Abstract
Nonalcoholic fatty liver disease (NAFLD) is a major factor in the pathogenesis of type 2 diabetes (T2D) and nonalcoholic steatohepatitis (NASH). The mitochondrial protonophore 2,4 dinitrophenol (DNP) has beneficial effects on NAFLD, insulin resistance, and obesity in preclinical models but is too toxic for clinical use. We developed a controlled-release oral formulation of DNP, called CRMP (controlled-release mitochondrial protonophore), that produces mild hepatic mitochondrial uncoupling. In rat models, CRMP reduced hypertriglyceridemia, insulin resistance, hepatic steatosis, and diabetes. It also normalized plasma transaminase concentrations, ameliorated liver fibrosis, and improved hepatic protein synthetic function in a methionine/choline–deficient rat model of NASH. Chronic treatment with CRMP was not associated with any systemic toxicity. These data offer proof of concept that mild hepatic mitochondrial uncoupling may be a safe and effective therapy for the related epidemics of metabolic syndrome, T2D, and NASH.
Nonalcoholic fatty liver disease (NAFLD) affects 15 to 30% of the world's population (1) and is a key predisposing factor for nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma. The role of hepatic steatosis in the pathogenesis of NASH and liver fibrosis remains undefined, and thus far, no therapeutic agents improve liver histology or hepatic protein synthetic function in animal models of NASH. In addition, NAFLD is strongly associated with hepatic insulin resistance and type 2 diabetes (T2D); however, efforts to ameliorate NAFLD or diabetes with pharmacologic agents have met with limited success.
The mitochondrial protonophore 2,4-dinitrophenol (DNP) has been investigated since the early 20th century for its ability to promote weight loss; however, after numerous reports of deaths in individuals taking DNP, production of the drug ceased in the United States in the late 1930s. Nevertheless, given its ability to promote insulin sensitivity in the rat (2), we investigated whether DNP could be pharmacologically manipulated to improve its safety margin. In a previous study (3), we showed that promoting subtle increases in hepatic mitochondrial uncoupling with a liver-targeted derivative of DNP ameliorates NAFLD and T2D in the rat. Although liver-targeted DNP was well tolerated, we hypothesized that we could further improve the safety and efficacy of DNP by developing a version of the drug with lower peak plasma concentrations and sustained-release pharmacokinetics.
To test this hypothesis, we first examined whether a 5-day continuous, low-dose intragastric infusion of DNP to achieve sustained plasma DNP concentrations in the 1 to 5 μM range would lead to reductions in hepatic steatosis and improve whole-body insulin sensitivity in high-fat–fed rats. This intragastric infusion of DNP resulted in steady-state plasma and liver DNP concentrations of ~3 and ~1 μM, respectively (fig. S1A). Nevertheless, these very low concentrations of DNP resulted in lower fasting plasma glucose and insulin concentrations as well as 80% reductions in plasma, liver, and skeletal muscle triacylglycerol (TAG) content (fig. S1, B to F).
Given the encouraging results of the intra-gastric infusion studies, we synthesized an orally available, controlled-release formulation of DNP, which is described in the supplemental materials, materials and methods. This formulation, called CRMP (controlled-release mitochondrial protonophore), was fed to rats in a small amount of peanut butter. In contrast to DNP, which caused a dose-dependent increase in body temperature at doses above 25 mg/kg, CRMP had a negligible effect on temperature at doses less than 100 mg/kg (fig. S2, A and B). To compare the safety and efficacy of CRMP and DNP, we performed 5-day parallel group dosing studies in high-fat–fed rats and found that the minimum effective dose of CRMP to decrease liver TAG was 0.5 mg/kg, whereas that of DNP was 5 mg/kg (fig. S2, C and D). In concert with this, the median lethal dose (LD50) of CRMP was more than 10-fold higher than that of DNP (fig. S2E). No changes to ala-nine aminotransferase (ALT), aspartate amino-transferase (AST), blood urea nitrogen (BUN), or creatinine were observed with any of the doses of CRMP below 125 mg/kg, whereas DNP treatment at doses above 0.5 mg/kg raised AST concentrations (fig. S3, A to H). Thus, the 5-day no observed adverse effect level (NOAEL) of CRMP was 100 mg/kg, as compared with 0.5 mg/kg for DNP.
