Abstract
The substantial health risk posed by obesity and compulsive drug use has compelled a serious research effort to identify the neurobiological substrates that underlie the development these pathological conditions. Despite substantial progress, an understanding of the neurochemical systems that mediate the motivational aspects of drug-seeking and craving remains incomplete. Important work from the laboratory of Bart Hoebel has provided key information on neurochemical systems that interact with dopamine (DA) as potentially important components in both the development of addiction and the expression of compulsive behaviors such as binge eating. One such modulatory system appears to be cholinergic pathways that interact with DA systems at all levels of the reward circuit. Cholinergic cells in the pons project to DA-rich cell body regions in the ventral tegmental area (VTA) and substantial nigra (SN) where they modulate the activity of dopaminergic neurons and reward processing. The DA terminal region of the nucleus accumbens (NAc) contains a small but particularly important group of cholinergic interneurons, which have extensive dendritic arbors that make synapses with a vast majority of NAc neurons and afferents. Together with acetylcholine (ACh) input onto DA cell bodies, cholinergic systems could serve a vital role in gating information flow concerning the motivational value of stimuli through the mesolimbic system. In this report we highlight evidence that CNS cholinergic systems play a pivotal role in behaviors that are motivated by both natural and drug rewards. We argue that the search for underlying neurochemical substrates of compulsive behaviors, as well as attempts to identify potential pharmacotherapeutic targets to combat them, must include a consideration of central cholinergic systems.
Keywords: Addiction, Acetylcholine, Cocaine, Dopamine, Feeding, Motivation
Introduction
More than three decades of research into the neurobiological substrates of reward have focused attention on the nucleus accumbens (NAc), a ventromedial component of the basal ganglia, as a key structure in the neural systems responsible for translating motivation to action [1–4]. The preponderance of this research effort has centered on dopamine (DA) as the primary neurotransmitter in this regard. Many laboratories have contributed to our understanding of the role of DA in motivated behavior, and certainly the efforts of Dr. Bartley Hoebel in his laboratory at Princeton have been particularly noteworthy. Studies by Hoebel and colleagues have demonstrated increases in the release of DA in the NAc as a function of a variety of behaviors including feeding [5,6], rehydration [7], models of binge eating [8,9] and hypothalamic stimulation [10]. Moreover, his laboratory has been instrumental in demonstrating that many drugs with abuse liability (including cocaine, amphetamine, opiates, nicotine and alcohol) share a common mechanism in their ability to elevate DA levels in the NAc.
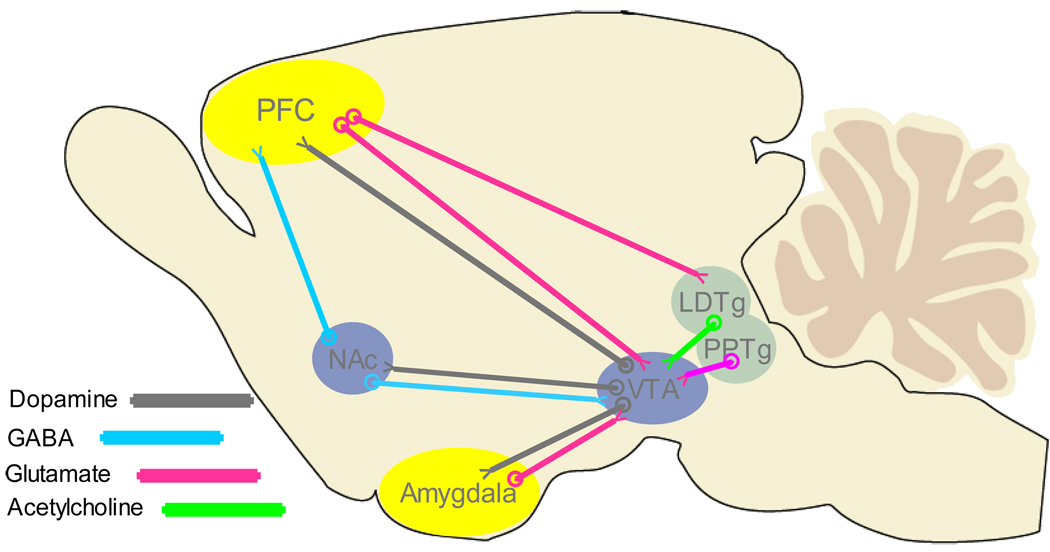
Of the many perspectives Hoebel brought to the study of neurochemical modulation of motivated behavior, perhaps the most salient for this review – and one that inspired much of the work presented herein – has been his view (along with that of Dr. Pedro Rada) that acetylcholine constitutes an integral component of the mesolimbic system [11]. Cholinergic pathways interact with key regions in the brain reward circuit, as illustrated in Figure 1. ACh projections from the nucleus basalis (NB) provide input to cortical and subcortical regions of the hippocampus and amygdala. These projections may play a role in learning and memory processes that are implicated in drug-induced dysfunctions like craving and relapse [12,13]. From the DA cell body region of the ventral tegmental area (VTA), DA terminals synapse on medium spiny gamma-aminobutyric acid (GABA)-containing cells and a smaller population of large, aspiny acetylcholine (ACh) -containing interneurons in the NAc [14]. Cholinergic interneurons have large dendritic arbors and an extensive network of axons that contact many cell bodies and terminals within both the core and shell subdivisions of the NAc [15,16]. Thus, in conjunction with DA inputs from VTA, ACh interneurons can modulate the activity of the GABA projection neurons, the primary output neurons of the NAc [17,18].
Figure 1.
ACh fibers (green) innervate key components of the mesolimbic system, including projections from nucleus basalis (NB) to sub-cortical areas (Hipp & Amygdala). ACh interneurons reside in the NAc and striatum. Another ACh projection system from the lateral dorsal tegmental nucleus (LDTg) projects to the DA cell body region of the ventral tegmental area (VTA).
Cholinergic cells in the laterodorsal tegmental nucleus (LDTg) and the posterior component of the pedunculopontine tegmental nucleus (PPTg) project to the VTA [19,20] where they modulate the activity of DA neurons and reward processing [21–26]. The anterior portion of the PPTg sends cholinergic projections to the substantia nigra [20]. Together with glutamate fibers, the ACh connection from the LDTg to the VTA forms a loop between the midbrain DA cells, the pons and the PFC. The ACh input onto DA cell bodies (in the VTA) is therefore a critical link for gating information flow through the mesocorticolimbic and mesostriatal systems.
