Abstract
The search for a homologous template is a fundamental, yet largely uncharacterized, reaction in DNA double-strand break repair. Two reports now demonstrate that broken chromosomes increase their movement and explore large volumes of nuclear space searching for a homologous template. Break mobility requires resection and recombination enzymes, as well as damage-checkpoint components.
Essential in all organisms is the mechanism by which discontinuities in DNA molecules are repaired by copying the missing information from a homologous molecule. Known as homologous recombination, this process involves a genome-wide search by the end of the broken molecule for a homologous sequence. Once it is found, the sequence is copied onto the broken molecule and bridges the break, allowing an intact molecule to be formed by homologous recombination. In spite of the tremendous importance of and interest in this process, the mechanism of the search for homology is not understood. Two papers on pages 502 and 510 of this issue provide some of the first intimate insights into this process in live budding yeast.
Miné-Hattab and Rothstein1 used fluorescence microscopy to follow the motion of a TetO/TetR–RFP (red fluorescent protein)-marked chromosome cleaved by the I-SceI endonuclease to create a double-strand break (DSB). The assay was performed on diploid cells, so that the DSB was efficiently repaired using the homologous chromosome marked by LacO/LacI–YFP. In the absence of DNA damage, the chromosomes are constrained to ~3% of the nuclear volume. However, following DSB induction, the broken chromosome explored ~30% of the nuclear volume, a 10-fold larger space. Similar observations were made by Dion et al.2, who tracked the movement of yellow fluorescent protein (YFP)-tagged version of a protein essential for homologous recombination, Rad52, that accumulates at I-SceI-induced DSBs at a LacO/CFP–LacI-marked locus. This assay was performed in haploid cells, so DSBs were not repairable and presumably stayed in ‘homology search mode’ for hours. Again, damaged chromosomes toured a much larger volume of the nucleus than undamaged ones. Therefore, both repairable and non-repairable DSB ends are mobilized.
What evidence indicates that increased mobility of DSB ends facilitates a search for homology? First, Miné-Hattab and Rothstein1 show that yeast homologous chromosomes typically reside far apart from each other, but when one breaks, it moves to pair with its homologue1. It is expected that once the broken chromosome pairs with its homologue, its mobility decreases; however, the authors do not investigate this in the present report. They also observe that once chromosomes have paired, Rad52 foci disassemble before the homologues separate, indicating that the damage is repaired. Second, the mobility of a DSB end depends entirely on a strand exchange protein of the RecA family that is capable of homology search3. Finally, strong evidence comes from the finding that deleting RAD9 decreases the mobility of DSB ends and delays recombination with a distant (but not proximal) homologous template2.
The study by Dion et al.2 implies that only the broken chromosome is more mobile. This observation is consistent with previous estimations of chromosome dynamics using chromosome conformation capture showing that both intra- and inter-chromosomal interactions of the broken chromosome change, whereas those of the unbroken chromosomes do not4. In contrast, the study by Miné-Hattab and Rothstein1 indicates that the unbroken homologous chromosome, and even non-homologous chromosomes, increase movement in the presence of damage elsewhere, albeit to a lesser degree than the broken chromosome. One possible explanation for this increased movement of all chromosomes in diploid cells is mating-type heterozygosity that increases the activity of Rad51 (ref. 5), which is needed for DSB-end mobility. Therefore, in diploid cells that have distant homologues, increased activity of Rad51 could stimulate DSB-end movement and consequently also collisions with, and motility of, all chromosomes.
These studies1,2 identify multiple enzymes required for the mobility of DSB ends (Fig. 1). Resection enzymes, which degrade one strand of DSB ends, and the key recombination proteins Rad51 and Rad54, are all important for DSB-end mobility. Although the role of Rad51 in homology search is expected3, the requirement of Rad54 for DSB-end mobility is somewhat surprising, because synapsis between MAT and HMR loci during mating-type recombination is successful in rad54Δ mutants6. A possible explanation for this contradiction is that MAT switching is an intra-chromosomal recombination event, so identification of a donor might be easier. Besides recombination proteins, the upstream DNA-damage checkpoint kinase Mec1 (ATR in mammals) and checkpoint mediator Rad9 are needed for increased mobility of DSB ends2. Checkpoint activation releases tethering of the chromosomes to nuclear pores through transcribed genes7, and this could also facilitate DSB-end mobility. Tethering release, however, requires the checkpoint signalling kinase Rad53, which is dispensable for DSB-end mobility2. Therefore, the established roles of Mec1 and Rad9 in the activation of Rad53 are unrelated to their functions in DSB-end mobility. Although these studies1,2 identify a number of proteins needed for DSB-end mobility, more work is needed to understand how they function in this process. Furthermore, it remains unexplored whether DSB-end mobility is an active process requiring ATP hydrolysis, or whether chromatin remodelling is also required. DSB-end movement is likely to generate entanglements between chromosomes that can inhibit further chromosome mobility. Thus, it will be interesting to investigate the role of topoisomerases in DSB-end mobility.