We next examined whether the improved safety of CRMP might be related to differences in pharmacokinetic properties (fig. S4, A to F). Peak plasma DNP concentrations at each toxic dose of DNP were significantly higher than equimolar doses of CRMP, whereas the area under the curve of DNP concentration was higher after treatment with CRMP, likely accounting for CRMP's improved efficacy and reduced toxicity (fig. S4, E and F). Detailed pharmacologic data can be found in the supplementary materials (fig. S5, A to H).
To further evaluate the safety margins of CRMP as compared with DNP, we treated rats for 6 weeks with oral DNP or CRMP. Six weeks of CRMP treatment at 1 mg/kg was well tolerated and did not result in any alterations in behavior, food intake, body weight, body temperature, liver or kidney histology, or induction of neuropathy (fig. S6, A to I). In addition, no toxic effects were seen with doses up to 100 mg/kg CRMP, whereas increases in AST were seen at 1 mg/kg DNP treatment (fig. S6, D to G). Thus, the 6-week NOAEL for CRMP is at least 100-fold greater for CRMP (more than 100 mg/kg) than for DNP (less than 1 mg/kg). Taken together, our data indicate that the toxicity of a DNP derivative is predicted by the maximum concentration of DNP (fig. S4F), whereas its efficacy is predicted by the area under the curve of plasma DNP concentrations (fig. S4E).
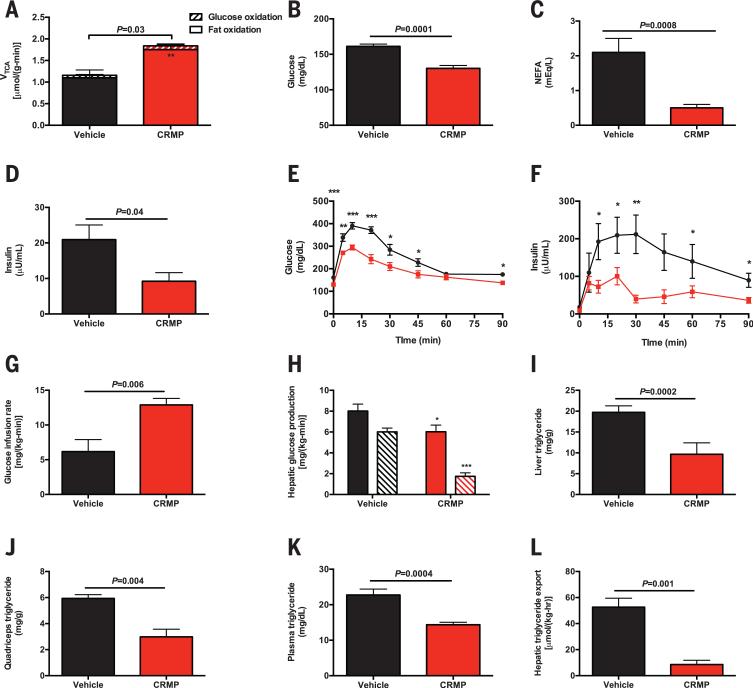
To examine the impact of CRMP on rates of hepatic mitochondrial glucose and fat oxidation, we assessed these rates using a combined liquid chromatography–mass spectrometry (MS)/MS–nuclear magnetic resonance method (4). We observed a 60% increase in rates of hepatic mitochondrial tricarboxylic acid cycle flux (VTCA) flux in CRMP-treated rats, which could be attributed to a 65% increase in rates of fat oxidation (Fig. 1A). In contrast, there were no differences in fat oxidation relative to VTCA in kidney, brain, heart, or skeletal muscle, indicating that the uncoupling effect of CRMP is confined to the liver (fig. S7A). To examine whether uncoupling with CRMP reduces tissue lipid content and improves insulin sensitivity, we treated a high-fat–fed rat model of NAFLD and insulin resistance with daily CRMP (1 mg/kg) or vehicle for 5 days. Despite identical body weight and fat content at the time of study, CRMP-treated rats exhibited 30 to 40% reductions in fasting plasma glucose, fatty acid, and triglyceride concentrations; a 30% increase in high-density lipoprotein concentration; and a 50% reduction in plasma insulin concentration, without any difference in hepatic gluconeogenic protein expression (Fig. 1, B to D, and fig. S7, B to H).