Several lines of evidence suggest that stimulation of both nicotinic and muscarinic cholinergic receptors can affect mesolimbic DA levels and modify the reinforcing value of self-administered drugs. In this regard, the action of nicotine (the prototypical agonist at nicotinic ACh receptors) on the mesolimbic DA system has been studied extensively (for review see [27]) as has its interaction with other drugs of abuse such as alcohol [28,29] and cocaine [30,31]. These studies demonstrate that nicotinic activation increases extracellular DA in the NAc, stimulates locomotor activity with repeated exposure, and potentiates the reinforcing value of cocaine.
In comparison to the rather clear-cut situation for nicotinic activation, the effect of muscarinic drugs on stimulus reinforcement is less clear. There is evidence that muscarinic receptor activation can decrease amphetamine-induced hyperactivity [32] and inhibit amphetamine-induced DA release in the NAc [33]. Conversely, muscarinic antagonists enhance the locomotor stimulating effects of amphetamine and cocaine [32,34,35]. Systemic administration of muscarinic agonists and partial agonists have been reported to decrease cocaine self-administration rates in mice [36], whereas co-administration with the muscarinic antagonist, scopolamine decreases cocaine self-administration in rhesus monkeys [37]. In addition, mice lacking the muscarinic M5 type receptor self-administer less cocaine and show reduced cocaine conditioned place preference compared to their wild-type counterparts [38], suggesting that muscarinic receptors may mediate some component of cocaine reward.
Identifying the precise location of cholinergic effects on psychostimulant reward has been difficult because systemic administration of drugs and genetic deletion models affect receptors throughout the nervous system. However, recent work has focused attention on the NAc as a site of interaction between cholinergic mechanisms and cocaine reinforcement [39]. ACh interneurons in the NAc are known to be responsive to cocaine self-administration [40,41]. Hikida et al (2001) reported that selective ablation of cholinergic interneurons within the NAc using immunotoxin-mediated cell targeting resulted in increased cocaine-induced locomotor activity and reward value (as measured by conditioned place preference). In contrast, augmentation of ACh levels with acetylcholinesterase inhibitors in lesioned mice had the opposite effect [42,43]. These studies provide strong evidence that cholinergic cells in the NAc play an important role in modulating the reinforcing value of psychostimulants, but the nature of the cholinergic receptors that are critical for this effect remains unclear.
Acetylcholine-Dopamine Interactions and Ingestive Behavior
Seminal studies by Hernandez & Hoebel (1988) demonstrated that food reward increased DA in the NAc as measured by microdialysis [5], and Radhakishun and colleagues reported a similar result in the same year [44]. A key question that remained to be addressed, however, was whether DA output in the NAc was part of a general stimulus-induced arousal process or, alternatively, did DA play a role in the transduction of ingestive reward valence. To address these issues, Hoebel and colleagues performed two studies that examined the responses of NAc DA to conditioned taste stimuli. In the first experiment, extracellular dopamine in the NAc of rats was measured before and after they had developed a conditioned aversion to the taste of saccharin. In rats that had not developed an aversion, intra-orally applied saccharin increased DA. In contrast, DA decreased in response to saccharin in rats that had developed an aversion [45]. A complementary study was performed in rats with a conditioned taste preference to a normally aversive tastant, using a procedure eloquently developed by Sclafani and colleagues [46]. DA in the NAc was found to be elevated in response to an appetitively conditioned taste [47]. These studies represented some of the first reports that ingestive-related learning could modify DA output in the NAc.
The fact that mesolimbic DA plays a major role in modulating ingestive related behavior is now well documented. However, the neurochemical history of the corpus striatum is replete with suggestions that DA does not act in isolation but combines its action at least in part with that of the cholinergic system [11,48–51]. In addition to a mesencephalic dopamine input, the caudate and accumbens nuclei contain cholinergic interneurons [52–54], and there are numerous indications that the two systems functionally interact in the striatum [55–59]. Moreover, in the clinical arena, the prescription of dopaminergic antagonists (e.g. for the treatment of psychosis) is usually accompanied by co-administration of anticholinergic agents. Given the history of cholinergic-dopaminergic interactions in the striatum and the similar anatomical connectivity of the subjacent nucleus accumbens, it seemed reasonable to explore the possibility that both DA and ACh may have a combined role in the modulation of motivated behaviors.
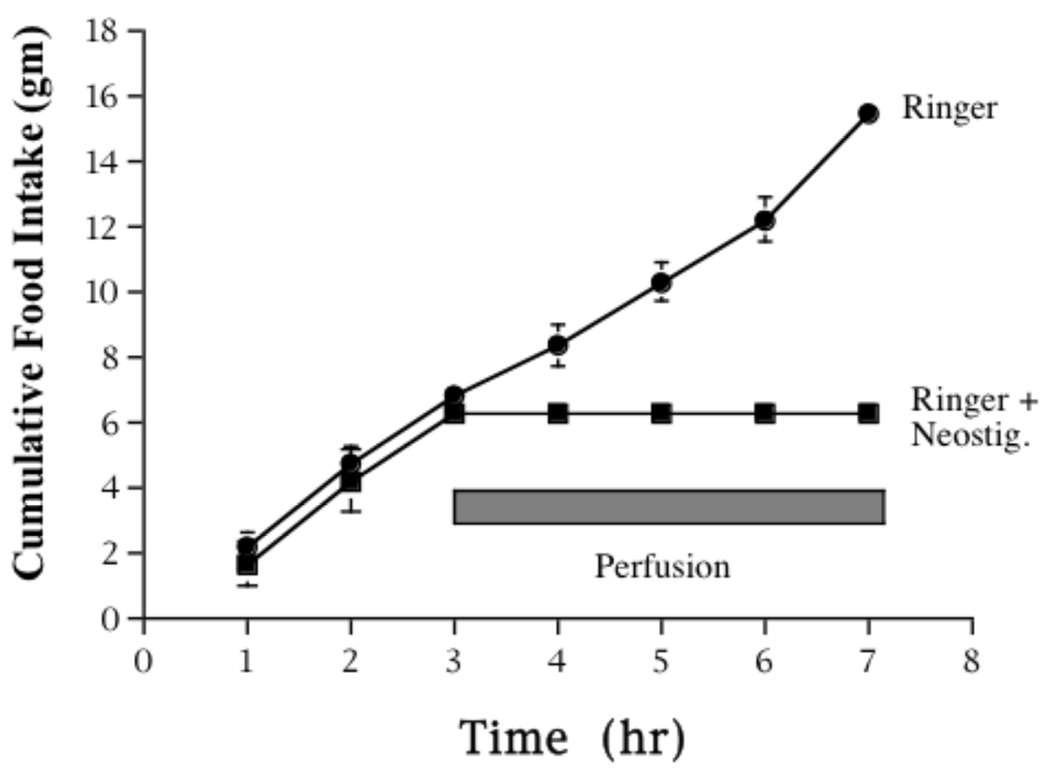
Evidence from the Hoebel laboratory has suggested that a particular balance between DA and ACh may indeed exist within the NAc and that changes in this balance may contribute to alterations in ingestive behavior (for a perspective, see [11]). In rats, presentation of an aversively conditioned taste results in an increase of extracellular ACh in the NAc [60], in contrast to the resulting decrease in DA mentioned above. Moreover, it is becoming increasing apparent that discrete manipulations in the level of ACh activity in NAc can have profound effects on the motivation to eat. For example, hungry rats stop feeding if the DA/ACh balance in the NAc is tilted in favor of excess cholinergic tone (Fig. 2). In this study, rats were implanted with bilateral microdialysis probes in the NAc and allowed to feed ad libitum over the course of their night cycle. Probes implanted in control animals were perfused throughout the night with a standard perfusion medium (Ringer), whereas experimental subjects had the perfusion solution switched to one containing the indirect cholinergic agonist neostigmine after 3 hr. Results showed a dramatic (and nearly complete) cessation of feeding during neostigmine exposure. Lack of eating was apparently not due to incapacitation or immobility, since water intake was unaffected in these animals (data not shown). Conversely, a situation in which ACh is depleted can cause hyperphagia (Fig. 3). For these experiments, rats were trained to lever-press for access to 45 mg food pellets during a 10 min interval each day for several weeks. After a stable baseline of responding was established, one half of the subjects received bilateral NAc injections of ethylcholine azirdinium mustard (AF64A), a compound which has relatively selective toxic effects on ACh neurons, while control animals received vehicle injections. Again, results were profound but in the opposite direction as observed before. In this case, AF64A-treated animals exhibited a 2-fold increase in responding for food. Activity on a food-inactive lever was unaffected (data not shown) indicating that the increase in bar pressing was not simply a consequence of hyperactivity.
