Abstract
Synthetic drug-like molecules that directly modulate the activity of key clock proteins offer the potential to directly modulate the endogenous circadian rhythm and treat diseases associated with clock dysfunction. Here, we demonstrate that synthetic ligands targeting a key component of the mammalian clock, the nuclear receptors REV-ERBα and β, regulate sleep architecture and emotional behavior in mice. REV-ERB agonists induce wakefulness and reduce REM and slow-wave sleep. Interestingly, REV-ERB agonists also reduce anxiety-like behavior. These data are consistent with increased anxiety-like behavior of REV-ERBβ null mice, in which REV-ERB agonists have no effect Also consistent with these effects being mediated by REV-ERB, the effect of the agonist on sleep and anxiety was suppressed by lithium treatment. These results indicate that pharmacological targeting of REVERB may lead to the development of novel therapeutics to treat sleep disorders and anxiety.
Introduction
Circadian rhythms play an essential role in aspects of physiology and behavior including the sleep-wake cycle, body temperature, blood pressure, and renal function. At the molecular level these circadian rhythms oscillate as a function of a feedback loop in gene expression where heterodimers of BMAL1 and CLOCK (or NPAS2) (the positive limb) activate the expression of the Cryptochrome (CRY) and Period (PER) genes (the negative limb). Once CRY and PER have reached a critical level of expression they are able to block the stimulatory effect of the CLOCK/BMAL1 complex on their own promoters, completing the loop. The nuclear receptors REV-ERBα and REV-ERBβ play an important role in regulation of the molecular clock where they directly suppress the expression of genes of the positive limb of the clock including CLOCK, BMAL1, and NPAS21–3. Rev-erbα/Rev-erbβ-deficient mice display profound alterations in circadian behavior as well as expression of clock genes4.
Normal oscillations in metabolic function are regulated by the circadian clock and disruption in the normal function of the clock leads to metabolic disorders including diabetes, obesity, and atherosclerosis5–8. We recently demonstrated that synthetic REVERB agonists altered both central and peripheral clock gene expression as well as modulated the metabolic state in vivo 9,10. Pharmacological activation of REV-ERB led to increased energy expenditure, loss of fat mass and improved plasma lipid profiles in mice9. We also observed increased oxidative capacity in skeletal muscle in mice that was associated with increased exercise endurance after treatment with a REV-ERB agonist11. Thus, direct targeting of a molecular clock component can alter the metabolic state and improve metabolic function.
Dysregulation of the circadian rhythm is associated with many disorders including metabolic diseases and neuropsychiatric disorders including bipolar disorder, anxiety, depression, schizophrenia and sleep disorders 12,13,14,15. In this report, we examined the effects of REV-ERB agonists on patterns of sleep and wakefulness and found that the compounds increase wakefulness. Furthermore, during this analysis we found that the compounds also decreased anxiety-like behavior. Thus, we hypothesize that targeting components of the mammalian clock with small molecules may provide therapeutics for treatment of sleep disorders and anxiety disorders.
RESULTS
Regulation of Sleep by Synthetic REV-ERB Agonists
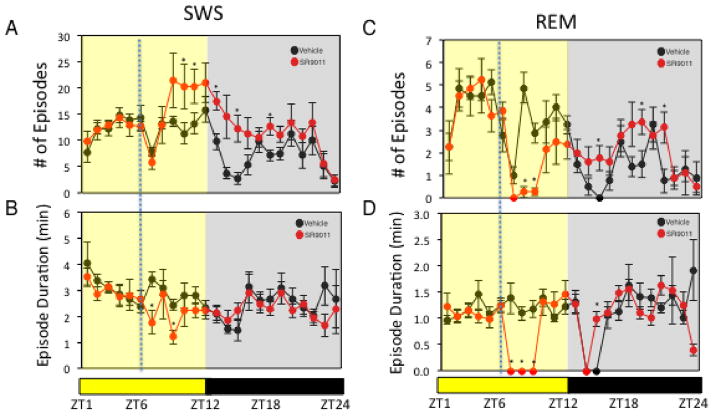
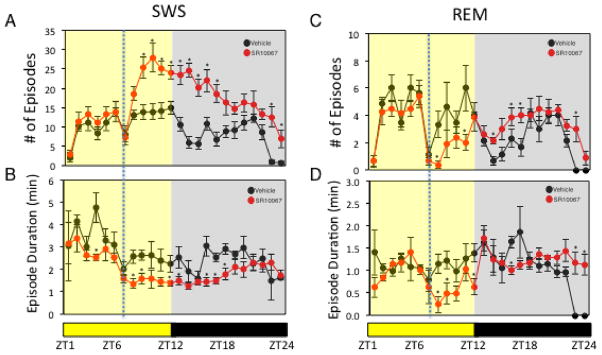
The “master” circadian clock located in the suprachiasmatic nucleus of the hypothalamus plays an essential role in regulation of sleep16 and given this close relationship, we sought to investigate the effect of pharmacological activation of REV-ERB on patterns of sleep and wakefulness. Although we observed alterations in circadian wheel running activity previously9, the effect on sleep was not clear. In order to investigate this we administered a REV-ERB agonist, SR9011, to mice and examined the effect on sleep and wakefulness by electroencephalogram (EEG). As illustrated in Fig. 1A, mice displayed a normal nocturnal profile with low wakefulness and high slow wave sleep (SWS) and paradoxical sleep – rapid eye movement (REM) sleep during the daytime (zeitgeiber time 0 (ZT0) to ZT12). Mice were administered SR9011 (100 mg kg−1, i.p.) or vehicle at ZT6 when Reverb expression is at its peak9. SR9011 treated mice displayed a large increase in wakefulness that was maintained for 2h post injection (Fig. 1A top panel). As expected this corresponded to a decrease in SWS and REM sleep during the same time period (Fig. 1A bottom panels). Latency to enter SWS and REM sleep after administration of SR9011 was increased as illustrated in Fig. 1B. At the onset of the dark period (ZT12) vehicle treated mice displayed a normal, rapid increase in wakefulness (and decrease in SWS and REM sleep) while this effect was delayed in SR9011-treated mice (Fig. 1A). A normal pattern of sleep was observed after this recovery period approximately 12h after the initial injection. Analysis of sleep architecture after the single injection of SR9011 at ZT6 revealed effects on both SWS and REM sleep architecture (Fig. 2). Following injection of SR9011 the number of episodes of SWS increased while their duration was shortened (Fig. 2A & 2B). REM sleep was also affected and was more impressive with REM sleep episodes and duration being nearly completely suppressed for 3 hours following administration of SR9011 (Fig. 2C & 2D). The sleep recovery period that was observed following transition to dark was also observed in the sleep architecture. Episodes of SWS were increased in SR9011 treated mice from ZT13-15 while SWS duration remained constant (Fig. 2A & 2B). Episodes of REM sleep were also elevated after transition to dark with SR9011 treatment (Fig. 2C). No effect of SR9011 treatment was observed on EEG power (Supplementary Fig. 1A).