Fig. 1. CRMP improves insulin sensitivity in high-fat–fed rats.
(A) Hepatic VTCA from fat oxidation (solid bars) and glucose oxidation (striped bars) in chow fed rats. (B to D) Fasting plasma glucose, NEFA, and insulin concentrations. (E and F) Plasma glucose and insulin concentrations during an intraperitoneal glucose tolerance test. *P < 0.05, **P < 0.01, ***P < 0.001 by means of Student's t test. (G) Glucose infusion rate during a hyperinsulinemiceuglycemic clamp. (H) Basal (solid bars) and clamped (striped bars) rates of hepatic glucose production. (I to K) Liver, quadriceps, and plasma triglyceride concentrations. (L) Liver VLDL export. In (A) to (L), data are mean ± SEM of n = 6 to 8 rats per group.
Rats treated with CRMP manifested improved glucose tolerance, with lower plasma glucose and insulin concentrations throughout an intraperitoneal glucose tolerance test (Fig. 1, E and F, and fig. S7, I and J). To evaluate the effect of CRMP on whole-body insulin sensitivity, we performed hyperinsulinemic-euglycemic clamps with radiolabeled glucose (fig. S8, A and B). Consistent with improved whole-body insulin sensitivity, the CRMP-treated rats required twofold more glucose to maintain euglycemia (Fig. 1G and fig. S8C). This improvement in insulin-stimulated whole-body glucose metabolism in the CRMP-treated animals could be attributed to increases in both liver and muscle insulin sensitivity, as reflected by a 2.5-fold increase in insulin-stimulated peripheral muscle glucose uptake and a threefold greater suppression of hepatic glucose production in CRMP-treated rats during the hyperinsulinemiceuglycemic clamp (Fig. 1H and fig. S8D).
Previous studies have shown a strong causal relationship between ectopic diacylglycerol (DAG) accumulation and insulin resistance in liver and skeletal muscle. Consistent with this, we found that CRMP-treated rats had lower TAG and DAG content and decreased protein kinase Cε (PKCε) and PKCθ translocation in liver and skeletal muscle, respectively (Fig. 1, I and J and fig. S8, E to J). The reduction in skeletal muscle triglycerides was associated with 40% lower plasma triglyceride concentrations and an 80% reduction in liver very-low-density lipoprotein (VLDL) export (Fig. 1, K and L), explaining the reduced muscle lipid content as a result of liver-specific mitochondrial uncoupling. However, these reductions in TAG and DAG content were dissociated from any changes in liver or muscle acylcarnitine or ceramide content, liver glycogen, plasma inflammatory markers, FGF-21, or adiponectin concentration, or markers of uncoupling in brown fat (fig. S9, A to O).
CRMP also prevented the development of NAFLD: Rats fed a high-fat diet for 2 weeks and concurrently fed CRMP had lower fasting plasma glucose and nonesterified fatty acid (NEFA) and insulin concentrations associated with 50 to 90% reductions in triglyceride concentrations in plasma, liver, and skeletal muscle (fig. S10, A to F). To examine the effect of CRMP treatment on whole-body energy metabolism, we performed Comprehensive Lab Animal Monitoring System (CLAMS) metabolic cage studies in mice and observed no differences in any parameter examined (fig. S11, A to H). These data demonstrate that low levels of mitochondrial uncoupling confined to the liver are sufficient to reduce liver fat content and improve whole-body insulin resistance, without affecting food intake or whole-body energy expenditure.
