Abstract
Background
Physical inactivity is a modifiable risk factor for cardiovascular disease. However, the relationship between physical activity and risk of end-stage kidney disease (ESKD) is not clear.
Methods
We analyzed data on a prospective cohort of 59,552 Chinese adults aged 45-74 years enrolled in the Singapore Chinese Health Study. Information on physical activity was collected with a structured questionnaire. Physically active individuals were defined as those who engaged in any moderate activities for 2 hours or more per week, and any strenuous activities 30 minutes or more per week. Incident ESKD was identified via record linkage with the Singapore Registry of Birth and Death and Singapore Renal Registry. Cox proportional hazards regression method was used for analysis for risk of incident ESKD alone or ESKD plus death associated with physical activity. Multivariable models were used to account for the potential confounding effect of sociodemographic, life style factors, and known co-morbidites on the physical activity-ESKD risk association.
Results
During a median follow-up of 15.3 years, a total of 642 incident ESKD occurred, and 9808 study participants died. A 24% lower adjusted risk of ESKD [hazard ratio (HR): 0.76; 95% confidence interval (CI): 0.62-0.93] was associated with moderate or strenuous physical activities compared to no regular physical activity. This association appeared to be dose dependent with the lowest risk for subjects at highest intensity of physical activity (p trend <0.003). Similar results were observed for risk of ESKD plus death.
Conclusions
Higher levels of physical activity are associated with lower risk of ESKD. Our findings highlight the role of physical activity for prevention of ESKD, which deserves further evaluation in intervention trials.
Keywords: end stage kidney disease, exercise, physical activity
Introduction
End stage kidney disease (ESKD) is a rising public health threat globally, and is associated with high morbidity and mortality, as well as significant social and economic consequences.1 The number of people requiring or nearing the need for expensive renal replacement therapy is rapidly increasing in part due to the aging populations coupled with prolonged exposure to risk factors for ESKD.2 Physical inactivity is recognized as one of the leading risk factors for cardiovascular disease (CVD).3, 4 Whether the same association exists with ESKD is not well established. Cross sectional studies indicate chronic kidney disease (CKD) is associated with reduced physical activity and muscle wasting.5, 6, 7, 8, 9, 10 Cohort studies also suggest that physical inactivity is associated with decline in glomerular filtration rate.11, 12, 13 Furthermore, physical inactivity is associated with greater mortality among patients with CKD than those without CKD.14, 15, 16 However, there is dearth of studies evaluating the predictive relationship between physical activity and the hard outcome of ESKD. Moreover, it is not known whether this relationship, if present, is dose-dependent in terms of intensity of physical activity.
A clear understanding of the association between physical activity and ESKD would be important for developing clinical and public health guidelines for prevention for ESKD. In this study, we analyzed data from the Singapore Chinese Health Study, a population based cohort of 63,257 Chinese men and women, linked with the Singapore Renal Registry which maintains a record of all patients nationwide with ESKD, treated with renal replacement therapy or managed conservatively, with the following objectives: 1) To determine the association between habitual physical activity with risk of ESKD; 2) and to assess whether there is a dose-dependent relationship between intensity of physical activity and risk of ESKD.
We hypothesized that higher levels of physical activity would be associated with a lower risk of ESKD; and that this risk would be even lower at higher intensity physical activity.
Methods
Study population
The Singapore Chinese Health Study is a population-based prospective cohort established between April 1993 and December 1998. The study recruited a total of 63,257 Chinese men (n=27,959) and women (n=35,298) aged 45-74 years, and residing in public housing estates, where 86% of Singapore resided at that time. This represented a response rate of about 85% of all eligible participants that we had contacted. The participants were men and women of Chinese origin from one of the two major dialect groups, Hokkien or Cantonese, who originated from Fujian and Guangdong, respectively, two contiguous provinces in southern China. Written informed consent was obtained from all participants. Each subject was interviewed in person by a trained interviewer using a structured questionnaire, which focused on questions on lifestyle including current alcohol and tobacco use, diet, habitual physical activity and medical history.17 The study was approved by the Institutional Review Board at the National University of Singapore.