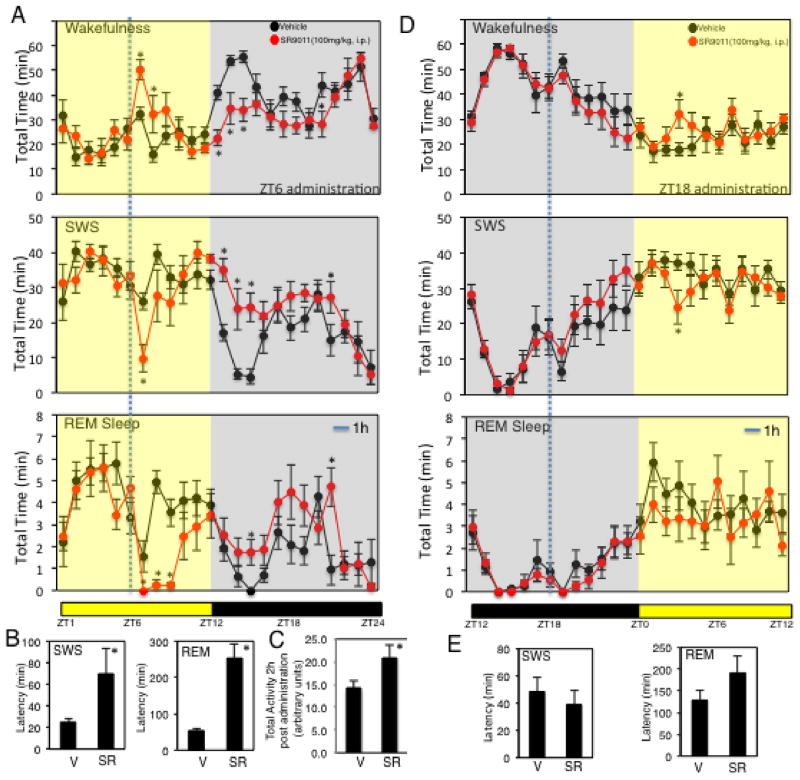
Figure 1. SR9011 Induces Wakefulness and Suppresses Sleep.
A, Mice injected with The REV-ERB agonist SR9011 (i.p. 100 mg kg−1) at ZT6 display an increase in wakefulness as detected by EEG. B, SR9011 increases SWS and REM sleep latency. C, Locomotor telemetry data indicating an increase in movement during the period 2h after ZT6 injection of SR9011. D, Mice injected with SR9011 (i.p. 100 mg kg−1) at ZT18 fail to demonstrate increased wakefulness. E, SR9011 does not alter SWS and REM sleep latency (ZT18 injection). Data are expressed mean ± SEM. All EEG graphs are plotted per 1 h for a 24 h period. Values are mean ± SEM (n=8 for EEG studies (A,B,D,E) and n=6 for telemetry studies (C)). Within the EEG studies (wakefulness, SWS and REM sleep), potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test. In panels examining latency and activity, differences between treatment groups (vehicle vs. SR) were assessed by a two tailed t test (Student’s) with significance *P < 0.05. In all experiments mice were maintained on a 12h:12h L:D cycle.
Figure 2. Administration of SR9011 Alters Sleep Architecture.
Effect of SR9011 administered at ZT6 (analysis of data from Figure 1A) on SWS (number of episodes (A) and episode duration (B)) and on REM sleep (number of episodes (C) and episode duration (D)). potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test with significance *P < 0.05
In a separate experiment, we implanted mice with transmitters to detect locomotion by telemetry. Using the same paradigm (12h:12h L:D and injection at ZT6) we monitored locomotor activity following injection of SR9011 or vehicle. Mice receiving SR9011 displayed considerably more locomotor activity following injection than vehicle consistent with an increase in wakefulness (Fig. 1C). The telemetry units we utilized also enabled measurement of core temperature and we assessed core temperature under the same paradigm that we assessed sleep – wakefulness patterns. As shown in Supplementary Figure 2 we observed the expected circadian pattern of body core temperature with higher temperatures noted during periods of darkness that are associated with wakeful mice. Upon administration of SR9011 or vehicle at ZT6 we observed an abrupt increase in core temperature, which we attribute to the waking of the animals due to administration that we observed in the EEG as well (Supplementary Fig. 2A). Following administration, mice treated with vehicle displayed core temperatures that returned to levels consistent with pre-administration levels, however, core temperatures in the SR9011-treated mice remained elevated relative to vehicle treated mice for ~2 hours (Supplementary Fig 2A). This elevation was approximately 0.5°C, which is smaller than the elevation typically observed with wakefulness during the dark period. Interestingly, there was no difference in core temperatures of mice treated with vehicle or SR9011 during the dark period where we have clearly observed a sleep recovery period by EEG. This indicates that although the mice are recovering in terms of sleep, the core temperature is elevated to levels equivalent to wakefulness.
Next we examined the effect of a REV-ERB agonist on sleep patterns when administered during the animals’ wakeful period. SR9011 or vehicle was administered to mice at ZT18, a time when the percentage of mice in a wakeful state is very high. We observed no distinction in sleep – wakefulness patterns between SR9011 and vehicle treatment and furthermore, beginning at ZT0 the mice entered their sleep phase normally (Fig. 1D). Latency to enter SWS and REM sleep was unaffected when the REV-ERB agonist was administered at ZT18 (Fig. 1E). Thus, our data indicate that pharmacological activation of REV-ERB leads to increased wakefulness, but this effect is dependent upon the time of day the compound is administered.
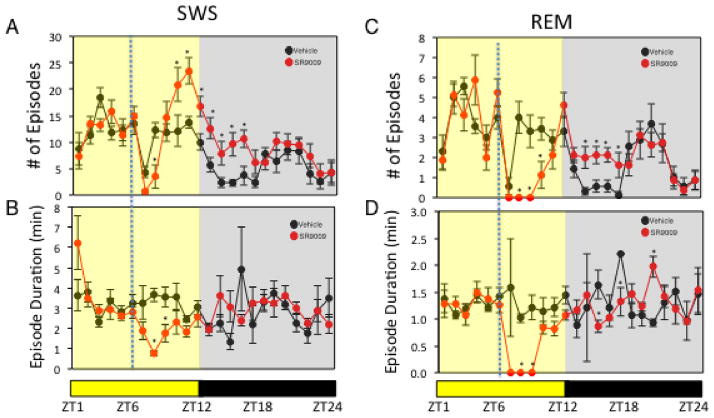
In order to further assess the specificity of this effect for REV-ERB agonists, we examined the effects of a distinct synthetic REV-ERB agonist, SR9009, on sleep architecture under L:D conditions. As shown in Fig. 3A, the effect of SR9009 was very similar to SR9011 with induction of wakefulness and suppression of SWS and REM sleep followed by a recovery period during the subsequent dark period. Latency to enter SWS and REM sleep was also extended following SR9009 injection (Fig. 3B). Similar to SR9011 treated mice, mice treated with SR9009 returned to normal patterns of wakefulness/sleep 12h after administration. Assessment of sleep architecture revealed a similar profile to that observed with administration of SR9011. After administration of SR9009 at ZT6 there was a general increase in episodes of SWS with a decrease in episode duration (Fig. 4A & 4B). The only distinction between SR9011 and SR9009 is a short delay in the increased SWS episode number where the number of episodes is actually decreased for one time point. REM sleep is also similarly affected with REM sleep episodes and episode duration suppressed following administration of SR9009 (Fig. 4C & 4D). The effect on sleep recovery was also observed after SR9009 treatment where the number of episodes of SWS and REM sleep were elevated after transition to the dark period (Fig. 4A and 4C). No effect on EEG power was observed (Supplementary Figure 1B).
