mechanical interfacial shear properties in Table 2. These findings were compared as regards the (a) primary vs. revision setting, (b) presence of bone graft, and (c) implant surface.
Table 1.
Histomorphometric properties. Mean value (SD)
| Treatment | Without bone graft | With bone graft | ||
|---|---|---|---|---|
| Ti | HA | Ti | HA | |
| Primary implants | ||||
| Bone in contact (%) | 0.5 (0.9) | 16 (12)1 | 31 (8.2)3 | 43 (16) |
| Bone in zone 1 (%) | 7.7 (5) | 18 (11)2 | 33 (13) | 35 (10) |
| Bone in zone 2 (%) | 20 (11) | 20 (15) | 47 (20)4 | 41 (14) |
| Revised implants | ||||
| Bone in contact (%) | 0 | 7.3 (11)5 | 12 (12) | 40 (23)8 |
| Bone in zone 1 (%) | 5.1 (6.6) | 11 (10) | 26 (16)6 | 33 (18) |
| Bone in zone 2 (%) | 22 (8.7) | 20 (8) | 33 (6.8)7 | 38 (12) |
Without a bone graft, HA implants differed from Ti implants (p = 0.008)
Without a bone graft, HA implants differed from Ti implants (p = 0.04)
Ti implants with a bone graft differed from HA implants without graft (p = 0.04)
Ti implants with a bone graft differed from HA implants without graft (p = 0.02)
Without a bone graft HA implants differed from Ti implants (p < 0.001)
Ti implants with a bone graft differed from HA implants without graft (p = 0.05)
Ti implants with a bone graft differed from HA implants without graft (p = 0.005)
With a bone graft, HA implants differed from Ti implants (p = 0.01)
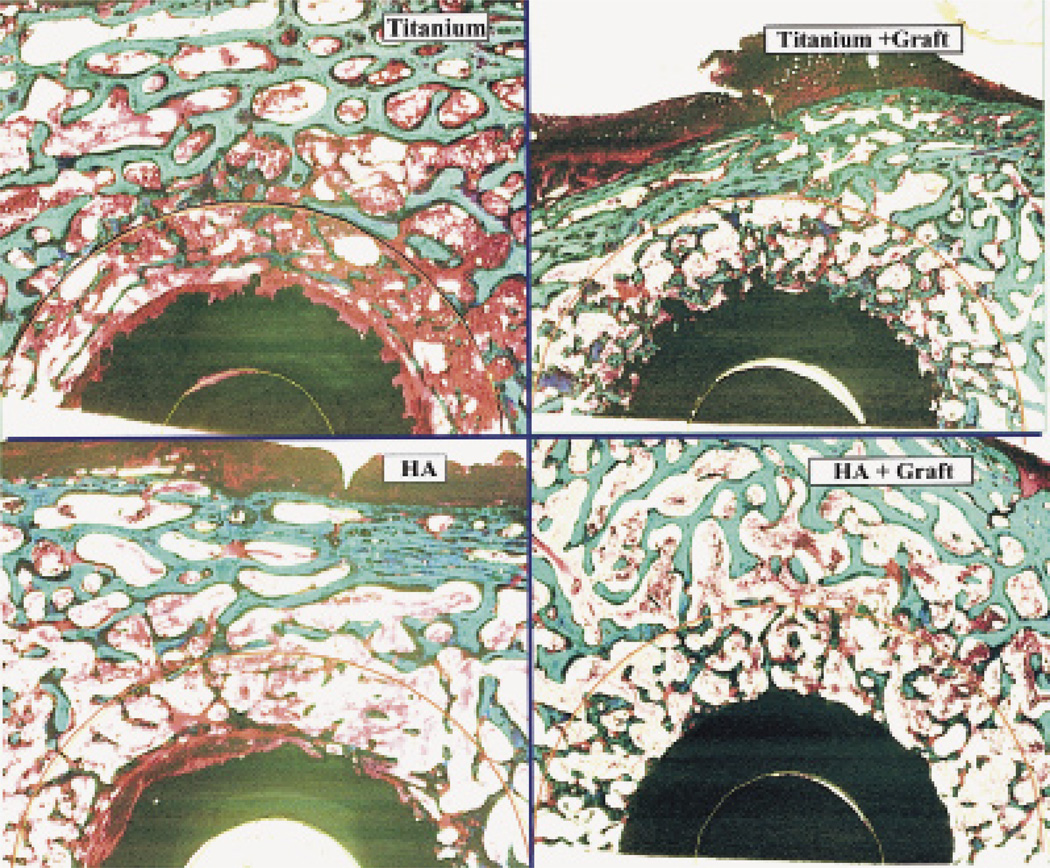
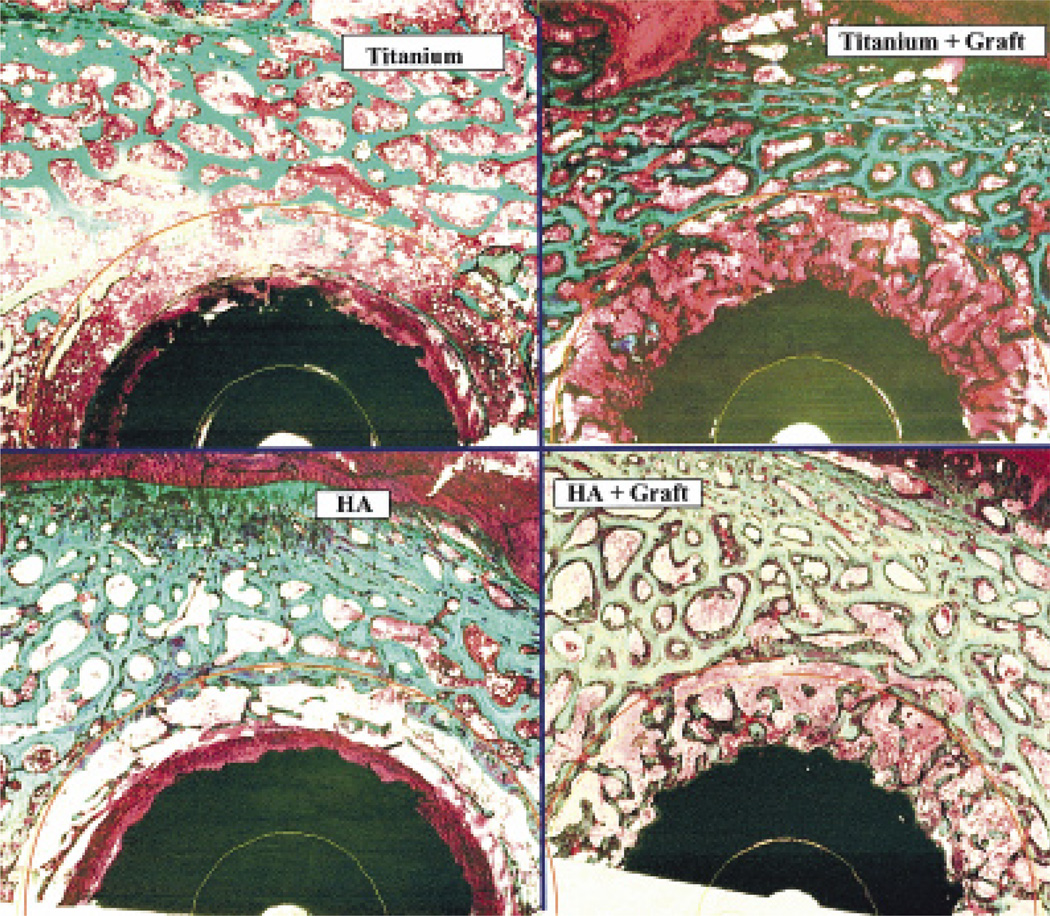
Figure 3.
Typical ground sections of implants in each of the primary and revision treatment groups, with Ti and HA implant surfaces, and with or without an allograft (1× magnification, basic fuchsin and light green, 50-micron thickness).
(revision group)
Table 2.