Figure 2.
Bilateral microdialysis probes were implanted into the NAc of rats and probes were perfused with a standard Ringer’s solution over the course of 8 hr during dark cycle in non-food deprived rats (N=10). Food intake was measured hourly for three hr, at which point half of the rats had the dialysis perfusion medium switched to a Ringer solution plus the cholinesterase inhibitor, neostigmine (100 µM). The remaining rats were maintained on the standard Ringer perfusion medium. Addition of neostigmine to the perfusion Ringer caused a cessation of eating in free-feeding rats.
Figure 3.
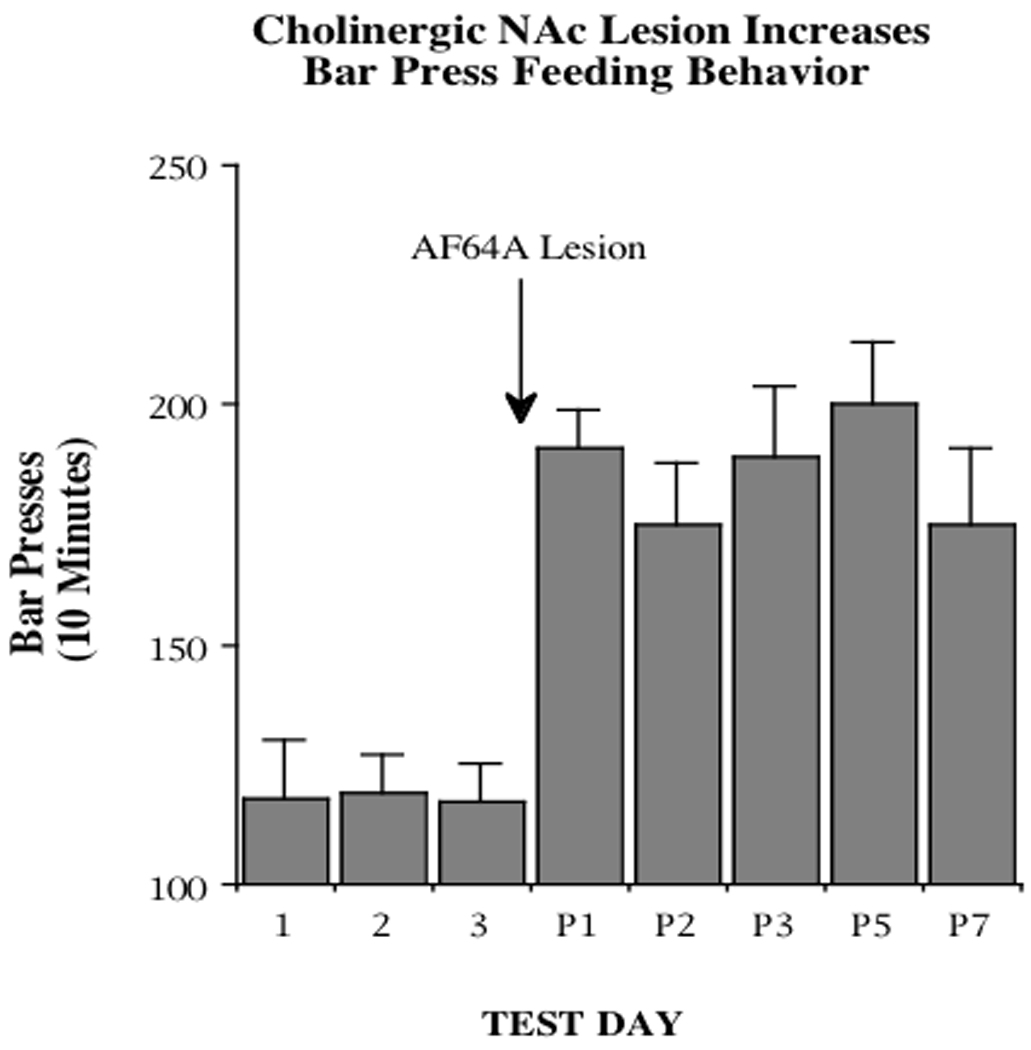
Lever pressing for 45 mg food pellets was measured for 10 min daily in non-food-deprived rats (N=8). Bilateral NAc injection of AF64A, a cholinergic cell toxin, caused a substantial increase in bar pressing for food on the first test day after the lesion and persisted for up to one week (P1–P7).