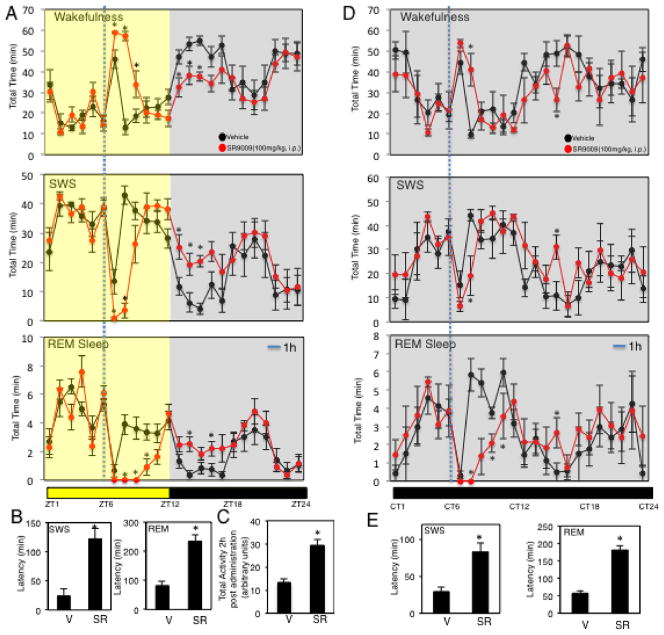
Figure 3. SR9009 Induces Wakefulness and Suppresses Sleep.
A, Mice injected with the REV-ERB agonist SR9009 (i.p. 100 mg kg−1) at ZT6 display an increase in wakefulness as detected by EEG. B, SR9009 increases SWS and REM sleep latency C, Locomotor telemetry data indicating an increase in movement during the period 2h after ZT6 injection of SR9011. Mice in A, B, and C were maintained under 12h:12h L:D conditions. D, Mice injected with SR9009 (i.p. 100 mg kg−1) at CT6 under constant dark conditions show increased wakefulness. E, SR9009 increases SWS and REM sleep latency when injected at CT6 under constant dark conditions. Data are expressed mean ± SEM. All EEG graphs are plotted per 1 h for a 24 h period. Values are mean ± SEM (n=8 for EEG studies and n=6 for telemetry studies). (C)). Within the EEG studies (wakefulness, SWS and REM sleep), potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test. In panels examining latency and activity, differences between treatment groups (vehicle vs. SR) were assessed by a two tailed t test (Student’s) with significance *P < 0.05. Experiments were performed at least twice.
Figure 4. Administration of SR9009 Alters Sleep Architecture.
Effect of SR9009 administered at ZT6 (analysis of data from Figure 3A) on SWS (number of episodes (A) and episode duration (B)) and on REM sleep (number of episodes (C) and episode duration (D)). potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test with significance *P < 0.05
With this particular experiment, we continued to observe the pattern of sleep – wakefulness for 48 additional hours to determine if there was any effect on circadian patterns of sleep. As shown in Supplementary Figure 3, we did not observe any alterations in the pattern of sleep in response to SR9009 after the 1st day of administration. Assessment of core body temperature following administration of SR9009 yielded similar results as did SR9011 administration (Supplementary Figure 2B). Immediately following administration of SR9009 core temperature was elevated, but not to levels that are normally associated with wakefulness during darkness. Additionally, as observed with SR9011 administration, increased sleep after transition to the dark period was not associated with lower core temperatures (Supplementary Fig. 2B).
Assessment of locomotion by telemetry in mice injected with SR9011 or vehicle revealed a response that was consistent with increased wakefulness induced by SR9011 treatment (Fig. 3C). Previously, we demonstrated that the effects of REV-ERB agonists on wheel running behavior were distinct based on whether the mice were maintained on a 12h:12h L:D cycle or in complete darkness9. We performed an identical experiment to that described in Fig. 3A monitoring EEG except the animals were maintained in complete darkness, which was initiated 2 days prior to injection. The effects of SR9009 administration at CT6 under constant darkness were very similar to those obtained under L:D conditions with increased wakefulness and decreased SWS and REM sleep observed (Fig. 3D). Increased latency to enter SWS and REM sleep following injection was also observed with SR9009 treatment (Fig. 3E).
Regulation of Anxiety by Synthetic REV-ERB Agonists
Polymorphisms in clock genes (RORA, CRY1, RORB, NR1D1, NPAS2, CRY2, PER2, PER3, BMAL1) have been linked to bipolar, depressive and anxiety disorders in human 17–24. Thus, the ability to modulate the patterns of expression of the clock genes by the REV-ERB agonists may provide a mechanism to influence these disorders. Mice with a disrupted Clock gene display a behavioral profile similar to human mania that included hyperactivity, decreased sleep, lowered anxiety, and an increase in reward value for cocaine25. A second group established that the Clock mutant mice exhibited increased exploratory activity and escape seeking behavior consistent with lower levels of anxiety26. The positive limb of the circadian clock is under direct positive transcriptional control by the retinoic acid receptor-like orphan receptors (RORs)27 and consistent with loss of Clock gene expression associated with reduced anxiety-like behavior, mice harboring a null mutation in the Rorβ gene also are less prone to display anxiety-like behavior28. These data suggest that targeting components of the molecular clock may be an effective method to influence anxiety behavior. More specifically, based on the genetic data described above, suppression of the positive limb of the clock, such as by activation of REV-ERB activity, may suppress anxiety.