Exposure assessment
All individuals were interviewed in-person using a structured questionnaire with information on physical activity. This questionnaire was modeled after the European Prospective Investigation in Cancer (EPIC) study physical activity questionnaire. The latter has been shown to be valid and repeatable.17 Subjects were asked “on average, during the last year how many hours in a week did you spend in the following activities?” The moderate activities included brisk walking, bowling, bicycling on level ground, Tai Chi (Chinese martial arts) or Qi Gong (traditional Chinese repeated coordinated movements involving strengthening and stretching exercise). Strenuous aerobic activities included jogging, bicycling on hills, tennis, squash, swimming laps or aerobics.
The time spent in moderate activities was recorded into pre-specified intervals of never, 30 minutes to <2 hours, 2 to <4 hours, 4 to <7 hours, 7 to <10 hours, 10 to <20 hours, 20 to <30 hours and 30 hours and above per week. The time spent in strenuous activities was recorded as never, 30 to <90 minutes, 90 to <150 minutes, 150 minutes or more minutes/week of sternuous activity/week. For the purpose of analysis, and consistent with previous report on association of physical activity with CVD in this dataset, the active group was defined as individuals who engaged in any moderate activity for 2 hours or more per week, or any strenuous activity on a weekly basis for at least 30 minutes.
Outcome ascertainment
The cohort database was linked with the population-based Singapore Renal Registry and the Singapore Registry of Births and Deaths for identification of incident ESKD and deaths, respectively. In Singapore, each citizen or permanent resident is assigned a unique number (the National Identification Card Number or IC Number) and this number is used for all medical records. Linkage is done by perfect matching of the NRIC, and verified by name. The Singapore Renal Registry has been shown to be comprehensive in its recording of ESKD cases since 1999.18 The registry defines ESKD that met at least one of the following criteria: 1) serum creatinine level of more than or equal to 500 μmol/l (5.7 mg/dl), 2) estimated glomerular filtration rate (GFR) of less than 15 ml/min/1.73 m2 (based on either the Modification of Diet in Renal Disease (MDRD) Study equation, Cockcroft Gault equation, or 24 hour creatinine clearance), 3) undergoing hemodialysis or peritoneal dialysis, and 4) had undergone kidney transplant. Criteria 1 to 3 had to be persistent for more than 3 months for qualifying as ESKD. As of 31 Dec 2010, 47 subjects from this cohort were known to be lost to follow-up due to migration out of Singapore or for other reasons. We identified 110 patients with ESKD whose initial diagnosis was made prior to the enrollment, thus those patients were excluded from the present analysis. In addition, we excluded 3595 participants who died within the first 5 years post enrollment to minimize the potential bias toward inactivity due to existing advanced chronic kidney disease. A total of 642 incident ESKD cases were identified from the remaining 59,552 participants included in the final analyses.
Statistical analysis
The primary outcome was incident ESKD. The secondary outcome was a composite of ESKD or death. We examined baseline characteristics of the entire cohort by habitual physical activity status. For each study subject, person-years were counted from the first date of fifth year post baseline interview to the date of ESKD diagnosis, the date of death, date of last contact (for the few subjects who migrated out of Singapore) or December 31, 2010, whichever occurred first. The incidence rates of ESKD and their 95% confidence intervals (CIs) by any physical activity, and with the intensity of physical activity (moderate and strenuous as ordinal variables) were generated using Poisson distribution. Survival analysis was performed to generate the Kaplan-Meier curves for primary and secondary outcomes, and log-rank test was used to compare the curves of those physically active versus not physical active.
Univariable and multivariable regression models were constructed and Cox proportional hazards regression analysis was performed to examine the associations between physical activity and the primary outcome of incident ESKD. The comparator group consisted of individuals who were not physically active (less than 2 hours moderate and less than 30 minutes strenuous aerobic activity per week). Deaths from competing causes (i.e. non ESKD deaths) were censored in the main analysis. We first examined the association of any physical activity with ESKD. For all analyses the reference group was physically inactive individuals. Next, we analyzed the association of different intensities of physical activity categorized as moderate activities, or strenuous aerobic activities with ESKD. The associations were measured by hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) and p values (two-sided).