Cholinergic interactions with mesencephalic dopamine systems and drug reward
Work done in the Hoebel laboratory over a decade ago was some of the first to demonstrate a relevant link between ACh in the VTA and feeding related behavior [61]. These findings suggested that an interaction between ACh from pontomesencephalic sources and VTA could also be particularly critical for determining the action of drugs such as cocaine, that increase DA signaling by reuptake blockade. The ability of cocaine to increase extracellular DA is impulse dependent, and the interaction of ACh-mediated excitation of DA neurons, coupled with DA reuptake blockade may effectively increase the impact of cocaine on the mesolimbic system. We have found support for this hypothesis in our finding that repeated exposure to nicotine increased break points for cocaine self-administration on a progressive ratio reinforcement schedule [31].
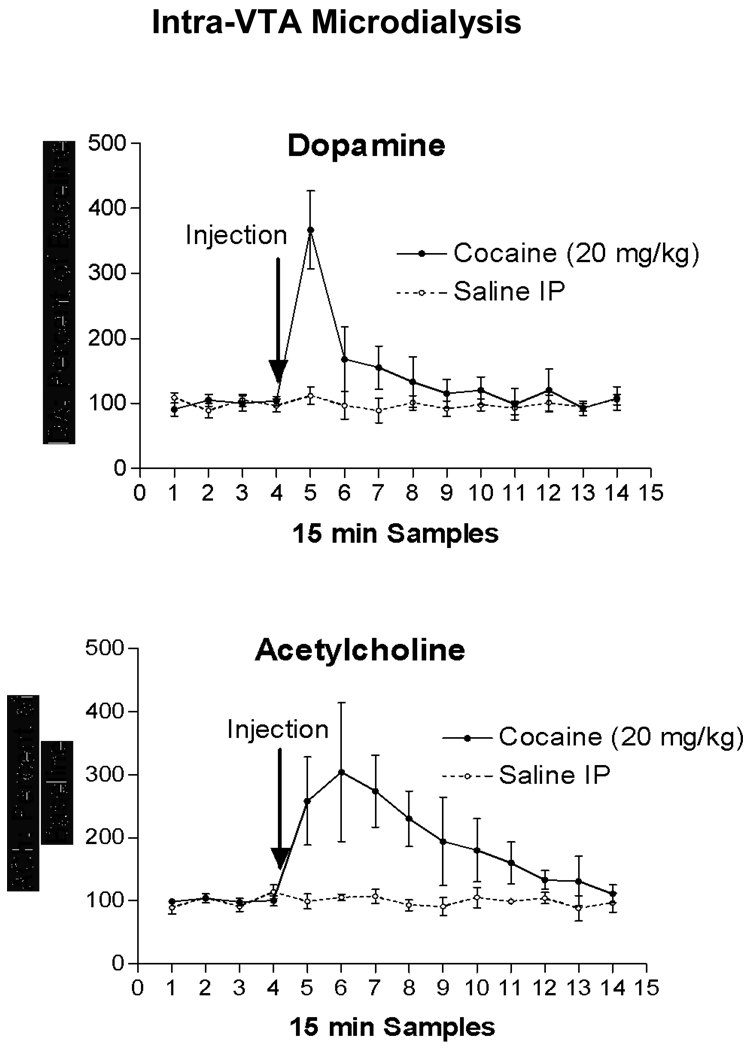
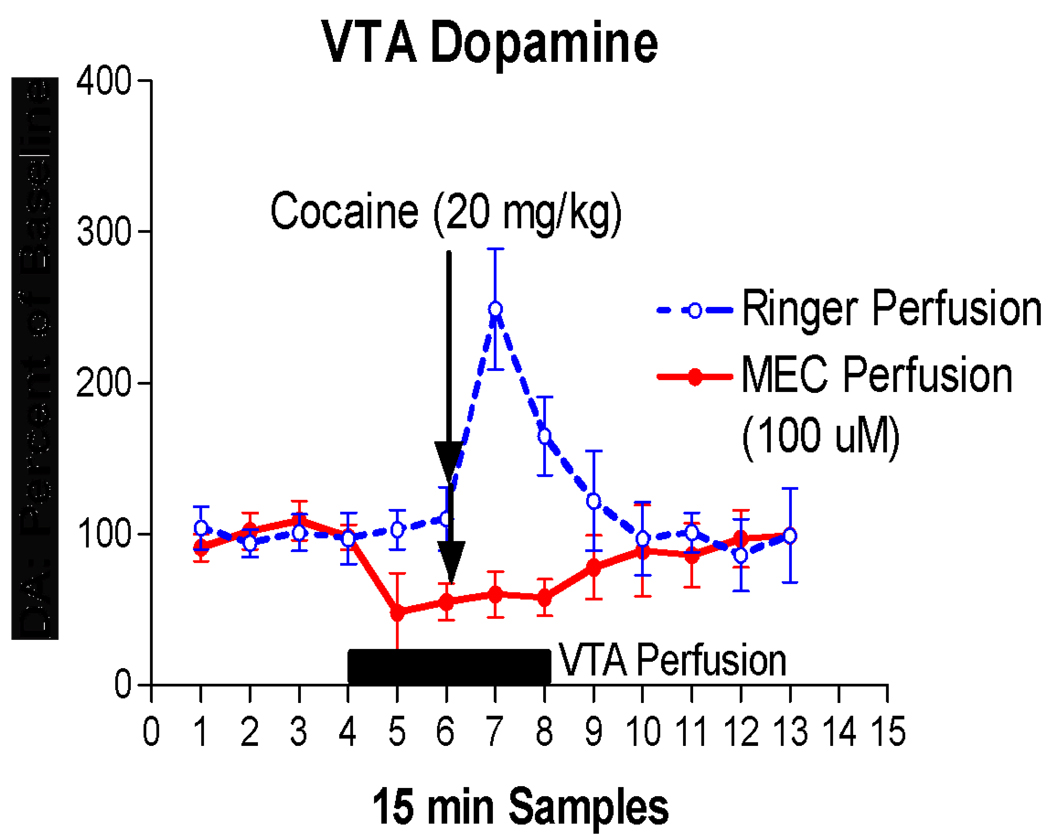
In a separate set of experiments, we measured the response to systemic injections of cocaine of DA released from dendrites in the VTA simultaneously with ACh released from terminals projecting from the pontomesencephalic cholinergic nuclei. Figure 4 shows that both ACh and DA increased robustly following 20 mg/kg cocaine. The time course of the ACh response was less phasic and the increase lasted longer than the DA response. Importantly, we have found identical responses (both in quality and time course) of DA and ACh to methamphetamine using microdialysis in the VTA of C57BL6 mice [62]. We also sought to determine if the increase in VTA DA following cocaine was dependent on cholinergic activity. We found that blockade of nicotinic cholinergic receptors (nAChR) within the VTA (using the nAChR antagonist mecamylamine; MEC) prevented the cocaine-induced increase in somatodendritic DA output (Fig. 5). These results collectively demonstrate that psychostimulants potently activate the cholinergic projections to the VTA and may be important finding for understanding the mechanism of ACh/DA interactions in the VTA.