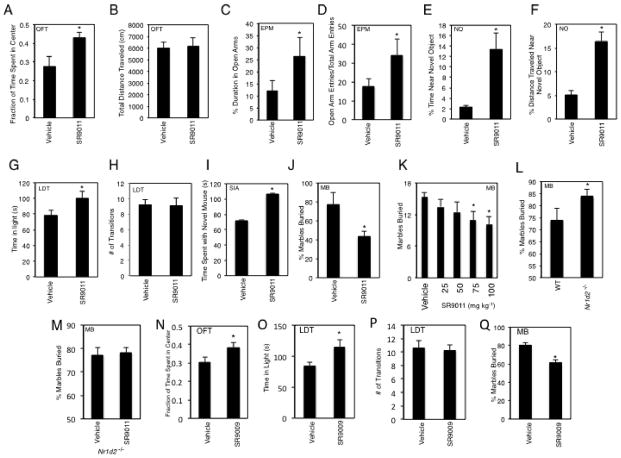
In previous experiments with S9011 and SR9009 where we examined the effects of REV-ERB agonism on metabolic rate9 (chronic twice per day dosing (b.i.d.) (ZT0 and ZT12)) we noted alterations in behavior that suggested alterations in anxiety-like behavior. Based on the links between the circadian rhythm and anxiety discussed above as well as our preliminary observations, we examined the effects of REV-ERB agonists on anxious behavior more closely. Mice were administered SR9011 chronically as was performed for previous assessments of effects on metabolic parameters (3–10 days; 100 mg kg−1, b.i.d., i.p.; details provided in the Methods as illustrated in Supplementary Figure 4) prior to beginning an open field assay to assess anxiety-like behavior. In an open field assay, mice treated with SR9011 spent considerably more time in the center of the field, but displayed no change in total distance traveled (Figs. 5A and 5B). This anxiolytic effect was confirmed in several other behavioral assays. Consistent with the anxiolytic activity of SR9011, mice treated with the drug spent considerably more time in the open arms in an elevated plus maze assessment (Fig. 5C). Additionally, the mice displayed a greater number of open arm entries (Fig. 5D). We also assessed the effect of SR9011 on neophobia exhibited by mice in a novel object assay. Mice treated with SR9011 spent 6.5-fold more time near the novel object consistent with anxiolytic activity (Figs. 5E & 5F). Anxiolytic activity was also assessed in a light-dark box assay and as shown in Fig. 5G. Mice administered SR9011 spent considerably more time in the light portion of the box, again consistent with anxiolytic activity of the REV-ERB agonist. Mice displayed no change in the number of transitions between the two boxes in this assay (Fig. 5H). In a social interaction assay (SIA) mice treated with SR9011 displayed increased interaction with a novel mouse relative to vehicle treated animals (Fig 5I). Finally, we assessed the effect of the drug in the marble burying assay, where mice are introduced into cages with marbles on the surface of the bedding. Mice will naturally bury the marbles to remove the stressful stimuli and effects of anxiolytics can be readily assessed by reduced marble burying activity. As demonstrated in Fig. 5J, mice administered SR9011 buried only 50% of the marbles that mice administered vehicle did. This anxiolytic effect was dose-dependent (Fig. 5K) and the potency (ED50=61 mg kg−1) compares favorably to the potency of SR9011 effects on circadian rhythm (ED50=56 mg kg−1) and gene expression in vivo (ED50=67 mg kg−1)9. We also observed anxiolytic activity of SR9009 in several of these assays (Figs. 5N–5Q). The magnitude of effects we observed with SR9011 and SR9009 was similar to those observed when mice were treated with a benzodiazepine (chlordiazepoxide, CDP) (Supplementary Fig. 5). These data, taken together, suggest that pharmacological activation of REV-ERB reduces anxiety-like behavior while increasing wakefulness rather than decreasing wakefulness as do most anxiolytic agents. Based on our initial observations of potential anxiolytic activity of SR9011 in chronically dosed mice in metabolic studies, we performed the range of experiments described above using chronically dosed mice. However, we also examined the potential acute anxiolytic effect of SR9011 in the marble burying assay by administration of 100 mg kg−1 SR9011 followed by initiation of the 30 min assay ½ hour after administration. We observed no alteration in marble burying activity (data not shown) suggesting that chronic administration is required for the anxiolytic activity.
Figure 5. REV-ERB Regulates Anxiety-Like Behavior.
SR9011 displays anxiolytic activity in a range of behavioral assays. A, Results from the open field assay demonstrating that mice administered SR9011 spend a significantly greater amount of time in the center field during the first 5 minutes after placing the animals in the apparatus than mice treated with vehicle. B, SR9011 treated mice display equivalent locomotor activity as vehicle treated mice in the open field assay. Results from elevated plus maze demonstrates that SR9011 treated mice spend a greater percentage of time exploring the open quadrants (C) and exhibit a greater frequency of open arm entries (D) than vehicle treated mice. Results from the novel object (neophobia) assay demonstrating that SR9011 treated mice spend more time near the novel object (E) and travel more distance near the novel object (F) than vehicle treated mice. Results from the light–dark box assay demonstrating that mice treated with SR9011 spend considerably more time in the light box than vehicle treated mice (G) while displaying no difference in the total number of transitions between the boxes (H). Results for the social interaction assay indicating that mice treated with SR9011 display greater social interaction with a novel mouse than mice treated with vehicle (I). Results from the marble burying assay demonstrate that SR9011 treated mice bury fewer marbles than vehicle treated mice (J). and this effect is dose-responsive (K). L, Rev-erbβ−/− mice display more anxiety in the marble burying assay consistent with data indicating that activation of REV-ERB leads to anxiolytic activity. M, The REV-ERB agonist, SR9011, does not display anxiolytic activity in Rev-erbβ null mice. Rev-erbβ null mice were treated with SR9011 in an identical manner as described in L and subjected the marble burying assay to examine the potential anxiolytic activity of SR9011. Consistent with the actions of SR9011, a distinct REV-ERB agonist, SR9009, also reduces anxiety-like behavior in the open field assay (N) the light-dark transition assay (O and P) and the marble burying assay (Q). Values are mean ± SEM, n=12 mice per group for all experiments except the marble burying test where n=8. With the exception of panel K differences between treatment groups (vehicle vs. SR) were assessed by a two-tailed t test (Student’s) with significance *P < 0.05. In panel K, differences between groups were assessed using one-way ANOVA followed by Tukey’s post hoc test with significance *P<0.05.
We hypothesized that if the effects of SR9011 and SR9009 on reducing anxiety-like behavior were indeed mediated by REV-ERB that mice lacking the receptor may be more anxious and that the compound would lack activity in the null mouse. In order to investigate this we generated an nr1d2 null mouse (Supplementary Figure 6) and examined its activity in the marble burying assay. As shown in Fig. 5L, these mice displayed greater marble burying activity consistent with increased anxiety-like behavior. Furthermore, treatment the null mice with SR9011 did not alter their behavior (Fig. 5M) indicating that the actions of the compound were at least partially mediated by REVERBβ.
REV-ERB Agonists Alter Reward Seeking Behavior
Many anxiolytics, such as benzodiazepines, are associated with abuse liability related to their positive reinforcing ability. This can be detected in rodents using a conditioned place preference (CPP) assay29–32. We assessed the activity of SR9011 in the CPP assay using an identical dose that displayed anxiolytic activity and found no conditioned place preference or aversion (Fig. 6A) suggesting that anxiolytics that target REV-ERB may display lower abuse potential than benzodiazapenes. Interestingly, we previously noted that administration of REV-ERB agonists results in loss of wheel running activity even though locomotor behavior was not affected9. The EEG data described above confirm this observation demonstrating that the mice are wakeful and exhibit locomotor activity during periods where we have observed loss of wheel running activity. This suggested to us that the REV-ERB agonists might be altering motivation to run on the wheel. Wheel running in rodents is a well-defined rewarding behavior33–35 and thus we hypothesized that REV-ERB agonists may be interfering with the central reward pathway. To investigate this we performed an additional CPP assay where we compared the effect of administration of cocaine alone to coadministration of SR9011 with cocaine. As illustrated in Fig. 6B, a clear condition place preference was observed with cocaine that was inhibited by coadministration of SR9011. These data suggest that REV-ERB agonists suppress the rewarding behavior associated with wheel running and cocaine, yet the agonist by itself does not induce aversive behavior.
Figure 6. REV-ERB Activation Alters Reward Seeking Behavior.
