Abstract
Introduction
Clinic-based tracing efforts and public health surveillance data can provide different information about HIV care status for the same patients. The relative yield and how best to use these sources to identify and re-engage out of care patients is unknown.
Methods
At a large public HIV clinic in San Francisco, we selected a 10% random sample of active patients who were at least 210 days “late” for an HIV primary care visit as of April 1, 2013, for clinic-based outreach. Patients were considered out of care if they did not have an HIV primary care visit in the 210 days prior to April 1, 2013. We then matched the sample with the San Francisco Department of Public Health HIV surveillance registry. Patients with a CD4 or viral load result in the 210-day period were classified as in care. We compared results from both sources and estimated the cumulative incidence of disengagement from care for the full cohort of clinic patients.
Results
Of 940 patients lost to follow-up, 95 were sampled. Clinic tracing found 60 (63%) in care, 23 (24%) not located, 9 (10%) out of care, 2 (2%) incarcerated, and 1 (1%) had died. Of 42 individuals surveillance classified as out of care, tracing found 22 (52%) were in care. Of 52 patients found to be in care by surveillance, 12 (23%) were out of care by clinic tracing or unable to be located. The naïve estimate of the cumulative incidence of disengagement from care at three years for the active clinic cohort was 41.1% (95% Confidence Interval [CI]: 37.6%–44.5%). The use of surveillance data reduced this estimate to 12.7% (95% CI: 18.2%–25.4%) and when further corrected using tracing outcomes, the estimate dropped to only 6.4% (95%CI: 3.4%–9.4%).
Conclusions
Clinic-based tracing and surveillance data together provide a better understanding of care status than either method alone. Using surveillance data to inform clinic-based outreach efforts may be an effective strategy, though tracing efforts are most likely to be successful if conducted in real time.
Keywords: retention in HIV care, loss to follow-up, clinic-based tracing, HIV surveillance
Introduction
In national estimates of the HIV care continuum, or, the HIV care cascade, the biggest apparent drop-off along the sequential steps of the cascade occurs with retention in care.1,2 While considerable progress has been made with regard to HIV diagnosis and linkage to care, retention in care is considered by many to be perhaps the biggest obstacle to successful HIV treatment, especially since some level of retention is necessary for virologic suppression.3 Yet challenges remain in measuring retention. First, it is possible for HIV-infected individuals to be retained at some points in time and not at others and existing metrics do not always summarize these fluctuating states completely.4 In addition to the potential episodic nature of engagement, retention is further complicated by the epidemiologic phenomenon of “churn,”5 in which geographic mobility leads to care entry and exit across a population and can result in misleading prevalence data.
Identifying and re-engaging HIV-infected individuals who are truly out of care is a priority area for clinicians and public health departments alike, given evidence regarding the treatment and prevention benefits of antiretroviral therapy (ART) regardless of CD4 cell count.6,7 Despite the importance of retention in HIV care, uncertainties remain about the optimal approach to measuring retention in the fragmented U.S. health system. Efforts to estimate retention have used clinic-based data sources, i.e. kept and missed primary care visits;8–10 as well as CD4 and HVI viral load laboratory test results reported by law to public health departments for surveillance purposes. 11–13
Each of these data sources has its pros and cons. Clinic visit data is more granular in that it identifies whether the rendering provider can prescribe ART, while surveillance laboratory data reveals little about clinical context, even if the medical site ordering the test is known. Missed primary care visits can function as a warning sign that a patient is at risk for dropping out of care altogether. However, loss to follow up at the clinic level is complicated by silent transfers of care, relocation, and incarceration. Consequently, clinic-based retention estimates may reflect “retention in clinic” rather than true retention in care.14 Surveillance laboratory data can partially address this problem because of reporting from multiple care locations, however, these population-level estimates can be similarly hampered by outmigration.15 Surveillance laboratory data can also suffer from incomplete reporting, different clinical practices with regard to laboratory monitoring, and care provision in research settings, resulting in overly pessimistic estimates of the number of individuals out of care.16
Given that clinic visit data and surveillance laboratory data can provide different information about care status for the same individuals, substantial interest exists in how best to use these data sources in order to identify and re-engage out-of-care HIV-infected patients. Recently, health departments in Seattle, New York City, and San Francisco conducted outreach to HIV-infected individuals who by surveillance records did not appear to have had laboratory monitoring in the past 9–12 months and/or have detectable viral loads on last monitoring.17–19 A key feature of these initiatives has been working closely with clinics to identify more accurately the patients who are truly out of care, either by embedding health department staff within the clinic or by contacting individual providers. These collaborations have also enabled clinics to more effectively target their outreach to patients lost to follow up, such as in Washington, D.C., where prior to a Ryan White provider-led “recapture blitz,” surveillance identified that 376/1375 (27%) of patients not seen for more than six months appeared to be receiving care elsewhere.20
We sought to build upon the findings of these clinic-public health partnerships in a novel fashion by measuring the relative yield of clinic-based outreach vs. surveillance laboratory data in ascertaining care outcomes for patients putatively lost to clinic follow up. Our objectives were: 1) to determine via maximal tracing efforts the true care status of a sample of patients at a large public hospital HIV clinic in San Francisco who by electronic medical record query of primary care visits appeared lost to follow up; 2) to use San Francisco Department of Public Health HIV surveillance registry data to classify these patients as in care or out of care; 3) to compare results from both data sources, and; 4) to use these data sources to estimate disengagement from care for the full cohort of clinic patients.