Figure 4.
Systemic injections of cocaine increase ACh and dendritic DA in VTA. Intraperitoneal injections of cocaine caused a robust increase in DA released from dendrites in the VTA (Top panel). Measured simultaneously, ACh levels in the VTA also increased dramatically (Bottom panel). The time courses of the ACh and DA responses differed; there was a sharp, phasic spike in DA compared to a slower, tonic ACh response.
Figure 5.
Perfusion of the non-specific nicotinic receptor antagonist mecamylamine into the VTA blocks DA response to systemic injection of cocaine. The DA response was measured by microdialysis in the VTA. Systemic cocaine (20 mg/kg IP) caused an increase in VTA DA levels under normal perfusion conditions (Ringer: Blue line) but cocaine did not increase DA levels when mecamylamine was perfused through the VTA by reverse dialysis (Red line). This strongly suggests that the ability of cocaine to increase DA in VTA is dependent on the functional mecamylamine-sensitive nicotinic ACh receptors. Note also that perfusion of VTA with mecamylamine caused a moderate reduction in the amount of DA recovered before the cocaine injection (samples 5 & 6). This suggests that nicotinic cholinergic receptors have an effect on basal DA release from dendrites in the VTA.
Another proposal we tested was whether inactivation of ACh would reduce the behavioral reinforcing effect of cocaine (as measured by decreased cocaine self-administration). Specific pharmacological agents that block or enhance muscarinic receptors in the LDTg change ACh levels in the VTA. We determined that ACh output in the VTA was attenuated by direct, bilateral microinjection of the selective muscarinic type-2 (M2) autoreceptor agonist oxotremorine·sesquifumarate (OxoSQ; Fig. 6). Due to its ability to reduce an excitatory (cholinergic) input onto DA neurons, we hypothesized that OxoSQ would reduce the motivation of rats to self-administer both natural and drug rewards. Animals were therefore tested on progressive ratio (PR) schedules of reinforcement for food pellets and cocaine. We found that OxoSQ microinjection in the LDTg, compared to aCSF, significantly reduced both the number of self-administered pellets and cocaine infusions during the initial half of the session and this reduction was dose-dependent [63]. These data suggest that Inactivation of ACh input to the VTA results in lower motivation for both food and cocaine self-administration.
Figure 6.
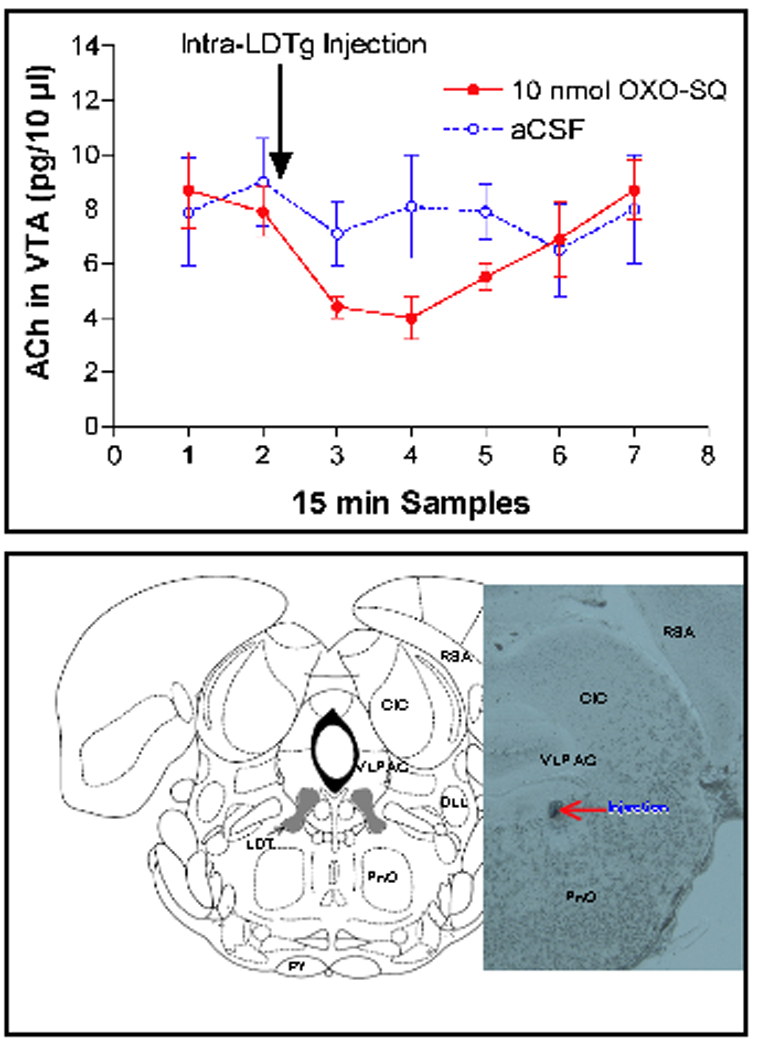
Intra-LDTg injection of the ACh autoreceptor agonist OXO-SQ reduced VTA ACh output. Top panel: Intra-LDTg injection of oxotremorine-sesquifumerate (OXO-SQ: an M2 cholinergic autoreceptor agonist) decreased the level of ACh measured in the VTA by microdialysis. Artificial cerebrospinal fluid (aCSF) did not have this effect (N=4/group). These results show the feasibility of inducing an ACh “deficit” in the VTA using microinjections in the LDTg. The histological slice shows the site of a thionin injection that was infused immediately after the conclusion of the microdialysis. Bottom panel: Coronal section of unstained (and unfixed) section from a rat that received an injection of OXO-SQ into the LDTg. Thionin stain was injected immediately after the conclusion of the microdialysis experiment. The red arrow shows the injection site, here compared to a plate taken from the atlas of Paxinos & Watson, (Bregma −8.72).