All multivariable models included age at recruitment (years), year of recruitment (1993-1995, 1996-1998), gender, dialect (Hokkien or Cantonese), self-reported history of physician-diagnosed hypertension, diabetes, coronary heart disease or stroke as dichotomous variables, education (no formal education, primary school, secondary school or higher), body mass index (BMI; <20.0, 20.0-23.9, 24.0-27.9, ≥28.0 kg/m2), alcohol drinking (none/monthly, weekly, daily), smoking status (never, ever), intake of ginseng (never, 1-3 times/month, 1-3 times/week, 4 or more times/week), and dietary protein intake (quartiles, % kcal/day) as categorical variables. These factors are known to be associated with ESKD in other studies and our previous report.19 For the analysis of intensity of physical activity with ESKD, we performed tests for trend by entering ordinal categorical variable of physical activity as continuous variables in the Cox regression models, and assessing p trend linear and quadratic. We also tested for interaction between baseline covariates and any physical activity to determine whether the relationship of physical activity with ESKD varies according to baseline characteristics including age, sex, obesity, hypertension, or diabetes status. The association between regular physical activity and the composite outcome of ESKD or death was evaluated in the dataset without censoring deaths. Time to (first) event analysis was performed.
The proportional hazards assumption in all Cox models was assessed by fitting log covariates in the multivariable model and checking graphically by plotting the Schoenfeld residuals, but no variables (except history of diabetes) violated the proportionality assumption.
Sensitivity analysis was performed using cut-off of 90 minutes for strenuous activity, and among subjects without history of diabetes.
All regression analyses were conducted using SAS statistical software version 9.2 (SAS institute, Cary, NC). Two-sided Ps < 0.05 were considered statistically significant.
Results
A total of 63,257 (approximately 85% of eligible subjects) who were invited to enrol in the Singapore Chinese Health Study responded positively. Figure 1 shows the flow chart of 59,552 subjects included in the final analysis. Of these, 44.1% were men. Table 1 shows baseline characteristics of the participants by intensity of physical activity. A total of 13,200 (22%) individuals engaged in habitual physical activity: 8,947 (15%) in moderate activity only and 4,254 (7%) in strenuous aerobic activity, regardless their status for moderate activity. Individuals who were physically active were more likely to be educated, consumed less alcohol, were more likely to be non-smokers, less likely to have hypertension, or heart disease.
Figure 1.
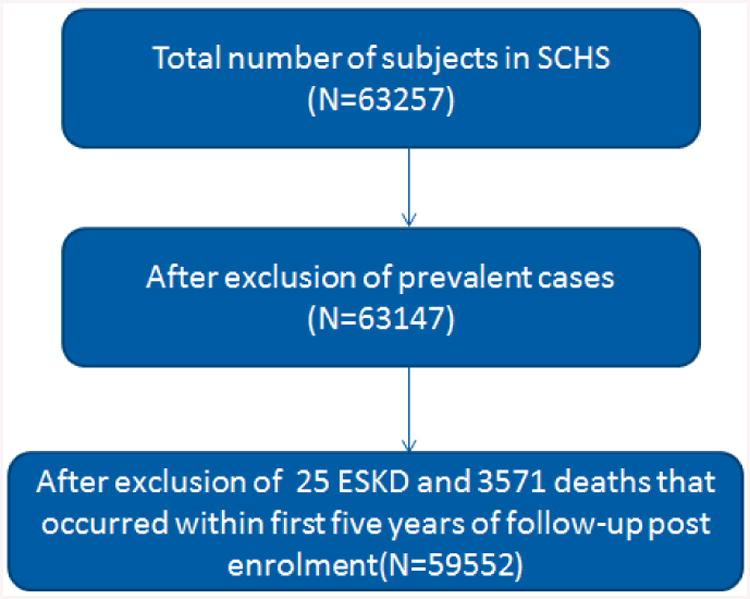
Flow chart of number of subjects included in the analysis
Table 1. Baseline characteristics of Study Participants by Physical Activity, The Singapore Chinese Health Study.