Methods
Patient population
We defined the active clinic cohort as patients who had at least one kept primary care visit at the HIV clinic of San Francisco General Hospital (SFGH), Ward 86, on or after April 1, 2010. We defined patients lost to follow-up group as those who were at least 210 days “late” for a primary care visit as of April 1, 2013, and were not known to have died. We chose this definition of lost to follow-up based on the following: 1) the median interval between scheduled primary care visits in our clinic is relatively short at 28 days (IQR 7–56 days), and; 2) the Department of Health and Human Services metric for retention is one visit in each six-month period of a 24-month observation period.3
Tracing a sample of lost patients
We randomly sampled 10% of the lost to follow-up patients and employed an “ascertainer” to conduct health care systems and community-based tracing for these patients. The ascertainer investigated care status through a sequential process of chart review, provider contact, phone calls to the patient and/or emergency contacts, email, U.S. Postal Service mail, and in-person outreach from April to December 2013. The ascertainer conducted this outreach according to protocols developed by the SFGH Ward 86 linkage and re-engagement team and documented outreach results in the clinic electronic medical record (EMR) to benefit clinical care. The full lost to follow up list was then matched to San Francisco Department of Public Health (SFDPH) HIV surveillance registry data to obtain CD4 and viral load test results, specimen collection dates, dates of receipt of lab value by the SFDPH, name of facility ordering the tests, and death dates. To ensure that surveillance laboratory data would have been complete for the traced sample if we had given the list to surveillance on the day tracing commenced rather than at its conclusion, we looked at the dates surveillance received the laboratory values and confirmed that all results were available as of April 1, 2013.
Ascertaining patient outcomes
We defined in care and out of care statuses for patients using clinic tracing and SFDPH surveillance data. From the clinic tracing perspective, individuals were considered in care if there was a chart note documenting transfer of care; there had been a drop-in (rather than a scheduled) visit with the primary care provider; the ascertainer obtained self-report of care elsewhere, or; the individual was incarcerated. When possible, the ascertainer also documented the location of care. The ascertainer assigned an out of care status if there were other types of visits without evidence of primary care visits; the chart documented out of care status through communication with the patient, or; the individual confirmed being out of care. Individuals were defined as in care from surveillance data if there was a CD4 or viral load value in the 210 days prior to April 1, 2013, and were defined as out of care if there were no laboratory values during this period. Virologic suppression was defined as a viral load value <200 copies/μL. We calculated summary statistics for basic demographics, i.e., age, gender, race/ethnicity, CD4 cell count, and viral load. We also calculated the median number of months from the last made appointment to the start of tracing.
Incidence estimation
We compared the proportions of patients in care and out of care by clinic-based tracing and surveillance. We also calculated and compared estimates of the cumulative incidence of disengagement from care (defined as not having had a primary care visit at the clinic for >210 days). Deaths occurring earlier than 210 days after the last visit counted as a competing risk, since early death precludes disengagement.21 Cumulative incidence estimates of disengagement from care were conducted from three data sources to allow comparison of these sources. The naïve estimate of disengagement from care used only the information available to the clinic on April 1, 2013, which consisted of an EMR query of data on clinic visits and reported deaths. In this naïve estimate, being lost to follow up was the equivalent of being disengaged from care. A second estimate made use of the SFDPH surveillance laboratory and death data described above for all the patients who appeared disengaged from care by virtue of being lost to follow up as of April 1, 2013. The third estimate of disengagement from care was calculated using the outcomes of the traced sample of 10%. Sampling-based probability weights (in which the weight is inverse to the probability of selection and successful outcome ascertainment) were used for this estimate. Confidence intervals were determined through bootstrapping.14,22
Clinic-based tracing was considered within the purview of clinical care; data collection and analysis were approved by the Committee on Human Research at the University of California San Francisco.