Cholinergic antagonist prevents escalation of cocaine self-administration in rats
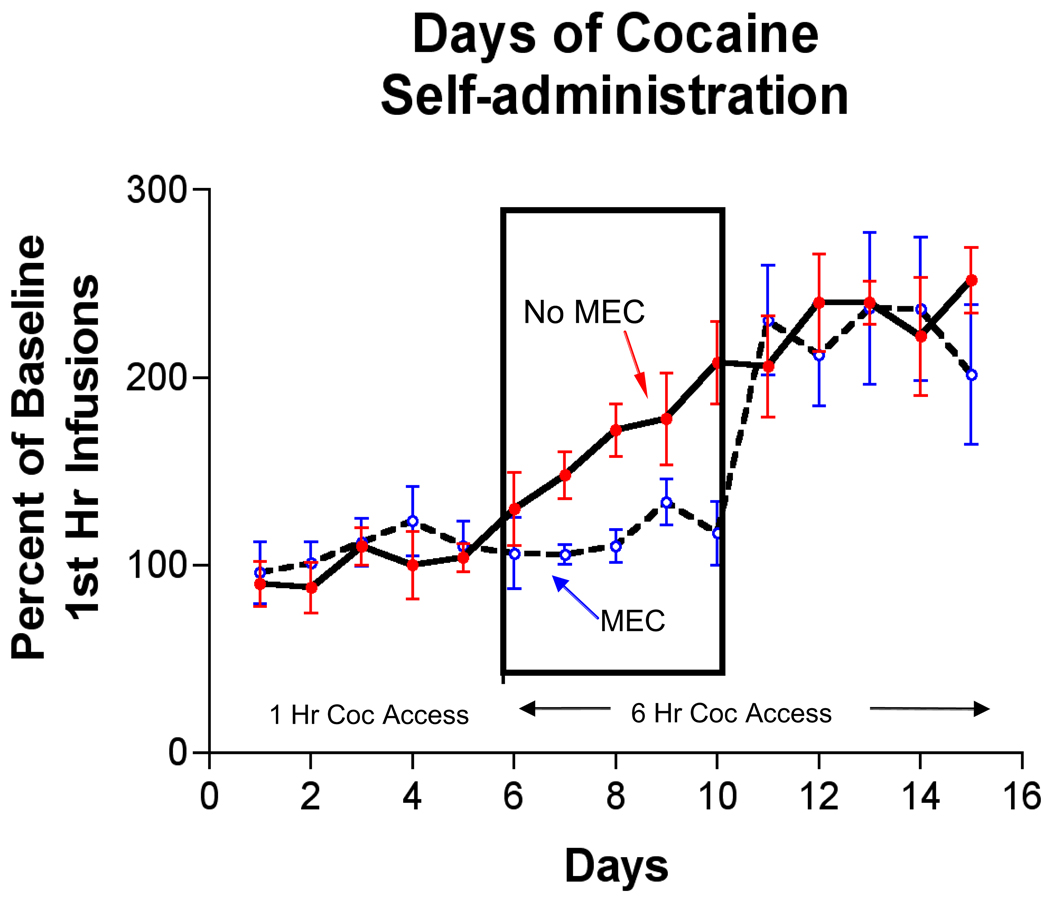
When rats are allowed increased access time to cocaine for self-administration (from 1-hr/day to 6-hr/day), they exhibit an escalation in their average daily intake of the drug [64,65]. Rats maintained on 1-hr/day access do not show an escalation. We have replicated this finding in our laboratory (See Figure 7; red circles, solid line). The escalation of drug intake phenomenon is a potential animal model with strong construct validity for the behavior of some human drug users who, as addiction develops, tend to increase drug intake when cocaine is readily available over extended time periods [66–68].
Figure 7.
The graph shows the number of cocaine infusions (normalized to baseline; 0.75 mg/kg/infusion) rats took in the first hr when access was limited to 1-hr (Days 1–4) or increased to 6-hr per day (Days 5–8). Rats tended to escalate cocaine intake with 6-hr access time (upper line) but if mecamylamine (MEC; 20 µg/kg/infusion) was added to the cocaine solution, escalation in cocaine intake did not occur. Note that MEC did not completely eliminate cocaine self-administration but prevented the expression of high-level (i.e. escalated) intake. Adapted from Hansen & Mark, (2007).
We examined whether a nicotinic receptor antagonist, mecamylamine (MEC) would affect escalation using the increased cocaine access-time procedure similar to the one described by Ahmed and colleagues. We found that when MEC was added to the cocaine solution that rats self-administered, escalation of intake did not occur (Figure 7; see also [69]). Moreover, we found that while MEC blocked the expression of increased cocaine self-administration, it may not have blocked its development because full escalation was observed the day after MEC was removed. These are exciting data because they show, first, that escalation of cocaine intake can be blocked without affecting normal (i.e. non-escalated) intake and, second, that neuroadaptations that underlie the development of increased cocaine-seeking behavior are independent of nicotinic receptors while those that are responsible for expression of higher drug-seeking require nicotinic receptor function.
Intra-VTA Injection of Mecamylamine reduces high-level cocaine intake
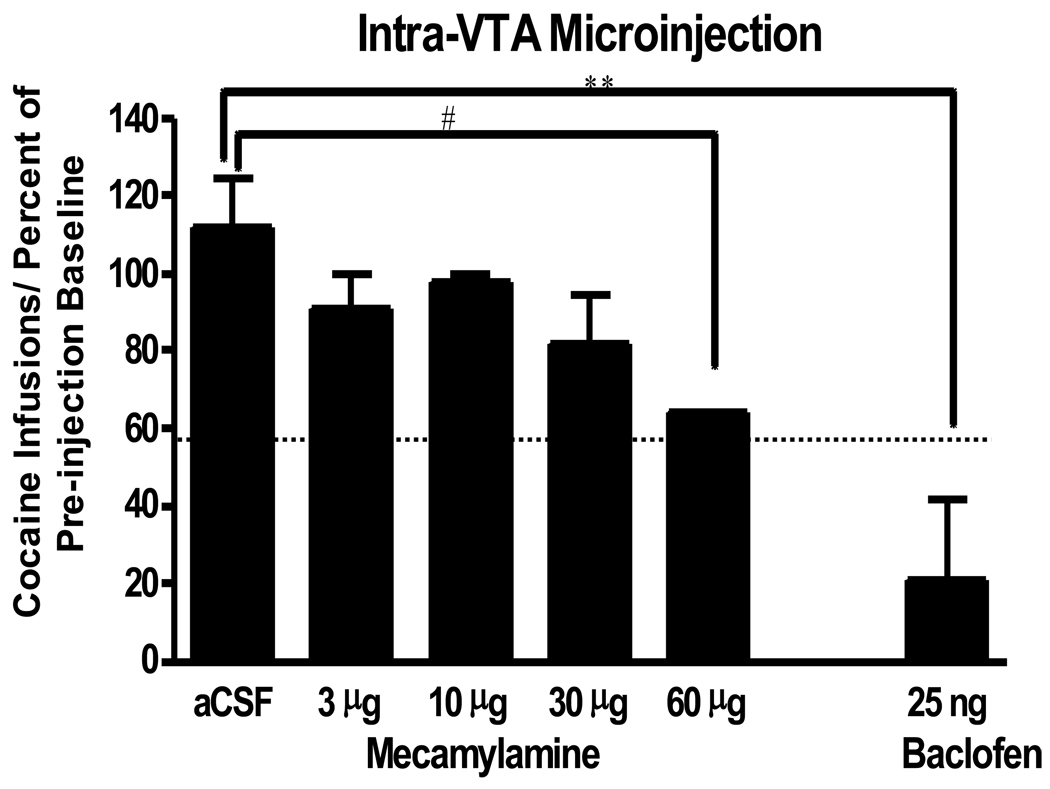
A key goal was to identify whether inactivation of cholinergic input to VTA can reduce expression of escalated cocaine intake. In a recent study, we found that microinjection of the nAChR antagonist mecamylamine (60 µg/side) reduced escalated cocaine intake (Figure 8) but did not reduce cocaine intake below pre-escalation levels. Rats self-administered cocaine for 1 hr per day in the pre-escalation phase. The average level of cocaine intake in 1 hr access sessions is represented by the dotted line. Rats were then given 6 hr per day access and cocaine intake increased. Microinjections of MEC were given between 8–12 days after the beginning of the 6 hr access sessions. Results are expressed as a percentage of pre-injection baseline, which was the average of the last 2 days of cocaine infusions on the 6 hr access schedule. At a concentration of 60 µg/side, intra-VTA injection of mecamylamine significantly reduced escalated intake but did not reduce cocaine self-administration below pre-escalation levels. As a control, we injected the GABAB agonist baclofen to confirm that intra-VTA injections of a drug known to reduce non-escalated cocaine intake would be effective at reducing cocaine intake below the pre-escalation baseline.