| Total (N=59552) | No physical activity (n=46352) | Any physical activity (n=13200) | P value | |
|---|---|---|---|---|
| Age at recruitment (mean, SD) | 56.1 (7.9) | 56.1 (7.8) | 56.1 (8.0) | 0.467 |
| Education (n, %) | ||||
| No formal education | 15987 (26.9) | 13661 (29.5) | 2326 (17.6) | <0.0001 |
| Primary | 26344 (44.2) | 21145 (45.6) | 5199 (39.4) | |
| Secondary &above | 17221 (28.9) | 11546 (24.9) | 5675 (43.0) | |
| Dialect (n, %) | ||||
| Cantonese | 31818 (53.4) | 25181 (54.3) | 6637 (50.3) | <0.0001 |
| Hokkein | 27734 (46.6) | 21174 (45.7) | 6563 (49.7) | |
| History of hypertension (n, %) | 13654 (22.9) | 10501 (22.7) | 3153 (23.9) | 0.003 |
| History of diabetes (n, %) | 4789 (8.0) | 3737 (8.1) | 1052 (8.0) | 0.730 |
| History of heart disease (n, %) | 2165 (3.6) | 1620 (3.5) | 545 (4.1) | 0.0006 |
| History of stroke (n, %) | 742 (1.3) | 584 (1.3) | 158 (1.2) | 0.565 |
| Smoking history (n, %) | ||||
| Never smoker | 42093 (70.7) | 32672 (70.5) | 9421 (71.4) | 0.049 |
| Ever smoker | 17459 (29.3) | 13680 (29.5) | 3779 (28.6) | |
| Alcohol (n, %) | ||||
| Never | 52625 (88.4) | 41163 (88.8) | 11462 (86.8) | <0.0001 |
| Weekly | 4888 (8.2) | 3566 (7.7) | 1322 (10.0) | |
| Daily | 2039 (3.4) | 1623 (3.5) | 416 (3.2) | |
| BMI (mean, SD) | 23.1 (3.3) | 23.1 (3.3) | 23.1 (3.2) | 0.680 |
| Ginseng (n, %) | ||||
| Never | 49477 (83.1) | 38812 (83.7) | 10665 (80.8) | <0.0001 |
| 1-3/month | 2744 (4.6) | 2041 (4.4) | 703 (5.3) | |
| 1-3/week | 5744 (9.7) | 4315 (9.3) | 1429 (10.8) | |
| 4-6/week and more | 1587 (2.6) | 1184 (2.6) | 403 (3.1) | |
| Protein intake (gm/Kcal/day) mean (SD) | 15.2 (2.5) | 15.2 (2.5) | 15.3 (2.4) | <0.0001 |
Any physically active group was defined as individuals who engaged in any moderate activity for 2 hours or more per week, or any strenuous activity on a weekly basis for at least 30 minutes.
During a median follow-up of 15.3 years, 642 (1%) individuals developed incident ESKD, and 9808 (16.4%) individuals died after excluding 25 incident ESKD and 3571 deaths which occurred within 5 years post enrollment. The overall crude incidence rate of ESKD was 74 (68-80) per 100,000 person years, and was significantly lower among individuals who were physically active compared to inactive individuals (60 versus 80 per 100,000). Compared to physically inactive individual, individuals engaging in any physical activity had significantly lower risk of ESKD after adjustment for multiple potential confounders [hazard ratio (HR), 0.76; 95% confidence interval (CI): 0.62-0.93] There was a statistically significant inverse relationship between the intensity of physical activity and risk ESKD (p for trend =0.003). (Table 3). Figure 2, panel A illustrates ESKD-free survival. Although event rate was low, ESKD-free survival was longer among those engaged in physical activity versus no physical activity (log-rank p=0.011).