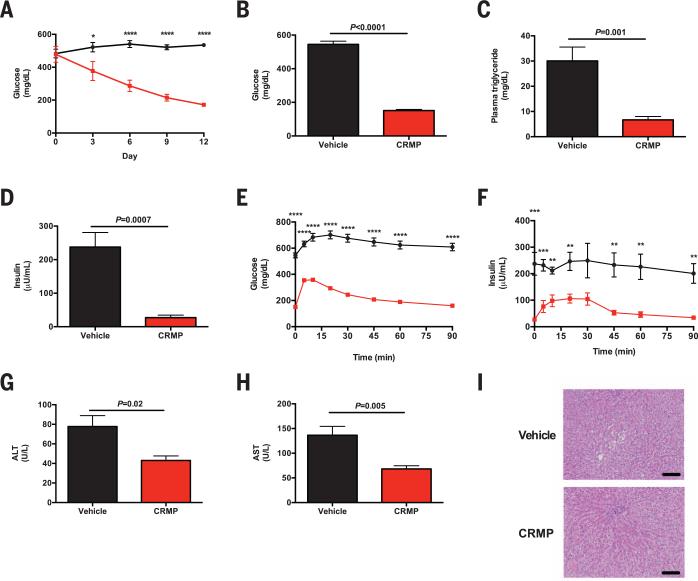
We next examined whether CRMP could reverse diabetes in the Zucker Diabetic Fatty (ZDF) rat. We treated high-fat–fed ZDF rats with CRMP daily for 14 days. CRMP treatment was associated with a progressive reduction in random plasma glucose concentrations and a 400 mg/dL decrease in fasting plasma glucose concentrations after 2 weeks of treatment along with marked decrements in fasting plasma insulin and triglyceride concentrations, despite identical body weight before and after treatment (Fig. 2, A to D, and fig. S12A). CRMP-treated rats also displayed a 60% reduction in hepatic acetyl coenzyme A (acetyl CoA), a key allosteric activator of hepatic gluconeo-genesis (fig. S12B) (4). The improved glycemia in CRMP-treated rats was associated with improved glucose tolerance during an intraperitoneal glucose tolerance test (Fig. 2, E and F, and fig. S12, C and D). These increments in insulin sensitivity and glucose tolerance were associated with 65 and 55% reductions in liver and quadriceps TAG, respectively (fig. S12, E and F). There was no detectable renal toxicity with this 2-week treatment (fig. S12, G and H). In addition to reducing ectopic fat content in liver and skeletal muscle, CRMP also reversed liver inflammation in ZDF rats, as reflected by normalization of liver enzymes (Fig. 2, G and H). Histologic analysis confirmed the resolution of NAFLD with CRMP treatment in this model of poorly controlled diabetes (Fig. 2I).
Fig. 2. CRMP improves glucose tolerance in diabetic rats.
(A) Random plasma glucose concentrations in vehicle-treated (black circles) and CRMP-treated rats (red squares). (B to D) Fasting plasma glucose, triglyceride, and insulin concentrations. (E and F) Glucose and insulin concentrations during an intraperitoneal glucose tolerance test. (G and H) Plasma ALT and AST concentrations. (I) Liver histology (hematoxylin and eosin stain). Scale bars, 100 μm. In (A) to (I), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by means of Student's t test. Data are mean ± SEM of n = 6 to 7 rats per group.
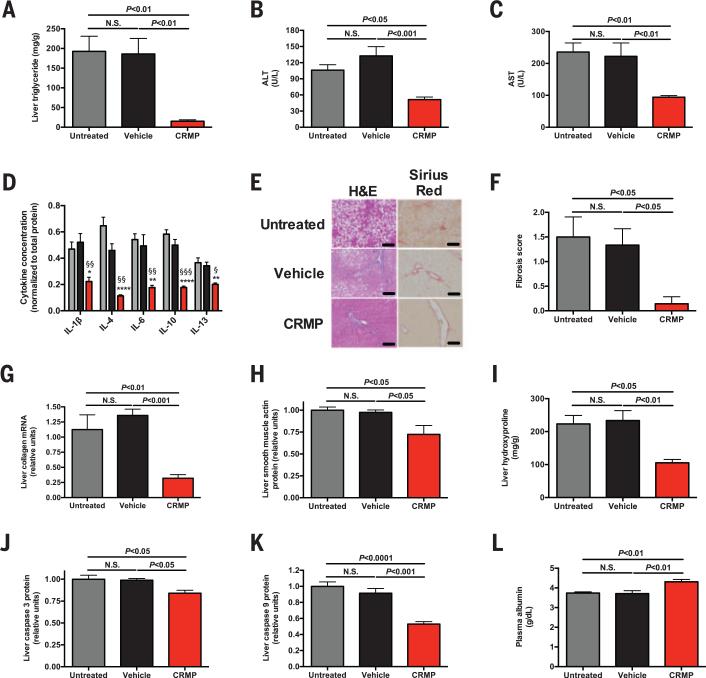
To investigate whether CRMP could ameliorate NAFLD-induced NASH and liver fibrosis, we fed rats a methionine/choline–deficient diet (MCD) for 8 weeks so as to induce NASH (5, 6) and subsequently treated the animals with CRMP for 6 weeks. CRMP reduced liver triglyceride concentrations by 90% and normalized plasma transaminase concentrations (Fig. 3, A to C). Consistent with this reduction in liver inflammation, CRMP-treated rats displayed lower concentrations of five inflammatory cytokines in the liver and reduced hepatic CD69 protein (Fig. 3D and fig. S13, A and B). Histological analysis confirmed the resolution of NAFLD and liver fibrosis in CRMP-treated rats, with a 90% reduction in the liver fibrosis score and accompanying reductions in collagen mRNA, smooth muscle actin, hydroxyproline, and caspase concentrations and unchanged terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining (Fig. 3, F to K, and fig. S13C). Because patients with liver cirrhosis manifest reduced hepatic glycogen content (7), we measured hepatic glycogen content in MCD-fed rat livers and found an 80% increase in glycogen content in CRMP-treated rats associated with reversal of fasting hypoglycemia (fig. S13, D and E). Additionally, CRMP improved liver protein synthetic function, indicated by increases in plasma albumin concentrations (Fig. 3L). By demonstrating an improvement in hepatic protein and carbohydrate synthetic function in addition to reversal of liver fibrosis in a NASH model, these data emphasize the potential efficacy of mitochondrial protonophores as a therapeutic agent for NAFLD-associated NASH to prevent liver cirrhosis and potentially hepatocellular carcinoma.