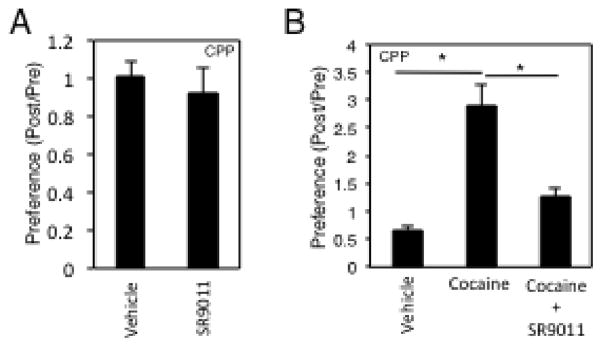
A, Results from a conditioned place preference (CPP) assay indicating lack of conditioned place preference or aversion activity of SR9011. Differences between treatment groups (vehicle vs. SR) were assessed by a two-tailed t test (Student’s) with significance *P < 0.05. B, Results of a CPP assay indicating that SR9011 coadministration inhibits the conditioned place preference displayed by cocaine. Differences between groups were assessed using one-way ANOVA followed by Tukey’s post hoc test with significance *P<0.05. Values are mean ± SEM, n=12 mice per group for all experiments.
Mice harboring a mutation in the clock gene have been shown to display a behavioral profile similar to human mania25. Furthermore, polymorphisms in the human gene encoding REV-ERBα (NR1D1) are associated with bipolar disorder 21. As previously discussed, REV-ERB agonists would be expected to reduce clock expression levels and may mimic the effect observed in these mice. According to the DSM V, a manic episode in humans is characterized by the presence of at least 3 symptoms of 7 that include reduced anxiety (elated mood) and increased wakefulness leading us to consider if the REV-ERB agonists may be inducing a behavioral profile similar to mania. Clearly, we observe reduced anxiety (Fig. 5) and increased wakefulness (Figs. 1 & 3). However, we do not observe an effect on depression like behavior in a tail suspension assay (Supplementary Figure 7) and we also observe a distinctly opposite effect on the reward value of cocaine (Fig. 6B).
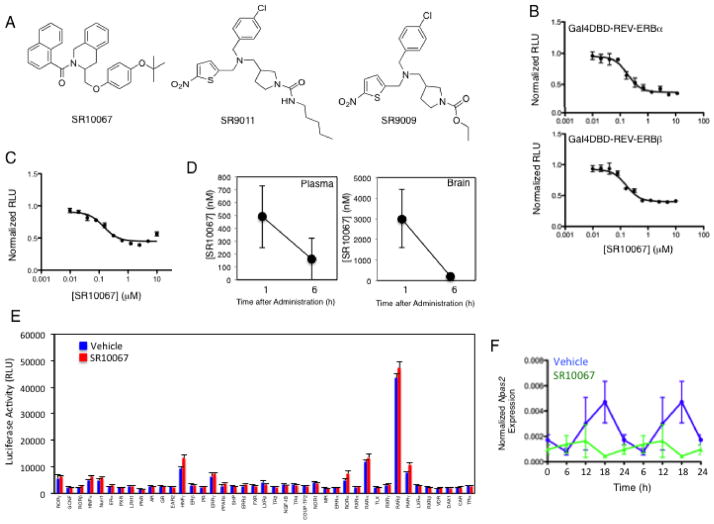
SR10067 is a Novel REV-ERB Agonist with high affinity
SR9011 and SR9009 were the first synthetic REV-ERB agonists developed with sufficient pharmacokinetic properties to be used as in vivo chemical tools9. However, they exhibit relatively low potency for the receptor with IC50’s of approximately ~700 nM9. We hypothesized that if activation of REV-ERB with synthetic agonists did indeed lead to the wakefulness and anxiolytic effects that development of higher affinity REVERB agonists with either equivalent or improved pharmacokinetic properties should yield improvements in the in vivo potency. In order to test this hypothesis, we evaluated a synthetic REV-ERB agonist (SR10067) derived from extensive modification of the SR9009/SR9011 scaffold37,38 (Fig. 7A and Supplementary Fig. 8 & 9) that displays substantially greater potency than SR9011 and SR9009 in vitro. The potency of SR10067 is considerably better than SR9011 and SR9009 in the Gal4DBD-REV-ERB ligand binding domain (LBD) cotransfection assay (SR10067: REV-ERBα IC50=170 nM, REV-ERBβ IC50=160 nM (Fig. 7B) vs. SR9011: REV-ERBα IC50=670 nM, REV-ERBβ IC50=800 nM9). Potency of SR10067 was also considerably better in a cotransfection assay utilizing full-length REV-ERBα along with the BMAL1 promoter luciferase reporter (SR10067 IC50=140 nM (Fig. 8C) vs. SR9011 IC50=620 nM9). SR10067 displayed no significant activity at any other nuclear receptor (Fig. 7E) or a range of other receptors, ion channels and transporters assessed in the NIMH Psychoactive Drug Screening Program (Supplementary Table 1. Assessment of plasma and brain concentrations of SR10067 one and six hours after i.p. injection (30 mg kg−1) revealed that levels of the compound remain above the IC50 for the receptor six hours after administration (Fig. 7D). As a marker of in vivo efficacy of REV-ERB agonists we previously demonstrated that a single injection of SR9011 or SR9009 suppressed the circadian rhythm of Npas2 gene expression in the mouse hypothalamus9, and when we performed this experiment with SR10067 we observed similar results (Fig. 7F). Synthetic REV-ERB agonists also suppress circadian wheel running activity after a single injection9, and this was observed with SR10067. As illustrated in Figs. 8A and 8B, administration of SR10067 at various concentrations yielded a dose-dependent effect on reduction in nocturnal wheel running activity as indicated in the actograms. The ED50 for suppression of wheel running activity was 20 mg kg−1 (Fig. 8B), which is more potent than that described for SR9011 (56 mg kg−1)9 and is consistent with the improved potency of SR10067 in the REV-ERB transcriptional assays. SR10067 induced wakefulness and reduced SWS and REM sleep when injected at ZT6 similar to what was observed with SR9011 and SR9009 (Fig. 8C). The effect of SR10067 on sleep architecture was also similar to what was observed with SR9011 and SR9009 with increased number of episodes of SWS with decreased duration and decreased number of episodes and duration of REM sleep (Fig. 9). No effect on EEG power was observed (Supplementary Figure 1C). SR10067 also induced locomotion when administered into mice at ZT6 in a manner similar to SR9009 and SR9011 consistent with an increase in wakefulness (Fig. 8D). The anxiolytic activity of SR10067 was assessed in the marble burying anxiety assay and SR10067 displayed superior potency than SR9011 (ED50 of 12 mg kg−1vs. 61 mg kg−1) (Fig. 8E). This 5-fold improvement in in vivo anxiolytic potency compares favorably with the in vitro improvement in potency (4–5 fold) when comparing SR10067 to SR9011. These data clearly suggest that the anxiolytic activity is mediated by activation of REV-ERB given that two synthetic REV-ERB selective agonists, with distinct chemical structures, display anxiolytic activity that correlates to their relative activity at REV-ERB.
Figure 7. The Activity of a REV-ERB Agonist is Suppressed by Treatment with Lithium.