Table 3. Unadjusted & Adjusted Risk of End Stage Kidney Disease According to Physical Activity, The Singapore Chinese Health Study.
| Physical activity | N | Person years | Number ESKD | Age & Sex adjusted Hazard Ratio (95% CI) | Multivariable * Adjusted Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Never vs | 46352 | 672080 | 525 | 1.00 | 1.00 |
| Any physical activity | 13200 | 192915 | 117 | 0.74 (0.61-0.91) | 0.76 (0.62-0.93) |
|
| |||||
| Never vs | 46352 | 672080 | 525 | 1.00 | 1.00 |
| >= 2 hrs moderate activity only | 8947 | 131771 | 96 | 0.83 (0.66-1.03) | 0.81 (0.65-1.01) |
| Strenuous aerobic activities | 4253 | 61144 | 21 | 0.50 (0.32-0.78) | 0.58 (0.37-0.90) |
| P trend | 0.0008 | 0.0034 | |||
Model adjusted for age, sex, interview year, body mass index, dialect (Hokkein, Cantonese), education level, self-reported history of physician diagnosed hypertension, diabetes, heart disease or stroke, alcohol use, smoking, intake of ginseng, protein intake.
Figure 2.
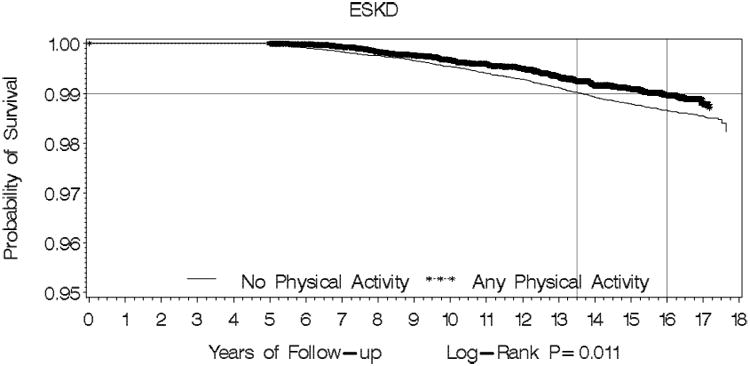
Panel A: Kaplan-Meier Survival Curve for Physical Activity vs No Physical Activity for ESKD
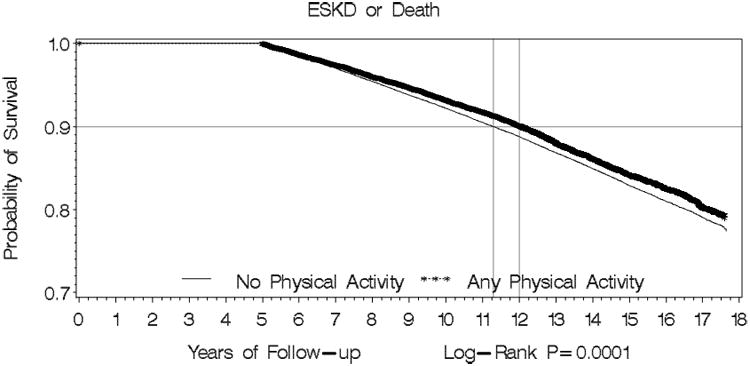
Panel B: Kaplan-Meier Survival Curve for Physical Activity vs No Physical Activity for composite of ESKD or Death
P value for difference in survival curve for the outcome of ESKD was 0.011, and for the composite outcome of ESKD or death was 0.0001.
The hazard rates (95% CI) of ESKD or death by physical activity status are shown in Tables 4. Compared to physically inactive individual, those who engaged in any physical activity had significantly lower adjusted risk of ESKD or death [hazard ratio (HR), 0.85; 95% confidence interval (CI): 0.81-0.90] in the multivariate analysis. Figure 2, panel B illustrates the cumulative risk for both ESKD incidence and death from any cause combined was lower among those who engaged in regular physical exercise relative to those who were physically less active (log-rank p=0.0001).