Results
The active clinic cohort consisted of 3,095 patients of whom 940 (30%) were lost to follow up. A 10% random sample of 95 patients was selected for tracing. The demographic and clinical characteristics of the active clinic cohort, the lost to follow up subgroup, the random sample selected for tracing, and the subset of the sample with outcomes ascertained by tracing or the match with surveillance are described in Table 1. The sample selected for tracing had a slightly higher proportion of white patients than the overall lost to follow up group from which they were selected (59.0% vs. 49.8%) and a higher proportion of patients who were virologically suppressed at last measurement before becoming lost to follow up (81.9% vs. 71.0%). Those located through tracing had been absent from the clinic for less time than those who were not located, median number of months absent 15.3 (IQR 10.2 to 25.3) vs. 20.2 (15.0 to 28.9), Wilcoxon-Mann-Whitney p-value 0.06.
Table 1.
Demographic and Clinical Characteristics of Study Patients
| Characteristic | Active Clinic Cohort (n=3095) | Lost to Follow Up (n=940) | Random Sample Selected for Tracing (n=95) | Outcome Ascertained by Tracing or Surveillance (n=79) |
|---|---|---|---|---|
|
| ||||
| Age, years, median (IQR) | 44 (36 to 50) | 41 (33 to 48) | 41 (35 to 48) | 41 (35 to 48) |
|
| ||||
| Male, % (n/N) | 87.5% (2703/3089) | 89.2% (836/937) | 88.4% (84/95) | 88.6% (70/79) |
|
| ||||
| Race/ethnicity, % (n/N): | ||||
| Asian/PI | 5.7% (175/3095) | 4.7% (44/940) | 5.3% (5/95) | 3.8% (3/79) |
| Black | 21.8% (674/3095) | 20.9% (196/940) | 14.7% (14/95) | 13.9% (11/79) |
| Latino | 23.2% (718/3095) | 21.8% (205/940) | 17.9% (17/95) | 17.7% (14/79) |
| White | 46.9% (1452/3095) | 49.8% (468/940) | 59.0% (56/95) | 60.8% (48/79) |
| Other | 2.2% (68/3095) | 2.7% (25/940) | 3.2% (3/95) | 3.8% (3/79) |
| Unknown | 0.3% (8/3095) | 0.2% (2/940) | 0% (0/95) | 0% (0/79) |
|
| ||||
| CD4 T cell count, % (n/N): | ||||
| <200 | 11.1% (331/2977) | 12.6% (116/922) | 12.9% (12/93) | 13.0% (10/77) |
| 200–350 | 17.4% (517/2977) | 16.8% (155/922) | 15.1% (14/93) | 15.6% (12/77) |
| 351–500 | 21.1% (627/2977) | 22.5% (207/922) | 21.5% (20/93) | 22.1% (17/77) |
| >500 | 50.5% (1502/2977) | 48.2% (444/922) | 50.5% (47/93) | 49.4% (38/77) |
|
| ||||
| Viral suppression at last measurement prior to becoming lost to follow up (VL<200), % (n/N) | 77.5% (2371/3059) | 71.0% (655/923) | 81.9% (77/94) | 80.8% (63/78) |
Clinic-Based Tracing
After EMR review and phone/email/mail outreach methods were exhausted for the 10% sample lost to follow up, the ascertainer conducted in-person outreach for 26 individuals by visiting the last known address, yielding care statuses for 4 individuals, three of whom were in care and one of whom was out of care. All individuals had a positive response to the in-person outreach. The ascertainer wrote field notes on these encounters, a selection of which follows.
XX is a heterosexual white female living in a hotel in the Tenderloin. The patient actively uses speed and was high at the time of my visit. She was excited that someone came to find her. She reported that she fired her PCP because she was not able to get fentanyl patches for her pain. She was in pretty bad shape. Her living space was in complete disarray and her front door appeared to have been kicked in. It was kind of hard to see someone living in that much chaos. She said she would be willing to return to care if she could get a different PCP.
XY is a gay white male living in the Castro with his partner. He appeared healthy and reported he was in care and virologically suppressed. He was gracious and gave me an apple and a glass of water before I left.