Mechanical interfacial shear properties. Mean value (SD)
| Treatment | Without bone graft | With bone graft | ||
|---|---|---|---|---|
| Ti | HA | Ti | HA | |
| Primary implants | ||||
| Shear strength (MPa) | 0.26 (0.21) | 2.3 (1.22)1 | 4.2 (2.3) | 3.4 (1.1) |
| Shear stiffness (MPa/mm) | 0.91 (0.87) | 15 (7.23)2 | 19 (9.4) | 19 (2.9) |
| Energy to failure (J/m) | 0.80 (0.30) | 6.8 (1.4)1 | 13 2.8) | 9.9 (1.2) |
| Revised implants | ||||
| Shear strength (MPa) | 0.16 (0.07) | 0.85 (0.72)3 | 1.0 (0.9) | 3.1 (0.6)5 |
| Shear stiffness (MPa/mm) | 0.43 (0.31) | 7.0 (6.7)4 | 4.1 (3.5) | 24 (6.2)6 |
| Energy to failure (J/m) | 0.5 (0.1) | 2.6 (0.8)3 | 3.1 (1.0) | 10 (0.6)7 |
Without a bone graft, HA implants differed from Ti implants (p = 0.003)
Without a bone graft, HA implants differed from Ti implants (p = 0.001)
Without a bone graft, HA implants differed from Ti implants (p = 0.04)
Without a bone graft, HA implants differed from Ti implants (p = 0.04)
With a bone graft, HA implants differed from Ti implants (p = 0.0003)
With a bone graft, HA implants differed from Ti implants (p = 0.00001)
With a bone graft, HA implants differed from Ti implants (p = 0.0001)
Primary versus revision
In all histomorphometric and mechanical parameters investigated, the magnitudes of the revision setting were smaller than the magnitudes of the primary setting. The sole exception was grafted revision HA implants, which had the same shear strength and stiffness and bone area as grafted primary implants.
Revised implants — in particular, those without a bone graft — had about half the shear strength and shear stiffness as the corresponding primary implants. However, revised HA implants and primary HA implants having a bone graft, maintained similar magnitudes for shear strength and shear stiffness. In contrast, revised Ti implants with a bone graft had lower shear strength and stiffness than primary ones. Histomorphometric parameters showed the same trend.
Bone graft
For all histomorphometric and mechanical parameters investigated, grafted implants were better than ungrafted ones. The sole exception was grafted revision HA implants, which had the same values as grafted primary ones.
Bone graft had the greatest effect on Ti implants (however, Ti implants were still worse than HA). Bone in contact with grafted primary Ti implants was 60-fold better than ungrafted primary Ti implants. Bone was found in contact with grafted revised Ti implants, unlike ungrafted Ti implants which had only fibrous membrane adjacent to the titanium alloy surface.
Hydroxyapatite versus titanium alloy
In all histomorphometric and mechanical parameters investigated, the magnitudes of HA were higher than those of Ti. The sole exception was the grafted revision HA implants, which had the same values as grafted primary implants.
In the absence of a bone graft, we found a 30-fold increase of bone in contact with primary HA implants as compared with primary Ti implants. The shear strength of HA implants increased 5–6 fold more when ungrafted, and up to 3-fold when grafted, as compared with the corresponding Ti implants.
Discussion
We found that HA combined with bone graft improved the fixation of revision implants (i.e., revised HA-coated grafted implants acquired the same quality of fixation of primary Ti- or HA-coated grafted implants). HA implants alone and bone graft alone were each found to improve fixation incrementally in the revision setting, but only the combination of HA and graft induced the same degree of fixation as primary implants. Although an HA coating is known to improve fixation of primary implants, to our knowledge our study is the first to evaluate its usefulness in a controlled model of revision implants.
The increase in fixation of HA-coated implants in our series accoards with the view that some of the HA surface is dissolved and that Ca++ and PO4 ions are released and taken up by the surrounding cells, and may increase bone formation (van Blitterswijk et al. 1985, Ducheyne et al. 1990). In vitro studies have shown morphological changes and an increase in cell proliferation and DNA synthesis in cultured fibroblastic and human bone cells when HA particles were added to the culture (Gregoire et al. 1987). The HA particles were found to be phagocytized by the fibroblastic cells within 24 h, and the changes in fibroblastic behavior may have been caused by release of Ca++ ions from the phagocytized HA particles because ionic calcium acts as a mediator and regulator in many cellular functions.
We found that bone-grafted implants were consistently better than their ungrafted counterparts. This has been previously shown in unloaded primary implants with fresh-frozen allograft (Søballe 1992b). The effectiveness of bone graft may be related to how it is incorporated in the surrounding bone. The early phases of incorporation (inflammation, revascularization and osteoinduction) occur rapidly (Goldberg 1987). Mesynchymal cells of the host are recruited to differentiate into osteoblasts, and new bone grows on preexisting dead trabeculae (Burchardt 1983, Goldberg 1987). The entrapped necrotic bone is then partially resorbed by osteoclasts, and the graft repaired and replaced by new bone in a process called creeping substitution (Burchardt 1983). The incorporation is preceded by revascularization and the source of stimulation and differentiation of osteogenic cells is probably the matrix in the form of bone morphogenetic protein.
While both Ti and HA surfaces benefited from the bone graft, the increase was much greater with Ti than HA implants. This had also been seen with unloaded primary implants (Søballe 1992a). The stimulating effects of the bone graft seemed to be more effective in the absence of the osteoinductive HA coating.