A, Treatment with lithium results in suppression of the wakefulness inducing effects of SR9009. Mice were maintained on either water or water with lithium chloride as described in the methods and vigilance states were monitored by EEG. Vehicle injection or SR9009 injection (100 mg kg−1, i.p.) is indicated. N=6 mice per group. B, Treatment with lithium suppresses the anxiolytic activity of SR9011 in a marble burying assay. Mice were maintained on either water or water with lithium chloride and treated with vehicle or SR9011 (100 mg kg−1 b.i.d., i.p.) as described in the methods. n=8 mice per group. Within the EEG studies (wakefulness, SWS and REM sleep), potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test. In the marble burying assay differences between treatment groups were assessed by a two tailed t test (Student’s) with significance *,P < 0.05.
Figure 8. Identification of SR10067 as a highly potent synthetic REV-ERB agonist.
A, Chemical structure of the synthetic REV-ERB agonist SR10067 compared to SR9011 and SR9009. B, Results from a Gal4-REV-ERB/Gal4 UASX5 luciferase reporter cotransfection assay in HEK293 cells displaying the potent REV-ERB agonist activity of SR10067. C, Results from a cotransfection assay in HEK 293 cells with full-length REVERBα and a BMAL1 promoter driven luciferase reporter displaying the potent agonist activity of SR10067. D, Plasma and brain concentrations of SR10067 2h following i.p. injection of 30 mg kg−1 of the compound. The 6h value for SR10067 in the brain is 150 ± 20 nM. E, Nuclear receptor specificity assay illustrating lack of activity of SR10067 on a wide range of nuclear receptors. The format of the assay was a cotransfection assay with Gal4 DNA binding domain – nuclear receptor fusions in HEK293 cells as previously described (see Methods). SR10067 was tested at 20 μM. Error bars indicate mean ± s.e.m. and n=3. There were no statistical differences between vehicle and drug treatment in any of the assays shown as assessed by a Student’s t test (unpaired two-tailed). F, Normalized (to Gapdh) expression of Npas2 mRNA isolated from the hypothalamic of mice injected with 30 mg kg−1 of SR10067 (i.p. at ZT0) demonstrating a SR10067-dependent alteration in the circadian rhythm of expression. Expression was monitored over 24h and the results are double plotted. n=5. *P<0.05. Mean ± SEM.
Figure 9. SR10067 Alters Sleep Architecture and Anxiety-like Behavior.
A, Actograms from wheel running cages demonstrating the effect of SR10067 injection (red bars, i.p. various concentrations) on wheel running activity in mice. B, Assessment of the dose-dependence of inhibition of wheel running activity in mice during the entire dark phase following administration of SR10067. n=6 to 8 mice per group. C, Assessment of the effect of SR10067 on wakefulness in mice. Wakefulness, SWS and REM sleep was monitored by EEG as indicated in Figs. 1 & 2. Mice were injected with SR10067 (30 mg kg−1, i.p.) or vehicle at ZT6. n=8 mice. D, Locomotor telemetry data indicating an increase in movement of mice during the period 2h after ZT6 injection of SR10067 (30 mg kg−1). n=6 mice. E, Results from the marble burying assay demonstrating that SR10067 dose-dependently reduces anxiety like behavior in the marble burying assay. n=8 mice. Values are mean ± SEM. In panel D differences between treatment groups (vehicle vs. SR) were assessed by a two tailed t test (Student’s) with significance *P < 0.05. In panels B and E, differences between groups were assessed using one-way ANOVA followed by Tukey’s post hoc test with significance *P<0.05. In panel C, potential differences between treatments were assessed by repeated measure two-way ANOVA followed by Bonferroni post hoc test with significance *P < 0.05.
DISCUSSION
The mammalian clock maintains the circadian rhythm that is essential for normal physiological function. Mutation of components of the clock alters normal physiological processes at multiple levels including sleep, metabolism, behavior, etc.7,39,40. Alteration of the normal circadian rhythm in humans due to shift work or experimental manipulation alters the metabolic and mental health of individuals. Additionally, circadian disruption is commonly noted in a range of psychiatric disorders including anxiety, schizophrenia, and depression12–15. It has been unclear if molecular components of the mammalian clock could be targeted for development of therapeutics, and recently small molecule regulators of the core clock have been discovered and developed10,41. Small molecule inhibitors42 and activators43 of CRY have been discovered and regulate the circadian rhythm in cell lines, but none have progressed to evaluation in animal models to determine their potential utility for treatment of disease. It has been suggested that REV-ERB be considered as a core component of the clock since mice lacking both REV-ERBα and REV-ERBβ are arrhythmic4. Our recent study demonstrated that synthetic REV-ERB agonists alter the circadian rhythm and improve the metabolic profile in mouse models of metabolic disease. Here, we show that pharmacological targeting of the core clock has further value demonstrating the ability of REV-ERB agonists to modulate sleep and emotional behavior.
In mice treated with various REV-ERB agonists, we observed alterations in patterns of sleep-wakefulness, sleep architecture, and the level of anxiety. Clock genes have been demonstrated to play an important role in regulation of sleep. Bmal1 null mice display increased sleep and this sleep displays increased fragmentation44. Clock mutant mice display reduced non-REM sleep while Cry1/Cry2 null mice display an increase in non-REM sleep45,46. Npas2 null mice as well as Per null mice also display alterations in the patterns of sleep47–49. Given the role that REV-ERB plays in regulation of these genes, we believed there would be an effect of pharmacological modulation of REV-ERB activity on sleep. All of the studies examining the effect of clock genes on sleep are genetic loss of function studies and not pharmacological studies, thus we were not sure what to expect with the first examination of pharmacological targeting of a clock gene on sleep. Administration of REV-ERB agonists stimulates wakefulness and suppresses sleep. This effect is acute, which is consistent with the short half-life of the compounds and the mice return to a normal sleep pattern within approximately 12h. The lack of effect on long term circadian behavior is consistent with our previous data indicating that REV-ERB agonists only transiently altered circadian wheel running behavior followed to a return to a normal circadian rhythm9. Sleep disorders affect approximately 70 million individuals in the United States and therapeutics like the REV-ERB agonists that induce wakefulness while also reducing anxiety should be examined for treatment of shift work sleep disorder and sleep apnea.
Previously, we demonstrated that REV-ERB agonists increase the metabolic rate and are quite effective in increasing oxidative metabolism in skeletal muscle9,11. This would likely be associated with an increase in core body temperature, however, a recent study also demonstrated that Ucp1 is a direct REV-ERB target gene and that in brown adipose tissue loss of REV-ERBα leads to a decrease in thermogenic brown adipose tissue activity50. Thus, it was unclear what the summation of effect of pharmacological activation of REV-ERB on core body temperature would be. We observed that pharmacological activation of REV-ERB led to a transient increase in core body temperature when administered at ZT6, but the elevation of temperature was not equivalent to that observed during normal wakefulness in the dark period. Interestingly, in the transition to darkness when mice administered REV-ERB agonists displayed sleep recovery they also displayed an increase in body core temperature that was indistinguishable from mice administered vehicle that displayed normal wakefulness. Alterations in sleep and wakefulness have been associated with changes in cellular energetics51 and although modulation of REV-ERB activity may indeed alter patterns of sleep and wakefulness by altering cellular energetics, we observed that these can be uncoupled, at least in terms of gross measurements of body core temperature.