Of the sample of 95 patients, clinic tracing found that 60 (63%) were in care, 23 (24%) were unable to be located, 9 (10%) were out of care, 2 (2%) were incarcerated, and 1 (1%) had died (Table 2). Of the 60 individuals in care, 25 (42%) had transferred care within San Francisco, 20 (34%) were in care outside of San Francisco, 11 (18%) had dropped in for primary care, 2 (3%) were in an HIV skilled nursing facility, and 2 (3%) did not divulge their care location.
Table 2. Updated Care Statuses based on Clinic-Based Tracing vs. Surveillance.
Patients were a random sample of 94 patients who were lost to follow-up from the Ward 86 Clinic and in whom additional information was sought either through a clinic-based tracer or the surveillance database maintained by the San Francisco Department of Health.
| Surveillance | |||||
|---|---|---|---|---|---|
| In Care by Surveillance with Suppressed VL | In Care by Surveillance with Detectable VL | Out of Care | Total | ||
| Patient Status As Ascertained by Clinic-based Tracer | In Care | 33 | 7 | 22 | 62 |
| Out of Care | 3 | 2 | 4 | 9 | |
| Unable to Be Located | 5 | 2 | 16 | 23 | |
| Total | 41 | 11 | 42 | 94 | |
Note: this table removes the deceased individual.
Surveillance Registry Match
Matching the 10% sample of 95 patients lost to follow up to the surveillance registry found that 52 (55%) were in care, 42 (44%) were out of care, and 1 (1%) had died (Table 2).
Comparing Outcomes from Clinic-Based Tracing and Surveillance Data
Clinic tracing found that 22 of the 42 classified as out of care using surveillance data (52%) were actually in care. Of these patients, 10 had moved out of state, 7 were in state but outside of San Francisco County, and 5 were in San Francisco County, but did not have labs during the period of interest. Roughly a quarter of patients (12/52 or 23.1%) found to be in care by surveillance were considered out of care or were unable to be located by clinic tracing. Of the 62 traced patients ascertained as in care, 22 (35.5%) were classified as out of care using surveillance data. There was agreement between clinic-based tracing and surveillance that 40 of 94 (43%) of surviving individuals were in care, of whom 33 (83%) had suppressed viral loads (Table 2 ).
A closer look at the patients classified as out of care by both clinic tracing and surveillance, as well as discrepancies in care status, reveals some important findings. Of the 4 patients classified as out of care by both clinic tracing and surveillance (Table 3a), 3 had clinic visits with undetectable viral loads just prior to the 210 day period of interest but returned to care 10–14 months later with detectable viral loads; 2 of these patients had been seen in the emergency department or urgent care clinic in the interim. Only one patient had a substantial lapse in care of 32 months. With regard to patients classified as out of care by clinic tracing and in care by surveillance (Table 3b), two patients remained virologically suppressed despite a lack of primary care visits during the 210 day period of interest, while three had no primary care visits and viral loads drawn in other contexts that were detectable.
Table 3a.
Patients who were Out of Care by Both Clinic Tracing and Surveillance (n=4)
| Demographics | Last Clinic Visit | Last Labs Prior to Loss to Follow-up | Last Lab Result | Missed Clinic Visit? | Date of Return to Clinic Care With Lab Result | Notes |
|---|---|---|---|---|---|---|
| 30-year-old male-to-female | September2010 | June 2010 | CD4 600 VL 5007 |
April2011 | May 2013 CD4 385 & VL 37, 324 |
Out of care for 32 months |
| 39-year-old male | August2012 | July 2012 | CD4 375 VL <40 |
February2013 | October 2013 CD4 353 & VL 2902 |
Emergency department visit February 2013 |
| 43-year-old male | July 2012 | July 2012 | CD4 399 VL <40 |
July2012 | May 2013 CD4 548 & VL 17,997 |
Urgent Care visit January 2013 |
| 41-year-old female | August 2012 | August 2012 | CD4 334 VL <40 |
January2013 | 7/13 from drug treatment program with VL<40 | Found by ascertainer |
Table 3b.