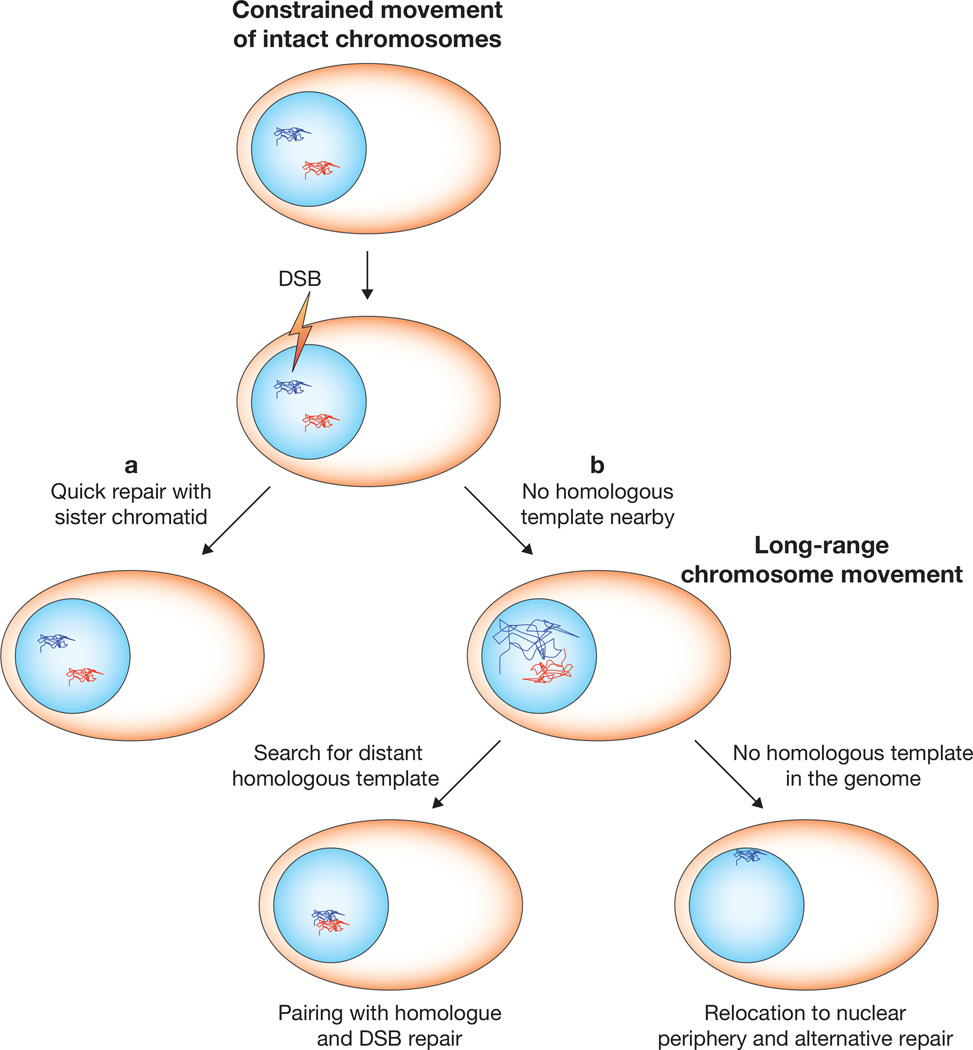
Figure 1.
DNA double-strand breaks (DSBs) promote chromosome movement. Homologous chromosomes (red and blue) reside far apart from each other and their movement is constrained. (a) When one is broken, it can be repaired with a sister chromatid template located nearby. (b) When there is no template available in the vicinity, the broken chromosome explores the nucleus in search of a homologous sequence, and then pairs with it for DSB repair. When a homologous sequence is not present, the broken chromosome relocates towards the nuclear envelope for alternative repair mechanisms.
Apart from playing a role in homology search, DSB-end mobility could possibly function in the relocation of non-repairable or slowly repaired breaks to the nuclear periphery4,8,9. It is unclear whether the mechanism of DSB-end mobility during homology search and relocation to nuclear periphery is the same. However, similar enzymatic requirements indicate that this may be the case. Relocation to the nuclear periphery requires resection and most of the same genes as DSB-end mobility. Furthermore, inner nuclear envelope proteins, including Mps3, are needed for the stable relocation of broken chromosomes to the nuclear periphery4,8,9. However, in contrast to Rad9, which facilitates homologous recombination with distant templates2, Mps3 has an inhibitory effect on ectopic recombination4. Perhaps Rad9 stimulates mobility in the nuclear lumen, whereas Mps3, recruited later, buries the DSB end at the nuclear envelope.