Table 4. Unadjusted & Adjusted Risk of End Stage Kidney Disease or Death According to Physical Activity, The Singapore Chinese Health Study.
| Physical activity | N | Person years | Number ESKD or Death | Age & Sex adjusted Hazard Ratio (95% CI) | Multivariable * Adjusted Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| Never vs | 46352 | 672080 | 8271 | 1.00 | 1.00 |
| Any physical activity | 13200 | 192915 | 2179 | 0.80 (0.76-0.84) | 0.85 (0.81-0.90) |
|
| |||||
| Never vs | 46352 | 672080 | 8271 | 1.00 | 1.00 |
| >= 2 hrs moderate activity only | 8947 | 131771 | 1805 | 0.85 (0.81-0.90) | 0.88 (0.84-0.93) |
| Strenuous aerobic activities | 4253 | 61144 | 374 | 0.62 (0.56-0.68) | 0.73 (0.66-0.81) |
| P trend | <0.0001 | <0.0001 | |||
Model adjusted for age, sex, interview year, body mass index, dialect (Hokkein, Cantonese), education level, self-reported history of physician diagnosed hypertension, diabetes, heart disease or stroke, alcohol use, smoking, intake of ginseng, protein intake.
No interactions between physical activity and baseline covariates were significant indicating the relationship between physical activity and ESKD does not vary by age, sex, obesity, hypertension or diabetes status. Sensitivity analysis using cut-off of 90 minutes for strenuous activity, and among subjects without history of diabetes yielded consistent results.
Discussion
Our in-depth analysis on data on 59,552 subjects followed over a median of 15.3 years with 642 incident ESKD cases in the Singapore Chinese Health Study is the first to show that habitual physical activity is associated with lower risk of ESKD requiring dialysis or transplantation. A 24% lower adjusted risk of ESKD was associated with any physical activity [hazard ratio (HR): 0.76; 95% confidence interval (CI): 0.62-0.93] compared to no physical activity. This association with lower risk of ESKD appeared to be dose dependent with higher intensity of physical activity (p trend <0.003). Strenuous activities were independently associated with lower risk of ESKD by 42% (HR: 0.58; 95% CI: 0.37-0.90).
The association of physical activity with the outcome of ESKD or death demonstrated consistent results HR: 0.85; 95% CI: 0.81-0.90 including a clear independent association of moderate activity with the composite outcome, as well as dose-related additional lowering of risk with strenuous activity. Our findings have substantial implications for public health and clinical practice recommendations for prevention of ESKD.
Previous studies demonstrating the association of physical activity and CKD have mainly been cross-sectional, raising the concerns regarding reverse causality.7, 8, 9, 10 While physical activity has been associated with reduced risk of decline in glomerular filtration rate (GFR) in the Cardiovascular Health Study, and few other longitudinal studies, these studies have primarily relied on the outcome of change in GFR. Our study is novel in establishing the predictive relationship of higher physical activity with lower risk of hard outcome of ESKD.11, 12 This association was independent of socio-demographic and clinical characteristics including education status, dietary protein intake tobacco use, BMI, hypertension, diabetes, and presence of CVD. The precise mechanism responsible for the independent relationship between physical activity and ESKD is not entirely clear. Physical activity is known to reduce blood pressure and improve glycemic control.20 Moreover, physical activity has been shown to be effective in improving cardiovascular health via enhancing endothelial function directly through response to insulin, angiogenesis and vascular regeneration by the up-regulation of endothelial nitric oxide production and other antioxidant enzymes.21, 22
It is entirely conceivable that the same would extend to the kidney vasculature leading to protection against glomerular filtration barrier defects, albuminuria, and declining kidney function.
Our findings have tremendous implications for public health policy globally, especially with number of people with and at risk of ESKD, such as those with diabetes and hypertension, in increasing rapidly. The American College of Sports Medicine currently recommends 150 min of moderate activity (30 min, 5 days/week) or 60 min of strenuous physical activity (20 min on 3 days) for all adults.23 Not with standing the need for an intervention trial, our results support these recommendations and underscore the potential role of strenuous activities in prevention of ESKD.