Patients who were Out of Care by Clinic Tracing and In Care by Surveillance (n=5)
| Demographics | Last Clinic Visit | Last Labs Prior to Loss to Follow-up | Last Lab Result | Missed Clinic Visit? | Date of Return to Clinic Care With Lab Results | Notes |
|---|---|---|---|---|---|---|
| 43-year-old male | April 2012 | October 2012 | CD4 561 VL <40 |
No | No | Planning move to Los Angeles |
| 49-year-old male | March 2012 | June 2012 | CD4 591 VL 1191 |
May 2012 | September 2013 – no labs available | Urgent Care & ED visits only |
| 47-year-old male | July 2012 | June 2012 | CD4 84 VL <40 |
March 2013 | April 2013 CD4 131 & VL <40 |
Remained on ART, pharmacy visit March 2013 |
| 47-year-old male | March 2013 | March 2013 | CD4 255 VL 433,863 |
Yes | May 2013 CD4 149 VL 9635 |
HIV specialty visits only |
| 40-year-old male | October 20122 | December 2012 | CD4 341 VL 21,793 |
January 2013 | April 2013 CD4 234 VL 40,878 |
Urgent Care visit |
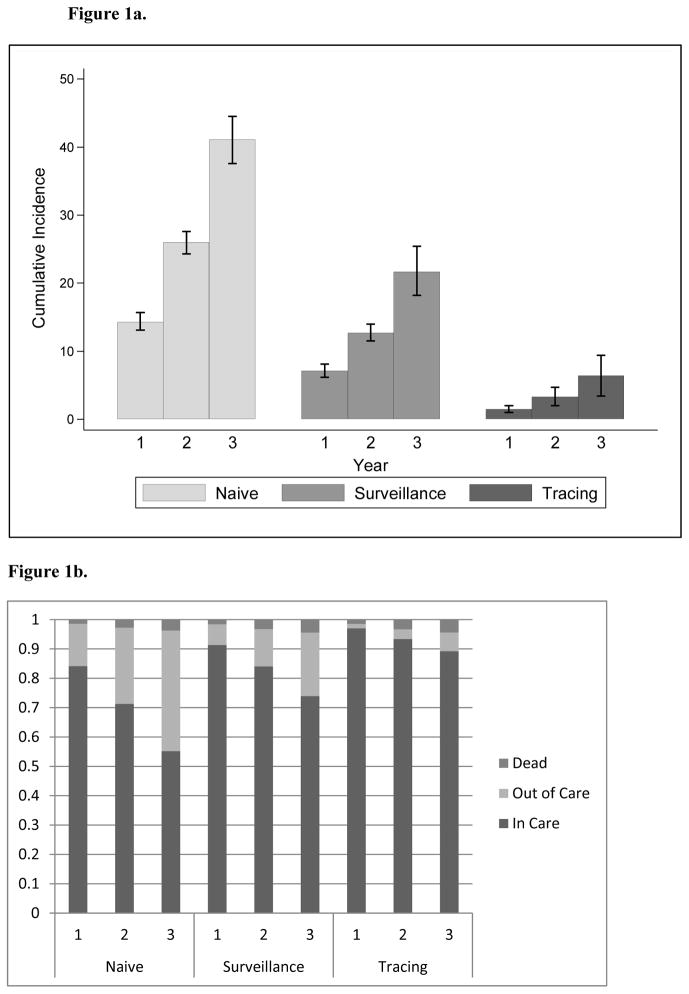
The naïve estimate of the cumulative incidence of disengagement from care (in which we considered being lost to follow up as equivalent to not being in care) for the active clinic cohort was 41.1% (Figure 1a) over three years (95% CI: 37.6%–44.5%). (The three-year time frame is a result of defining the active clinic cohort as of April 2010 and the lost to follow up group as of April 2013). This estimate is slightly different than the crude proportion of those lost to follow up (30%) as of April 2013 because the three-year estimate was scaled to three years of person-time, however, individuals could contribute less than three full years of person-time to the analysis since they entered and exited the cohort at different times. The second estimate utilizing SFDPH surveillance laboratory and death data dropped the cumulative incidence of disengagement to 21.7% over three years (95% CI: 18.2% to 25.4%). When estimates were further corrected using tracing outcomes and sampling probability weights, the estimated cumulative incidence of disengagement from care was only 6.4% over three years (95% CI: 3.4% to 9.4%). Similarly, the naïve estimate for those retained in care at three years was 55% (Figure 1b) while the use of surveillance data increased this estimate to 74% and the incorporation of clinic-based tracing resulted in a retention estimate of 89%.
Figure 1.
Figure 1a: Disengagement from HIV Care using three approaches to ascertaining outcomes. Cumulative incidence and 95% confidence intervals for disengagement from care at one, two and three years of observation and grouped by ascertainment approach. The “naïve” ascertainment approach uses only information documented in the clinic medical records and assumes those lost to follow-up are no longer in care. The “surveillance” approach supplements clinic-based information with HIV-related laboratory and vital status information routinely reported to the San Francisco County Department of Health. The “tracing” approach makes use of outcomes discovered through clinic-based effort to seek outcomes through phone calls and in-person visits to patient residences in the community.