Figure 8.
Intra VTA injection of mecamylamine (MEC; 60 µg/side) reduced cocaine self-administration in rats that had established escalated cocaine intake on a 6 hr schedule. Dotted line indicates the average pre-escalation cocaine intake. Note that MEC reduced escalated intake but did not reduce cocaine self-administration below pre-escalation levels. Baclofen was used as a positive control to show that VTA injections could reduce cocaine self-administration below pre-escalation levels, whereas MEC only reduced escalated intake. (N=5–6 per group; # P<0.05; ** P<0.01).
These findings have strong implications for the role of cholinergic systems in controlling drug intake and potentially for the development of treatment strategies. Escalation of self-administration behavior is particularly relevant to cocaine addiction, and the paradigm we (as well as other labs) have used has construct and predictive validity as a model of uncontrolled drug intake in humans. Our data may have important implications for the development of potential therapeutics. For example, it is possible that new treatments that target nAChRs could interfere with the redevelopment of escalation in addicts when they leave in-patient treatment programs. There is support for this hypothesis since addicts report lower levels of cocaine craving following administration of MEC [70].
Acknowledgments
I (GPM) would like to extend my sincerest gratitude to Dr. Bartley G. Hoebel for his guidance and support throughout the length of this work and all that preceded it. He has been an outstanding role model. The authors would like to thank Tammie Painter for her excellent technical assistance. This work was supported by NIH grants R01 DA14639, T32 DA07262, and P50 DA018165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 2.Fibiger HC, Phillips AG. Reward, motivation, cognition: Psychobiology of mesotelencephalic dopamine system. In: Mountcastle VB, editor. Handbook of Physiology, Section 1: The Nervous System. 1986. pp. 647–675. [Google Scholar]
- 3.Koob GF, Goeders NE. Neuroanatomical substrates of drug self-administration. In: Liebman JM, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Oxford: Oxford University Press; 1989. pp. 214–263. [Google Scholar]
- 4.Wise RA. Opiate reward: Sites and substrates. Neurosci Biobehav Rev. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Science. 1988;42:705–712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- 6.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Mark G, Rada P, Pothos E, Hoebel BG. Effects of feeding and drinking on acetylcholine release in the nucleus accumbens, striatum and hippocampus of freely-behaving rats. J Neurochem. 1992;58:2269–2274. doi: 10.1111/j.1471-4159.1992.tb10973.x. [DOI] [PubMed] [Google Scholar]
- 8.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Rada PV, Mark GP, Hoebel BG. Dopamine release in the nucleus accumbens by hypothalamic stimulation-escape behavior. Brain Res. 1998;782:228–234. doi: 10.1016/s0006-8993(97)01283-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. 2007;7:617–627. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–483. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- 13.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 14.Smith AD, Bolam JP. The neural netwark of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 15.Meredith GE, Chang HT. Synaptic relationships of enkephalinergic and cholinergic neurons in the nucleus accumbens of the rat. Brain Res. 1994;667:67–76. doi: 10.1016/0006-8993(94)91714-0. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons: chemical, physiological and morphological characterization. Trends Neurosci. 1995;12:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 17.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 18.de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. Eur J Neurosci. 2002;16:2279–2290. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- 19.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- 20.Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeomans JS. Role of tegmental cholinergic neurons in dopamine activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology. 1995;12:3–16. doi: 10.1038/sj.npp.1380235. [DOI] [PubMed] [Google Scholar]
- 22.Yeomans J, Baptista M. Both nicotinic and muscarinic receptors in ventral tegmental area contribute to brain-stimulation reward. Pharmacol Biochem Behav. 1997;57:915–921. doi: 10.1016/s0091-3057(96)00467-4. [DOI] [PubMed] [Google Scholar]
- 23.Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 25.Alderson HL, Latimer MP, Winn P. Intravenous self-administration of nicotine is altered by lesions of the posterior, but not anterior, pedunculopontine tegmental nucleus. Eur J Neurosci. 2006;23:2169–2175. doi: 10.1111/j.1460-9568.2006.04737.x. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti L, Picciotto MR, Zoli M. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology (Berl) 2007;190:189–199. doi: 10.1007/s00213-006-0598-6. [DOI] [PubMed] [Google Scholar]
- 27.Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- 28.Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 29.Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- 30.Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux J, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 31.Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162:178–185. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. J Pharmacol Exp Ther. 1990;255:549–553. [PubMed] [Google Scholar]
- 33.Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Res. 2002;958:176–184. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- 34.Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. J Pharmacol Exp Ther. 1993;267:16–24. [PubMed] [Google Scholar]
- 35.Hagan JJ, Tonnaer JADM, Rijk H, Broekkamp CLE, van Delft AML. Facilitation of amphetamine-induced rotation by muscarinic antagonists is correlated with M2 receptor affinity. Brain Res. 1987;410:69–73. doi: 10.1016/s0006-8993(87)80021-5. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen T, Sauerberg P, Nielsen EB, Swedberg MD, Thomsen C, Sheardown MJ, Jeppesen L, Calligaro DO, DeLapp NW, Whitesitt C, Ward JS, Shannon HE, Bymaster FP, Fink-Jensen A. Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. Eur J Pharmacol. 2000;402:241–246. doi: 10.1016/s0014-2999(00)00442-8. [DOI] [PubMed] [Google Scholar]