The major strength of this study is the large sample size of a population-based cohort with near-complete follow-up, and with valid and comprehensive outcome of ESKD identified via linkage with a robust national renal registry. In a sensitivity analysis, all results regarding key relationships with physical activity (overall and with regard to dose-response with intensity) were consistent in direction in the full dataset after including first 5 years of follow-up. Our analysis has some limitations. First, physical activity was self-reported and did not include an objective assessment. However, the questionnaire used was a modified version of validated EPIC physical activity questionnaire, and self-reported physical inactivity based on the same has also been shown to be independently predictive of cardiovascular disease in the Singapore Chinese Health Study24 Moreover, the robust association with secondary composite outcome of ESKD or death in our study, where the vast majority of endpoints are from deaths, lend further support to our main results. Therefore, we believe our findings of association of physical activity with ESKD are also valid. Second, information on baseline kidney function including serum creatinine or albuminuria was not available. This raises concern whether the observed association was mainly because individuals with pre-existing CKD had already reduced their physical activity level at baseline. However, individuals with advanced CKD and those who progressed fast to ESKD are likely to be excluded with first five years of follow-up not included in the analysis. Nevertheless, individuals with less advanced and slowly progressing CKD would potentially still be included our cohort. The benefit of physical activity has been demonstrated in individuals with documented CKD.13 Third, data on antihypertensive medications including blockers of renin-angiotensin, and reduction in blood pressure or albuminuria during follow-up were not available.25 We were therefore not able to determine any potential interaction between these factors with physical activity on ESKD. However, hypertension and diabetes at baseline, both leading causes of ESKD, as well as predictors of progression were accounted for in our multivariable models, albeit as self-reported co-morbidities. Finally, the outcome of ESKD was determined solely on the basis of data linkage. Linkage is done by perfect matching of the NRIC, and verified by name. However, this was not asked at the follow-up interview. Thus, we are unable to determine if any individuals were unmatched. However, there is robust implementation of mandatory registration of vital statistics and ESKD in Singapore. Thus, the information on ESKD and death is virtually complete, and the findings of the present study were valid and generalizable.
In summary, our large cohort is novel in demonstrating a predictive relationship between higher physical activity and lower risk of ESKD. Our results indicate that this association is dose dependent with even greater lowering of risk at higher intensity of physical activity. Our findings highlight the role of moderate and especially strenuous physical activity in clinical and public health guidelines for prevention of ESKD, which deserves further evaluation in intervention trials.
Table 2. Incidence of End Stage Kidney Disease among the study participants According to Physical Activity, The Singapore Chinese Health Study.
| All | No | Any physical activity | |
|---|---|---|---|
| Persons | 59552 | 46352 | 13200 |
| Person-years | 864995 | 672080 | 192915 |
| Median of follow-up | 15.3 | 15.3 | 15.4 |
| ESKD (N) | 642 | 525 | 117 |
| Incidence rate per 100,000py 95%CI | 74.22 (68.6-80.2) | 78.12 (71.6-85.1) | 60.65 (50.2-72.7) |
Acknowledgments
We thank Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study and Renwei Wang for maintenance of the cohort database. We also thank the Singapore Renal Registry for assistance with the identification of ESKD and mortality outcomes via database linkages. We acknowledge the founding Principal Investigator of the Singapore Chinese Health Study – Mimi C. Yu. We also thank John Allen for advice on statistical analysis on this paper.
Funding: The Singapore Chinese Health Study was supported by the U.S. National Institutes of Health Grant Numbers RO1 CA55069, R35 CA53890, R01 CA80205, and R01 CA144034. Dr Jafar's contribution to the paper was supported by a grant from Singapore Ministry of Education.