Figure 1b: Mortality and care status using three approaches to ascertaining outcomes. The probability of death, remaining in care and being out of care estimated at one, two and three years of observation and grouped by ascertainment approach.
Discussion
In this study we used different approaches to supplement knowledge of care outcomes and found that while surveillance data enabled the assessment of in care status, clinic-based tracing further extended the ability to classify outcomes. Among patients who had unknown outcomes at the clinic, the combination of clinic-based tracing and HIV surveillance registry data allowed for ascertainment of outcomes on 83% of the traced sample. More specifically, when information from clinic-based tracing was combined with surveillance laboratory results during the period of interest as evidence of care, more than three-quarters of those thought lost to follow up were in care and only 4% were out of care. When assessed separately, surveillance data led to a four-fold greater proportion of patients classified as out of care than tracing. This difference was largely due to migration out of county that was undetected by routine HIV surveillance activities. Nevertheless, matching the lost to follow up list with surveillance data prior to tracing would have determined that approximately half of the patients thought to be lost to follow up and out of care were receiving care, substantially reducing the outreach burden. Streamlining outreach in this way is an important point to consider if clinic-based tracing is to be integrated into the job duties of an existing staff position.
The incremental benefit of combining data sources is also evident in the estimates of the cumulative incidence of disengagement from care at three years. The naive estimate, which considers the lost to follow up as out of care, is nearly twice that of the estimate using surveillance data; however, the use of clinic-based tracing data with sampling probability weights further reduces the estimate of the cumulative incidence of disengagement from care at three years to 6.4%. One limitation of our analysis is that the traced sample had 23 individuals whose outcomes were not classified by the ascertainer and therefore assigned weights of zero. However, these patients were demographically and clinically similar to those with identified outcomes. Moreover, 5 of 7 individuals with available surveillance data were suppressed, thus we would expect any bias from lack of tracking outcomes to be minimal. Another limitation is that the patients selected for tracing had a higher proportion of virologically suppressed patients than the untraced patients who were lost to follow up. This observation suggests that despite random sampling patients who had good adherence to ART and were motivated to stay in care may be over-represented among the traced group, potentially resulting in a lower weighted estimate of disengagement from care.
While the number of individuals estimated to be out of care by this study is much lower than national retention estimates, it is comparable to other jurisdictions such as Seattle, where HIV surveillance has investigated the care status of those lost to follow up through medical record review and in person outreach.17 However, it is worth noting that appointment attendance alone does not necessarily equal optimal engagement in treatment. Previous research in our clinic has demonstrated high mortality (~10%) among individuals with CD4 cell counts <350 copies/μL,23 indicating that being “in” care by appointment attendance does not fully explain patient outcomes.
Indeed, in considering these findings, a key point is that both “in care” and “out of care” represent a spectrum rather than a binary status. Not all in care or out of care states are the same. A small fraction of individuals presumably remained suppressed even though they were not having primary care visits. However, some of the individuals classified as in care by surveillance but out of care by tracing had detectable viral loads, demonstrating sub-optimal engagement with care. Most of those who were out of care, either by absence of primary care visits or absence of laboratory data, were still touching the health care system through other types of visits, notably to the emergency department and the urgent care clinic. These visits represent an opportunity for re-engagement in HIV primary care if identified. The importance of these locations as sites for re-engagement has been demonstrated by the Louisiana Public Health Information Exchange (LaPHIE) intervention.24 In a network of seven safety-net hospitals, the presentation of an HIV-diagnosed individual who had no CD4 or viral load result in the past 12 months triggered a real-time EMR alert to the clinician logging into the patient’s record, offering a menu of options for confirming care status and providing opportunities for re-linkage. Over a 27-month period, 549 LaPHIE alerts resulted in 85% of patients with at least six months of follow up receiving at least one subsequent CD4 and/or viral load test.25
While periodic “sweeps” of primary care attendance records may remain useful in assessing retention in clinic, primarily by triggering chart review and clinic-based outreach to investigate care status, this study demonstrated the low yield of in person tracing, suggesting that tracing is most likely to be effective shortly after a pattern of missed visits has been demonstrated. Indeed, the longer an individual was absent from the clinic, the less likely the ascertainer was to find them. Our study suggests that rather than wait for a six to twelve month assessment of retention status, a strategy to prevent loss to follow up by providing active outreach to catch individuals from falling completely out of care may be more effective. As such, our team is working on the development of a protocol in which a certain number of missed visits in combination with a shorter period of time away from clinic, e.g. three months, would trigger a standardized chain of outreach involving clinic phone calls, communication with case managers and the city jail, checks of HIV surveillance laboratory data, and, finally, in person tracing by a SFDPH-based team of retention experts. Retention remains the most challenging point of the HIV care cascade because rather than being a discrete event, it encompasses the lifelong experience of an individual living with HIV; moreover, some individuals with suboptimal or no care may not consider themselves as out of care.26
Our study also provides evidence that using routinely collected HIV surveillance data can enhance retention in care efforts. Community acceptance of the use of HIV surveillance data in support of retention in HIV care is necessary for the success of such initiatives. Concurrent with the development of our study, colleagues at Project Inform, a San Francisco-based HIV treatment advocacy organization, and the UCSF Center for AIDS Prevention Studies convened a national think tank with representatives from federal, state, and county government, health care, and community-based organizations to discuss existing models for the use of surveillance data to re-engage HIV-infected individuals in care and define best practices for meaningful stakeholder engagement. As described in this supplement,27 community representatives believed provider-led outreach to those lost to follow up maximized benefits while minimizing risks to HIV-infected individuals. We employed this approach in our study and among the patients located by the ascertainer, this type of in person outreach was highly acceptable.