Even more intriguing is our observation that REV-ERB agonists reduced anxiety-like behavior in mice in an array of behavioral assays that are models of generalized anxiety disorder and social anxiety disorder52. Although there is data to suggest that the mammalian clock is involved in anxiety, there have been no chemical tools available until recently to effectively address the potential to alter the central anxiety pathways by altering the clock. Anxiety disorders are among the most common mental disorders and nearly 30% of individuals will be directly affected by an anxiety disorder at some point in their lifetime, thus these disorders significantly burden our society53–55. Given the predominance of anxiety disorders in our society, there continues to be a focus on development of novel anxiolytic agents with improved efficacy and/or side effect profiles. Clearly the pharmacological profile of these compounds is paradoxical given their ability to combine anxiolytic activity with increased wakefulness, which could offer a clear clinical advantage under many circumstances. Drugs that typically increase arousal also increase anxiety (e.g. cocaine and amphetamines) while drugs that decrease anxiety decrease arousal (e.g. benzodiazepines and ethanol)56. Exceptions to this profile include nicotine57 and Neuropeptide S58, but the pharmacological profile of REV-ERB agonists and their ability to target the clock appear to be distinct from these pathways.
It is also important to note that SR9011 inhibited the condition place preference associated with cocaine-induced reward without itself inducing aversion. Our data indicating that mice treated with various REV-ERB agonists failed to wheel run during periods when they were awake led us to hypothesize that there may be suppression of reward seeking behavior. Drug addiction clearly has a circadian component59 and mice with mutations in clock genes such as Per1, Per2, Clock and Npas2 have altered responsiveness to the reward associated with cocaine, morphine and/or alcohol25,60–62. Thus, it is quite reasonable to expect that a small molecule regulator of the clock would modulate reward-seeking behavior. These data suggest that modulation of the clock by small molecule drugs, such as REV-ERB agonists, may have utility in treatment of addiction.
Using a variety of approaches including mouse genetic models, epidemiology, clinical research, circadian rhythms and the underlying molecular clock mechanism have been associated with a variety of disorders. With recent chemical biology approaches it has become possible to target certain components of the clock and begin to investigate whether pharmacological modulation of clock function and the circadian rhythm may offer an approach to treat human disease. In summary, our data indicate that pharmacological targeting of the clock holds promise for treatment of disorders associated with anxiety and sleep disorders as well as addiction.
METHODS
Synthesis and Preparation of SR9011 and SR9009
SR9011 and SR9009 were prepared as previously described 9.
Synthesis and Preparation of SR10067
DL-(1,2,3,4-Tetrahydroisoquinolin-3-yl)methanol
DL-1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (3.54 g, 20 mmol) was added portion wise to a suspension of LiAlH4 (3.04 g, 80 mmol) in THF (150 mL) at 0°C under argon. The reaction mixture was heated under reflux for 16 h. It was then cooled to 0°C and water (3 mL), 15% aqueous NaOH (3 mL) and water (9 mL) were added under stirring. The precipitate was then filtered and washed with diethyl ether (3 × 100 mL). The solvent was removed under vacuum and the product obtained as brown microcrystals (76%) was used in the next step without further purification. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.18 – 7.05 (m, 3H), 7.06 – 7.00 (m, 1H), 4.05 (s, 2H), 3.76 (dt, J = 10.9, 3.1 Hz, 1H), 3.51 (dd, J = 10.8, 7.9 Hz, 1H), 3.05 (ddq, J = 11.3, 7.4, 3.7 Hz, 1H), 2.69 (dt, J = 16.3, 3.4 Hz, 1H), 2.57 (dd, J = 16.4, 10.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ (ppm) 135.6, 134.1, 129.4, 126.4, 126.2, 126.0, 65.7, 55.2, 48.0, 31.1; HRMS calculated for C10H13NO (M+H)+ : 164.1069, Found: 164.1051.
(3-(Hydroxymethyl)-3,4-dihydroisoquinolin-2(1H)-yl)(naphthalen-1-yl)methanone
To a solution of DL-(1,2,3,4-Tetrahydroisoquinolin-3-yl)methanol (0.816 g, 55 mmol) in DCM was added 1-naphthoyl chloride (0.908 mL, 6 mmol), and triethylamine (1.36 mL, 10 mmol) and the mixture was stirred for 12 h. The reaction mixture was washed with HCl (2 N), saturated sodium bicarbonate solution and brine. The organic phase was separated, dried over anhydrous MgSO4 and the solvent was removed under reduced pressure. The residue was then purified by flash chromatography on silica gel (ethyl acetate/hexanes) to give the title compound as light brown microcrystals (78.8%). 1H NMR (400 MHz, Chloroform-d) δ (ppm) 1H NMR (400 MHz, CDCl3): [mixture of rotamers] δ (ppm) 8.05 (t, J = 9.5 Hz, 0.6H), 7.98 – 7.76 (m, 2.4H), 7.64 – 7.44 (m, 3.4H), 7.37 (dd, J = 20.3, 7.5 Hz, 1H), 7.25 – 7.17 (m, 2H), 7.16 – 7.01 (m, 1H), 6.75-6.62 (m, 0.5H), 5.69 –5.58 (m, 0.5H), 5.33-5.20 (m, 0.5H), 4.59 – 4.00 (m, 2H), 3.96 – 3.72 (m, 1H), 3.64 – 3.48 (m, 0.5H), 3.30 – 3.10 (m, 1H), 3.04 – 2.79 (m, 1H), 2.68 – 2.51 (m, 0.5H); 13C NMR (100 MHz, CDCl3): [mixture of rotamers] δ (ppm) 172.12, 171.33, 134.57, 134.50, 133.60, 133.35, 132.75, 131.51, 129.58, 129.53, 129.45, 129.32, 129.09, 128.92, 128.62, 128.55, 128.49, 128.33, 127.57, 127.49, 127.13, 127.08, 126.89, 126.74, 126.71, 126.65, 126.58, 126.49, 126.45, 125.70, 125.54, 125.37, 125.33, 125.16, 124.76, 124.48, 123.77, 63.81, 63.63, 61.66, 61.22, 54.39, 51.36, 51.32, 46.36, 42.05, 41.31, 41.22, 30.23, 30.07, 29.86, 29.82; HRMS calculated for C21H19NO2 (M+H)+ : 318.1489, Found: 318.1491.