- 37.Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology. 2002;161:442–448. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- 38.Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 39.Crespo JA, Sturm K, Saria A, Zernig G. Activation of muscarinic and nicotinic acetylcholine receptors in the nucleus accumbens core is necessary for the acquisition of drug reinforcement. J Neurosci. 2006;26:6004–6010. doi: 10.1523/JNEUROSCI.4494-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–1156. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 41.Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology. 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- 42.Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Nat Acad Sci. 2001;98:13351–13354. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci U S A. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radhakishun FS, van Ree JM, Westerink BHC. Scheduled eating increases dopamine release in the nucleus accumbens of food-deprived rats as assessed with on-line brain dialysis. Neuroscience Letters. 1988;85:351–356. doi: 10.1016/0304-3940(88)90591-5. [DOI] [PubMed] [Google Scholar]
- 45.Mark GP, Blander DS, Hoebel BG. A conditioned stimulus decreases extracellular dopamine in the nucleus accumbens after the development of a learned taste aversion. Brain Research. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- 46.Sclafani A, Nissenbaum JW. Robust conditioned flavor preferences produced by intragastric starch infusions in rats. American Journal of Physiology. 1988;255:R672–R675. doi: 10.1152/ajpregu.1988.255.4.R672. [DOI] [PubMed] [Google Scholar]
- 47.Mark GP, Smith SE, Rada PV, Hoebel BG. An appetitively conditioned taste elicits a preferential increase in mesolimbic dopamine release. Pharmacol Biochem Behav. 1994;48:651–660. doi: 10.1016/0091-3057(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 48.Butcher LL. Nature and mechanisms of cholinergic-monoaminergic interactions in the brain. Life Sci. 1977;21:1207–1226. doi: 10.1016/0024-3205(77)90001-7. [DOI] [PubMed] [Google Scholar]
- 49.De Souza H, Palmero-Neto J. A quantitative study of cholinergic-dopaminergic interactions in the central nervous system. Pharmacology. 1982;24:222–229. doi: 10.1159/000137600. [DOI] [PubMed] [Google Scholar]
- 50.Kasper KL. The anticholinergic biperiden in depressive disorders. Pharmacopsychiatry. 1981;14:195–198. doi: 10.1055/s-2007-1019597. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Iverson MT, Leclere J-F, Fibiger HC. Cholinergic-dopaminergic interactions and the mechanisms of action of antidepressants. Eur J Pharmacol. 1983;94:193–201. doi: 10.1016/0014-2999(83)90408-9. [DOI] [PubMed] [Google Scholar]
- 52.Butcher SG, Butcher LL. Origin and modulation of acetylcholine activity in the neostriatum. Brain Res. 1974;71:167–171. doi: 10.1016/0006-8993(74)90202-9. [DOI] [PubMed] [Google Scholar]
- 53.Hoover DB, Muth EA, Jacobowitz DM. A mapping of the distribution of acetylcholine, choline acetyltransferase and acetylcholinesterase in discrete areas of rat brain. Brain Res. 1978;153:295–306. doi: 10.1016/0006-8993(78)90408-0. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- 55.Damsma G, De Boer P, Westerink BHC, Fibiger HC. Dopaminergic regulation of striatal cholinergic interneurons. Naunyn-Schmiedeberg's Arch Pharmacol. 1990;342:523–527. doi: 10.1007/BF00169040. [DOI] [PubMed] [Google Scholar]
- 56.De Boer P, Damsma G, Schram Q, Stoof JC, Zaagsma J, Westerink BHC. The effect of intrastriatal application of directly and indirectly acting dopamine agonists and antagonists on the in vivo release of acetylcholine measured by brain microdialysis. Naunyn-Schmiedeberg's Arch Pharmacol. 1992;345:144–152. doi: 10.1007/BF00165729. [DOI] [PubMed] [Google Scholar]
- 57.Stadler H, Lloyd KG, Gadea-Ciria M, Bartholini G. Enhanced striatal acetylcholine release by chlorpromazine and its reversal by apomorphine. Brain Res. 1973;55:476–480. doi: 10.1016/0006-8993(73)90317-x. [DOI] [PubMed] [Google Scholar]
- 58.Stoof JC, De Boer T, Sminia P, Mulder AH. Stimulation of D2-dopamine receptors in rat neostriatum inhibits the release of acetylcholine and dopamine but does not affect the release of gamma-aminobutyric acid, glutamate or serotonin. Eur J Pharmacol. 1982;84:211–214. doi: 10.1016/0014-2999(82)90204-7. [DOI] [PubMed] [Google Scholar]
- 59.Tedford CE, Crosby G, Iorio LC, Chipkin RE. Effect of SCH 39166, a novel dopamine D1 receptor antagonist, on [3H]acetylcholine release in rat striatal slices. Eur J Pharmacol. 1992;211:169–176. doi: 10.1016/0014-2999(92)90525-9. [DOI] [PubMed] [Google Scholar]
- 60.Mark GP, Rada PV, Weinberg JB, Hoebel BG. Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res. 1995;688:184–188. doi: 10.1016/0006-8993(95)00401-b. [DOI] [PubMed] [Google Scholar]
- 61.Rada PV, Mark GP, Yeomans JJ, Hoebel BG. Acetylcholine release in ventral tegmental area by hypothalamic self-stimulation, eating, and drinking. Pharmacol Biochem Behav. 2000;65:375–379. doi: 10.1016/s0091-3057(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 62.Dobbs LK, Mark GP. Comparison of systemic and local methamphetamine treatment on acetylcholine and dopamine levels in the ventral tegmental area in the mouse. 2008 doi: 10.1016/j.neuroscience.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shabani S, Foster R, Gubner N, Phillips TJ, Mark GP. Muscarinic type 2 receptors in the lateral dorsal tegmental area modulate cocaine and food seeking behavior in rats. 2010;170:559–569. doi: 10.1016/j.neuroscience.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 65.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 66.Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- 67.O'Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12(Suppl 2):S36–S47. [PubMed] [Google Scholar]
- 68.Schaffer HJ, Eber GB. Temporal progression of cocaine dependence symptoms in the US National Comorbidity Survey. Addiction. 2002;97:543–554. doi: 10.1046/j.1360-0443.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 69.Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- 70.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]