Fig. 3. CRMP ameliorates liver disease in a rat model of NASH.
(A) Liver triglyceride content. (B and C) Plasma ALTand AST. (D) Liver inflammatory cytokine concentrations, normalized to total protein. n = 4 rats per group. (E) Liver histology. Scale bars, 100 μm. (F) Fibrosis score. (G) Liver collagen mRNA. (H) Liver smooth muscle actin protein. (I) Hepatic hydroxyproline content. (J and K) Liver caspase 3 and caspase 9 protein. (L) Plasma albumin. Unless otherwise specified, n = 6 to 8 rats per group. Data are mean ± SEM, with comparisons by means of analysis of variance.
We have shown that altering the pharmacokinetics of DNP to promote a low sustained systemic release can increase the therapeutic window of this agent by more than 500-fold. Daily CRMP administration reversed NAFLD, insulin resistance, T2D, NASH, and liver fibrosis in rats without detectable toxicity. Altering the pharmacokinetics of DNP by increasing the DNP area under the curve while reducing the peak plasma DNP concentrations with a sustained-release coating increased the ratio of toxic to effective dose 25-fold over liver-targeted DNP and 1250-fold over unaltered DNP. These data support the potential utility of mitochondrial protonophores and other mitochondrial uncoupling agents for the treatment of the related epidemics of NASH, metabolic syndrome, and T2D.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Dong, C. Soroka, J. P. Camporez, M. Jurczak, J. Stack, M. Kahn, C. Borders, Y. Kosover, A. Nasiri, G. Butrico, M. Batsu, and W. Zhu for their invaluable technical assistance; C. Frassetto and A. Barkley for their work to formulate the CRMP; B. Ehrlich for assistance with the thermal algesia studies; M. Kashgarian for expert analysis of renal histology; and A. Ray and C. Tay for toxicology advice. Yale University has applied for a patent (provisional patent application 61/919, 003) related to the use of CRMP and similar protonophores for the treatment of metabolic diseases, including NAFLD/NASH and T2D. This research was funded by grants from the United States National Institutes of Health (R01 DK-40936, R24 DK-085638, U24 DK-059635, T32 DK-101019, P30 DK-45735, P30 DK-34989, and UL1 TR-000142) and the Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, Copenhagen, Denmark.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/347/6227/1253/suppl/DC1
Materials and Methods
Figs. S1 to S13
References (8–17)
REFERENCES AND NOTES
- 1.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. J. Hepatol. 2010;53:372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Samuel VT, et al. J. Biol. Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 3.Perry RJ, et al. Cell Metab. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry RJ, et al. Nat. Med. 2014;20:759–763. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leclercq IA, et al. J. Clin. Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, et al. Am. J. Physiol. 1999;276:E529–E535. doi: 10.1152/ajpendo.1999.276.3.E529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.