In Drosophila melanogaster, DSBs in heterochromatin transiently relocate to its periphery for repair, thus reducing potential genome rearrangement of repetitive sequences10. Similarly in yeast, DSB ends in rDNA repeats located in the nucleolus migrate out of it11. Again, resection and the checkpoint response are essential for these relocation events. The role of Rad51 is less clear because Rad51 foci are not formed within heterochromatin, yet it is needed for relocation outside heterochromatin. It is possible that a small amount of Rad51, insufficient to form a visible focus, facilitates relocation and/or traps resected DSB ends outside heterochromatin for the time of repair. Furthermore, the Smc5/6 SUMO ligase is needed for the relocation of DSBs in both yeast and Drosophila10,11. Smc5/6 also prevents Rad51 focus formation and, consequently, suppresses spontaneous aberrant recombination in rDNA or heterochromatin. It will be important to distinguish proteins important for the DSB-end movement itself from proteins merely trapping DSB ends at the nuclear envelope, outside the nucleolus or at the periphery of heterochromatin.
Interestingly, not all types of DNA damage that recruit homologous recombination proteins cause increased movement. Rad52–YFP foci formed at camptothecin-induced DNA nicks that generate one-ended DSBs or spontaneous Rad52 foci all remain relatively constrained2. So why are they different? This damage is mostly repaired by recombination with sister chromatids, so there is no extensive search for a template (Fig. 1). Homology search in inter-sister recombination is probably limited to only a few minutes, because joint molecules between them occur just 15 min after DSB formation12. Repair between homologues takes much longer, because joint molecules are visible only 60–90 min after DSB induction12, consistent with the time needed for the pairing of two homologues as reported by Miné-Hattab and Rothstein1. Also, spontaneous damage repaired by homologous recombination is mostly induced by single-strand gaps formed during replication, and not by DSBs. Again in this situation, the sister chromatid serves as the template13. Thus, increased mobility characterizes DSB ends that are not repaired, or repaired slowly with templates other than sister chromatids. Similarly, only slowly repaired or unrepairable DSBs relocalize to the nuclear periphery4. Alternatively, perhaps the duration of the homology search between sister chromatids is too short to capture the mobility state using current methodology. It will be interesting to test the role of cohesins that hold sister chromatids together in DSB-end movement.
The finding that different forms of homologous-recombination-repaired DNA damage induce distinctive movements also helps to clarify the discordant estimations of repair-focus mobility in mammalian cells. Some previous studies have indicated that repair foci exhibit increased motion, whereas others showed constrained mobility. Spatial proximity of DSBs was shown to be a major determinant of translocations14; however, this analysis was done in G1-arrested cells, in which resection and Rad51 loading, both required for DSB-end mobility, is limited. In light of the observations reported by Dion et al.2 and Miné-Hattab and Rothstein1, the reported differences in DSB-end mobility might be explained by different types of DNA damage, distinct cell-cycle phase, sister chromatid availability, resection efficiency or contribution of non-homologous end-joining to DSB repair. Thus, it will be important to extend these analyses to mammalian systems in a carefully controlled manner to reveal whether these intriguing observations are broadly conserved and whether the resection and homologous recombination machinery has a similar role in facilitating damaged chromosome movement.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Grzegorz Ira, Email: gira@bcm.edu.
Philip J. Hastings, Email: hastings@bcm.edu.
References
- 1.Miné-Hattab J, Rothstein R. Nat. Cell Biol. 2012;14:510–517. doi: 10.1038/ncb2472. [DOI] [PubMed] [Google Scholar]
- 2.Dion V, Kalck V, Horigome C, Towbin BD, Gasser SM. Nat. Cell Biol. 2012;14:502–509. doi: 10.1038/ncb2465. [DOI] [PubMed] [Google Scholar]
- 3.Forget AL, Kowalczykowski SC. Nature. 2012;482:423–427. doi: 10.1038/nature10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Genes Dev. 2009;23:912–927. doi: 10.1101/gad.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan EA, Shah N, Symington LS. Mol. Cell Biol. 2002;22:6336–6343. doi: 10.1128/MCB.22.18.6336-6343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houston PL, Broach JR. PLoS Genet. 2006;2:e98. doi: 10.1371/journal.pgen.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermejo R, et al. Cell. 2011;146:233–246. doi: 10.1016/j.cell.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalocsay M, Hiller NJ, Jentsch S. Mol. Cell. 2009;33:335–343. doi: 10.1016/j.molcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Nagai S, et al. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiolo I, et al. Cell. 2011;144:732–744. doi: 10.1016/j.cell.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres-Rosell J, et al. Nat. Cell Biol. 2007;9:923–931. doi: 10.1038/ncb1619. [DOI] [PubMed] [Google Scholar]
- 12.Bzymek M, Thayer NH, Oh SD, Kleckner N, Hunter N. Nature. 2010;464:937–941. doi: 10.1038/nature08868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lettier G, et al. PLoS Genet. 2006;2:e194. doi: 10.1371/journal.pgen.0020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Cell. 2012;148:908–921. doi: 10.1016/j.cell.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]