In normal bone (e.g., primary implants), the osteogenic effect of HA seems to be sufficient to increase fixation. In bone which is less actively responsive (e.g., revision implants), the combination of bone graft and HA seems necessary in order to improve fixation to the level of a primary implant.
Both osteoconductive and osteoinductive processes will probably occur when a bone graft is used in combination with HA. Osteoinductive factors encourage osteoblast formation from undifferentiated mesenchymal cells, and include a variety of bone growth factors. Osteoconductive materials encourage bone cells to grow by serving as a passive scaffold, and they include bone allograft and implant surfaces, such as calcium phosphate and bioglass.
The combination of the osteoinductive growth factors in the bone allograft, the osteoconductive bone graft pieces, and the osteoconductive nature of the HA coating may explain the stronger fixation achieved in the present study’s revision setting. While the osteoconductivity of HA alone may be sufficient to strengthen fixation in normal bone, the additional stimulation of a bone graft seems to be needed when the activity of the host bone has been impaired—for example, due to a loose implant and the revision setting.
We found no histological signs of allograft rejection. Despite the fact that some of the antigenicity is retained by freezing (Burchardt 1983, Goldberg 1987, Stevenson et al. 1992), this did not seem to affect our results adversely. Clinically, the usefulness of an allograft is well established, and it has not been shown to be associated with rejection or antigenicity.
In view of the limitations inherent in extrapolating experimental results to clinical work, our findings confirm the value of combining a bone graft with HA-coated implants, when revising an aseptically-loosened implant. The clinically observed poorer function and higher loosening rate of revision implants may be improved when the dual stimulation of bone graft and HA is used.
Acknowledgments
This study was supported by grants from NIH (AR42051) and the Arthritis Foundation, and the Minnesota Supercomputing Institute. We thank Biomet, Inc. for donating titanium alloy- and HA-coated implants.
We also thank the following persons for their help: Prof. Fleming Melsen, MD, Annette Milton, and Barbara Wicklund B.S.. Robert E. Sherman, Ph.D. gave statistical advice.
References
- Bechtold JE, Søballe K, Kubic V, Gustilo R. A controlled experimental model of revision implants. Part I. Development. Acta Orthop Scand. 2001a;72(6):642–649. doi: 10.1080/000164701317269094. [DOI] [PubMed] [Google Scholar]
- Bechtold JE, Mouzin O, Kidder L, Søballe K. A controlled experimental model of revision implants. Part II. Implementation. Acta Orthop Scand. 2001b;72(6):650–656. doi: 10.1080/000164701317269102. [DOI] [PubMed] [Google Scholar]
- Bechtold JE, Kubic V, Søballe K. Bone ingrowth in the presence of particulate polyethylene. Synergy between interface motion and particulate polyethylene in periprosthetic tissue response. J Bone Joint Surg (Br) 2002;84:915–919. doi: 10.1302/0301-620x.84b6.12111. [DOI] [PubMed] [Google Scholar]
- Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200:165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Burchardt H. The biology of bone graft repair. Clin Orthop. 1983;174:28–42. [PubMed] [Google Scholar]
- Ducheyne P, Beight J, Cuckler J, Evans B, Radin S. Effect of calcium phosphate coating characteristics on early postoperative bone tissue growth. Biomaterials. 1990;11(8):531–540. doi: 10.1016/0142-9612(90)90073-y. [DOI] [PubMed] [Google Scholar]
- Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop. 1987;225:7–16. [PubMed] [Google Scholar]
- Goodman SB. The effects of micromotion and particulate materials on tissue differentiation. Bone chamber studies in rabbits. Acta Orthop Scand. 1994;258:1–43. doi: 10.3109/17453679409155227. [DOI] [PubMed] [Google Scholar]