Partnerships between public health surveillance and providers to re-engage HIV-infected individuals who are not in care have been endorsed by the Centers for Disease Control and Prevention as one of the strategies of high impact HIV prevention. “Data to Care” training manual and technical guidance are available on the website: http://www.effectiveinterventions.org/en/HighImpactPrevention/PublicHealthStrategies/DatatoCare.aspx. Our group was fortunate in that the logistics of combining datasets were relatively simple, which may not always be the case. We were able to successfully match 940 clinic patients to the SFDPH surveillance registry using name, medical record number, and date of birth in large part because SFDPH conducts active case surveillance at the clinic. As a result, surveillance is more likely to have complete and current identifiers and contact information. Successful matching may be more challenging in areas without the capacity for active surveillance or when matching lists of patients seen at medical facilities that report passively to HIV surveillance.
How to best use surveillance data to target clinic outreach merits further study. Not only are resources likely best focused on patients at risk for or with recent loss to follow up, but in addition, a more nuanced understanding of what surveillance laboratory data has been collected and what it can tell us is needed. For example, the presence of a viral load result is not necessarily enough to infer optimal engagement in HIV care, whereas knowledge that the viral load is suppressed may make this assumption more reasonable.
In summary, we found that using clinic-based tracing and surveillance data together provided better ascertainment of care status than using either method alone and resulted in much lower estimates of the number of individuals out of care. Use of surveillance data demonstrated the potential for increased efficiency in assessing retention in HIV care while clinic-based tracing improved accuracy. In addition, we provide additional proof of concept that clinics and HIV surveillance can work together effectively to bi-directionally share information. There were no logistical challenges in matching patients for outcome ascertainment and while the yield of in person outreach to those lost to follow up was low, it was highly acceptable. These findings should help develop new models for provider-public health partnerships in order to improve care and services for individuals living with HIV.
Acknowledgments
Source of Funding: Dr. Christopoulos has received investigator-initiated grant support from Bristol Myers Squibb. Supported by a supplement to the UCSF Center for AIDS Prevention Studies 5P30 MH062246 (S.F.M), K23MH092220 (K.A.C.) and R24 AI067039 (E.H.G).
The authors would like thank Lawrence Lowery for conducting patient outreach for this study.
Footnotes
Data were presented at the 9th International Conference on HIV Treatment and Prevention Adherence, Miami, Florida, June 8–10, 2014.
Conflicts of Interest: All other authors have no conflicts of interest.
Contributor Information
Katerina A. Christopoulos, Email: Katerina.Christopoulos@ucsf.edu.
Susan Scheer, Email: Susan.Scheer@sfdph.org.
Wayne T. Steward, Email: Wayne.Steward@ucsf.edu.
Revery Barnes, Email: Reverybarnes@gmail.com.
Wendy Hartogensis, Email: Wendy.hartnogensis@ucsf.edu.
Edwin D. Charlebois, Email: Edwin.Charlebois@ucsf.edu.
Stephen F. Morin, Email: Steve.Morin@ucsf.edu.
Hong-Ha M. Truong, Email: Hong-Ha.Truong@ucsf.ed.
Elvin H. Geng, Email: Elvin.geng@ucsf.edu.