Footnotes
Conflict on Interest: None
Information about presentations of this work: The abstract based on this work has been presented at the 46th Annual Kidney Week in November, 2013 at Atlanta, GA.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, Levin A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Wei M. Cardiorespiratory fitness, adiposity, and mortality. JAMA: the journal of the American Medical Association. 2008;299:1013. author reply 1014. [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharakhada N, Yates T, Davies MJ, Wilmot EG, Edwardson C, Henson J, Webb D, Khunti K. Association of sitting time and physical activity with ckd: A cross-sectional study in family practices. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012;60:583–590. doi: 10.1053/j.ajkd.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa T, F M. Factor analysis of lifestyle-related diseases including gfr and uric acid in japanese adults. International Medical Journal. 2010;17 [Google Scholar]
- 7.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM. Association between physical activity and kidney function: National health and nutrition examination survey. Medicine and science in sports and exercise. 2011;43:1457–1464. doi: 10.1249/MSS.0b013e31820c0130. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein J, Joshi A, Hise MK. Association of physical activity and renal function in subjects with and without metabolic syndrome: A review of the third national health and nutrition examination survey (nhanes iii) American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;48:372–382. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Kurella M, Ireland C, Hlatky MA, Shlipak MG, Yaffe K, Hulley SB, Chertow GM. Physical and sexual function in women with chronic kidney disease. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2004;43:868–876. doi: 10.1053/j.ajkd.2003.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Hallan S, de Mutsert R, Carlsen S, Dekker FW, Aasarod K, Holmen J. Obesity, smoking, and physical inactivity as risk factors for ckd: Are men more vulnerable? American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006;47:396–405. doi: 10.1053/j.ajkd.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Robinson-Cohen C, Katz R, Mozaffarian D, Dalrymple LS, de Boer I, Sarnak M, Shlipak M, Siscovick D, Kestenbaum B. Physical activity and rapid decline in kidney function among older adults. Archives of internal medicine. 2009;169:2116–2123. doi: 10.1001/archinternmed.2009.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronborg J, Solbu M, Njolstad I, Toft I, Eriksen BO, Jenssen T. Predictors of change in estimated gfr: A population-based 7-year follow-up from the tromso study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:2818–2826. doi: 10.1093/ndt/gfn148. [DOI] [PubMed] [Google Scholar]
- 13.Robinson-Cohen C, Littman AJ, Duncan GE, Weiss NS, Sachs MC, Ruzinski J. Physical activity and change in estimated gfr among persons with ckd. Journal of the American Society of Nephrology. 2014;25:399–406. doi: 10.1681/ASN.2013040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (nhanes iii) Clinical journal of the American Society of Nephrology: CJASN. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore chinese health study: Development, validation, and calibration of the quantitative food frequency questionnaire. Nutrition and cancer. 2001;39:187–195. doi: 10.1207/S15327914nc392_5. [DOI] [PubMed] [Google Scholar]
- 16.Chen IR, Wang SM, Liang CC, HL K. Association of walking with survival and rrt among patients with ckd stages 3-5. Clin J Am Soc Nephrol. 2014;9:1183–1189. doi: 10.2215/CJN.09810913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, Bauman A. Validity and repeatability of the epic physical activity questionnaire: A validation study using accelerometers as an objective measure. The international journal of behavioral nutrition and physical activity. 2008;5:33. doi: 10.1186/1479-5868-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Http://www.Nrdo.Gov.Sg/uploadedfiles/nrdo/health_factsheet_esrd_(inp-12-1).Pdf)]
- 19.Jin A, Koh WP, Chow KY, Yuan JM, Jafar TH. Smoking and risk of kidney failure in the singapore chinese health study. PloS one. 2013;8:e62962. doi: 10.1371/journal.pone.0062962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohno K, Matsuoka H, Takenaka K, Miyake Y, Nomura G, Imaizumi T. Renal depressor mechanisms of physical training in patients with essential hypertension. American journal of hypertension. 1997;10:859–868. doi: 10.1016/s0895-7061(97)00109-x. [DOI] [PubMed] [Google Scholar]
- 21.Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the nad(p)h oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- 22.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. The Cochrane database of systematic reviews. 2011:CD003236. doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colberg SR, Albright AL, Blissmer BJ, Braun B, Chasan-Taber L, Fernhall B, Regensteiner JG, Rubin RR, Sigal RJ. Exercise and type 2 diabetes: American college of sports medicine and the american diabetes association: Joint position statement. Exercise and type 2 diabetes. Medicine and science in sports and exercise. 2010;42:2282–2303. doi: 10.1249/MSS.0b013e3181eeb61c. [DOI] [PubMed] [Google Scholar]
- 24.Odegaard AO, Koh WP, Gross MD, Yuan JM, Pereira MA. Combined lifestyle factors and cardiovascular disease mortality in chinese men and women: The singapore chinese health study. Circulation. 2011;124:2847–2854. doi: 10.1161/CIRCULATIONAHA.111.048843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS. Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Annals of internal medicine. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]