- Gregoire M, Orly I, Kerebel L-M, Kerebel B. In vitro effects of calcium phosphate biomaterials on fibroblastic cell behavior. Biol Cell. 1987;59:255–260. doi: 10.1111/j.1768-322x.1987.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Kärrholm J, Malchau H, Snorrason F, Herberts P. Micromotion of femoral stems in total hip arthroplasty. A randomized study of cemented, hydroxyapatite-coated, and porous-coated stems with roentgen stereophotogrammetric analysis. J Bone Joint Surg (Am) 1994;76(11):1692–1705. doi: 10.2106/00004623-199411000-00013. [DOI] [PubMed] [Google Scholar]
- Kärrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses, review of methodology and clinical results. Clin Orthop. 1997;344:94–110. [PubMed] [Google Scholar]
- Lalor PA, Namba R, Mitchell SL, Bearcroft J, Beals N, Sledge CB, Spector M. Migration of polyethylene particles around stable implants in an animal model. J Long-Term Eff Med Implants. 1999;9(4):261–272. [PubMed] [Google Scholar]
- Linder L. Implant stability, histology, RSA and wear—more critical questions are needed. A viewpoint. Acta Orthop Scand. 1994;65:654–658. doi: 10.3109/17453679408994626. [DOI] [PubMed] [Google Scholar]
- McDonald DJ, Fitzgerald RH, Jr, Chao EY. The enhancement of fixation of a porous-coated femoral component by autograft and allograft in the dog. J Bone Joint Surg (Am) 1988;70:728–737. [PubMed] [Google Scholar]
- Nelissen R, Valstar E, Rozing R. The effect of hydroxyapatite on the micromotion of total knee prostheses. A prospective, randomized, double-blind study. J Bone Joint Surg (Am) 1998;80(11):1665–1672. doi: 10.2106/00004623-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Önsten I, Nordqvist A, Carlesson A, Besjakov J, Shott S. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components. A randomized RSA study in 116 patients. J Bone Joint Surg (Br) 1998;80(3):417–425. doi: 10.1302/0301-620x.80b3.7937. [DOI] [PubMed] [Google Scholar]
- Søballe K, Hansen ES, B-Rasmussen H, Pedersen CM, Bünger C. Bone graft incorporation around titanium alloy- and hydroxyapatite-coated implants on dogs. Clin Orthop. 1992a;274:282–293. [PubMed] [Google Scholar]
- Søballe K, Hansen ES, B-Rasmussen H, Bünger C. Tissue ingrowth into titanium- and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res. 1992b;10:285–299. doi: 10.1002/jor.1100100216. [DOI] [PubMed] [Google Scholar]
- Søballe K, B-Rasmussen H, Hansen ES, Bünger C. Hydroxyapatite coating modifies implant membrane formation. Controlled micromotion study in dogs. Acta Orthop Scand. 1992c;63(2):128–140. doi: 10.3109/17453679209154808. [DOI] [PubMed] [Google Scholar]
- Søballe K, Toksvig-Larsen S, Fruensgaard S, Hansen E, Ryd L. Migration of hydroxyapatite-coated femoral prostheses. A roentgen stereophotogrammetric study. J Bone Joint Surg (Br) 1993a;75(5):681–687. doi: 10.1302/0301-620X.75B5.8397213. [DOI] [PubMed] [Google Scholar]
- Søballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand (Suppl 255) 1993b;64:1–58. doi: 10.3109/17453679309155636. [DOI] [PubMed] [Google Scholar]
- Stevenson S, Li X-Q, Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg (Am) 1992;73(8):1143–1156. [PubMed] [Google Scholar]
- Thanner J, Kärrholm J, Herberts P, Malchau H. Porous cups with and without hydroxyapatite-tricalcium phosphate coating: 23 matched pairs evaluated with radiostereometry. J Arthroplasty. 1999;14(3):266–271. doi: 10.1016/s0883-5403(99)90050-5. [DOI] [PubMed] [Google Scholar]
- Thanner J, Kärrholm J, Herberts P, Malchau H. Hydroxyapatite and tricalcium phosphate-coated cups with and without screw fixation: a randomized study of 64 hips. J Arthroplasty. 2000;15(4):405–412. doi: 10.1054/arth.2000.2963. [DOI] [PubMed] [Google Scholar]
- Toksvig-Larsen S, Jorn LP, Ryd L, Lindstrand A. Hydroxyapatite-enhanced tibial prosthetic fixation. Clin Orthop. 2000;370:192–200. doi: 10.1097/00003086-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Turner TM, Urban RM, Sumner DR, Galante JO. Revision, without cement, of aseptically loose, cemented total hip prostheses. Quantitative comparison of the effects of four types of medullary treatment on bone ingrowth in a canine model. J Bone Joint Surg (Am) 1993;75:845–862. doi: 10.2106/00004623-199306000-00006. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk CA, Grote JJ, Kuipers W, Blok-van CJG, Daems WT. Bioreactions at the tissue/hydroxyapatite interface. Biomaterials. 1985;6:243–251. doi: 10.1016/0142-9612(85)90020-1. [DOI] [PubMed] [Google Scholar]