References
- 1.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013 Jul 22;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 3.Mugavero MJ, Amico KR, Horn T, Thompson MA. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Oct;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- 4.Rajabiun S, Mallinson RK, McCoy K, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21( Suppl 1):S20–29. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 5.Gill MJ, Krentz HB. Unappreciated epidemiology: the churn effect in a regional HIV care programme. International journal of STD & AIDS. 2009 Aug;20(8):540–544. doi: 10.1258/ijsa.2008.008422. [DOI] [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed on December 7, 2014]. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009 Jan 15;48(2):248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010 Oct;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007 Jun 1;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 11.Dombrowski JC, Kent JB, Buskin SE, Stekler JD, Golden MR. Population-based metrics for the timing of HIV diagnosis, engagement in HIV care, and virologic suppression. AIDS. 2012 Jan 2;26(1):77–86. doi: 10.1097/QAD.0b013e32834dcee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torian LV, Xia Q, Wiewel EW. Retention in care and viral suppression among persons living with HIV/AIDS in New York City, 2006–2010. American journal of public health. 2014 Sep;104(9):e24–29. doi: 10.2105/AJPH.2014.302080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowan SE, Burman WJ, Johnson SC, et al. Engagement-in-care during the first 5 years after HIV diagnosis: data from a cohort of newly HIV-diagnosed individuals in a large US city. AIDS patient care and STDs. 2014 Sep;28(9):475–482. doi: 10.1089/apc.2013.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA : the journal of the American Medical Association. 2008 Aug 6;300(5):506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buskin SE, Kent JB, Dombrowski JC, Golden MR. Migration distorts surveillance estimates of engagement in care: results of public health investigations of persons who appear to be out of HIV care. Sexually transmitted diseases. 2014 Jan;41(1):35–40. doi: 10.1097/OLQ.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dombrowski JC, Buskin SE, Bennett A, Thiede H, Golden MR. Use of Multiple Data Sources and Individual Case Investigation to Refine Surveillance-Based Estimates of the HIV Care Continuum. Journal of acquired immune deficiency syndromes. 2014 Nov 1;67(3):323–330. doi: 10.1097/QAI.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buskin SE, Barash EA, Bauer AL, Kent JB, Garcia-Smith HR, Wood WW. HIV infected individuals presumed not to be receiving medical care: a surveillance program evaluation for investigations and referrals in Seattle, WA. Journal of HIV/AIDS Surveillance and Epidemiology. 2011;3(1) [Google Scholar]
- 18.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost or just not following up: public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care. AIDS. 2013 Sep 10;27(14):2271–2279. doi: 10.1097/QAD.0b013e328362fdde. [DOI] [PubMed] [Google Scholar]
- 19.Buchacz K, Chen MJ, Parisi MK, Yoshida-Cervantes M, Antunez E, Delgado V, Moss NJ, Scheer S. Using HIV Surveillance Registry Data to Re-Link Patients to Care: the RSVP Project in San Francisco. 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014; Boston. [Google Scholar]
- 20.West TL. [Accessed on October 24, 2014];Strategic Information in DC: Uses of Public Health Data for Evidence Based Programming. 2011 Available at: http://www.uchaps.org/assets/DC_Strategic_Info_West.pdf.
- 21.Coviello V, Boggess M. Cumulative Incidence Estimation in the Presence of Competing Risks. The Stata Journal. 2004;4(2):103–112. [Google Scholar]
- 22.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6(7):e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowdy DW, Geng EH, Christopoulos KA, et al. Mortality among antiretroviral-eligible patients in an urban public clinic. Journal of acquired immune deficiency syndromes. 2011 Aug 1;57(4):297–300. doi: 10.1097/QAI.0b013e31822233aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herwehe J, Wilbright W, Abrams A, et al. Implementation of an innovative, integrated electronic medical record (EMR) and public health information exchange for HIV/AIDS. J Am Med Inform Assn. 2012 May;19(3):448–452. doi: 10.1136/amiajnl-2011-000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnus M, Herwehe J, Gruber D, et al. Improved HIV-related outcomes associated with implementation of a novel public health information exchange. Int J Med Inform. 2012 Oct;81(10):e30–38. doi: 10.1016/j.ijmedinf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Christopoulos KA, Massey AD, Lopez AM, et al. “Taking a Half Day at a Time:” Patient Perspectives and the HIV Engagement in Care Continuum. AIDS patient care and STDs. 2013 Apr;27(4):223–230. doi: 10.1089/apc.2012.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans D, Van Gorder D, Morin SF, Steward WT, Charlebois ED. Acceptance of the Use of HIV Surveillance Data for Care Engagement: National and Local Community Perspectives. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]