(3-((4-(Tert-butoxy)phenoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)(naphthalen-1-yl)methanone
To a solution of (3-(hydroxymethyl)-3,4-dihydroisoquinolin-2(1H)-yl)(naphthalen-1-yl)methanone (0.317 g, 1 mmol), triphenylphosphine (0.288 g, 1.1 mmol), and 4-(tert-butoxy)phenol (0.166 g, 1 mmol) in dry toluene (2 mL) at 0°C was added DIAD (0.216 mL, 1.1 mmol) dropwise. The mixture was stirred at ambient temperature overnight. The solvent was evaporated and the residue was then purified by flash chromatography on silica gel (ethyl acetate/hexanes) to give the title compound as a white microcrystals (50%). 1H NMR (400 MHz, CDCl3): [mixture of rotamers] δ 8.23 – 8.12 (m, 0.5H), 7.98 – 7.82 (m, 2H), 7.76 – 7.41 (m, 4H), 7.34 – 7.06 (m, 4H), 7.02 – 6.83 (m, 2H), 6.79 – 6.63 (m, 2H), 6.50 – 6.42 (m, 0.5H), 5.82 – 5.52 (m, 1H), 4.60 – 4.36 (m, 1H), 4.35 – 4.10 (m, 2H), 3.92 (dt, J = 12.6, 9.5 Hz, 0.5H), 3.70 (ddd, J = 31.9, 9.8, 6.1 Hz, 0.5H), 3.35 (dd, J = 16.3, 6.3 Hz, 0.5H), 3.31 – 3.05 (m, 1H), 2.84 – 2.61 (m, 0.5H), 1.36 – 1.25 (m, 9H); 1H NMR (400 MHz, d6-DMSO, 110 °C): δ 8.0 (d, 2H), 7.8 (br s, 1H), 7.62-7.58 (m, 4H), 7.2 (br s, 3H), 7.0-6.5 (br m, 4H), 5.5 (br s, 1H), 4.5-3.7 (m, 4H), 3.3-2.8 (m, 2H), 1.3 (s, 9H); 13C NMR (100 MHz, CDCl3): [mixture of rotamers] δ (ppm) 171.11, 171.07, 170.58, 154.88, 154.50, 154.30, 149.13, 149.10, 148.93, 134.38, 132.83, 129.41, 127.02, 126.91, 126.87, 125.56, 125.53, 125.47, 125.27, 114.86, 114.72, 114.51, 114.37, 78.31, 78.29, 78.21, 67.16, 66.79, 52.57, 52.03, 47.81, 41.28, 41.20, 34.79, 30.73, 28.85, 28.79, 28.77, 22.78, 22.08, 14.26; 13C NMR (100 MHz, d6-DMSO, 110 °C): 169.8, 154.5, 149.5, 135.1, 133.6, 129.2, 128.7, 127.2, 126.7, 126.6, 125.6, 124.8, 124.2, 115.4, 77.8, 67.8, 52.3, 30.1, 29.0; HRMS calculated for C31H31NO3 (M+H)+ : 466.2376, Found: 466.2373.
Pharmacokinetic studies
Pharmacokinetic studies were performed as previously described1.
Cotransfection assays
Cotransfection assays were performed as previously described in HEK293 cells (Gal4cotransfection assay) or in HepG2 cells (full-length nuclear receptors)1. Cells were recently obtained from the ATCC (within 4 passages) and monitored for mycoplasma contamination.
Mice
C57BL6 and Balb/c male mice were obtained from Jackson Laboratories (Bar Harbor, ME). All the procedures were conducted in the Scripps or SLU vivarium, which are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and were approved by the Scripps Institutional Animal Care and Use Committee and the SLU Institutional Animal Care and Use Commitee. All experiments were conducted at 22 to 23° C.
Compound Administration
For all experiments 8 week old C57BL6/J or Balb/c male mice were administered doses of SR9011 or SR9009 100 mg kg−1 (i.p., b.i.d.) for 3 to 10 days for behavioral assays. See Supplementary Figure 5 for a schematic of the dosing and testing regimen. For twice per day dosing, dosing was performed at ZT0 and ZT12. For the first testing regimen open field assay, light dark transfer assay, marble burying assay and tail suspension assays were performed after 3, 5, 8 and 10 days of dosing, respectively. These assays were typically performed 1–2 hrs after the ZT12 injection under red light. However, the open field and marble burying assays were also performed at ZT6–8 and under normal light conditions with no distinction in efficacy of SR9011 indicating that the anxiolytic action was not time of day dependent. For the 2nd regimen the novel object and elevated plus maze assays were performed after 3 and 5 days of twice per day dosing, respectively, at ZT 6–8 under normal light conditions. Initially, multiple assays were performed in this manner so as to allow the same cohort of animals to be examined in multiple assays. However, if single assays were run, the identical dosing regimen was employed for consistency. The CPP assay and SIA were performed after 5 days of dosing. SR10067 was administered at various doses as indicated in the figure legends. The specifics of administration of compounds for sleep studies is indicated in the figures/figure legends. Formulation of the REV-ERB agonists was performed as previously described9. For studies where LiCl was used, mice were provided LiCl in drinking water (600 mg l−1) while control mice received normal water for 10 days prior to initiation of the study. The LiCl drinking water was maintained during the study. For studies using cocaine, Cocaine hydrochloride (National Institute on Drug Abuse) was dissolved in sterile 0.9% saline solution. Mice were administered 10 mg kg−1 cocaine in the CPP studies and doses as indicated in the figure in the EEG studies.
Gene Targeting
Standard gene targeting procedures63 were used to generate mice with floxed alleles at the Rev-erbβ locus on a C57BL6/6J background. The floxed Rev-erbβ mice were generated at the transgenic core at the Pennington Biomedical Research Center in Baton Rouge, LA. The strategy for generation of the mice is shown in Supplementary Fig. 7A. For generation of the germ line deleted strain the floxed Rev-erbβ mice were mated with Ella-Cre mice.
Open Field and Neophobia Assays
Mice were handled during 2 min daily for 3 days before testing their spontaneous locomotor activity and reactivity to novelty in an activity chamber. The chamber where the mice were assessed is a rectangle (37cm X 57cm) that is divideded into nine virtual zones of identical size (~12cm X 19cm zones). The open field assay was initiated by placing the mice in the center of the chamber. The locomotor activity was monitored by a video camera mounted on the ceiling and a computerized tracking system (Ethovision 1.90, Noldus IT, Wageningen, The Netherlands) recorded the total distance moved, speed, percentage of time spent in each zone. Time occupied in the center zone (~12cm X 19cm) and latency to enter the center zone was assessed for the open field assay as well as total distance traveled. For the neophobia assay, mice that had already been assessed in the open field assay and thus familiar with the chamber were placed into the center of the chamber to which a novel object had been fixed. The novel object was a #7 black rubber stopper placed at the top center of the chamber (zone 2 if one considers the activity box consisting of 3 rows of three zones) and the reactivity of the animal toward the object was measured. Zone 2 was considered near the novel object and time spent in this zone was scored. The floor of the arena and the object were washed after each test with 70% ethanol solution to remove odors left by previous subjects.
Elevated Plus Maze
Anxiety-like behavior of mice were evaluated using the elevated plus maze (EPM) test. The EPM consisted of two opposing open arms (45cm×10cm) and two closed arms (45cm×10cm×50cm) that extended from a central platform (10cm×10cm) elevated 65cm above the floor. Mice were placed individually on the central platform facing a closed arm and were allowed to freely explore the maze for 5min. The behavior of each mouse was monitored using a video camera and the movement of the mice automatically registered and analyzed with a computerized tracking system (Ethovision 1.90, Noldus IT, The Netherlands). Entry into an arm was defined as entry of all four paws into the arm. Total distance moved, speed, time spent in the open and closed arms, number of times the animal entered each type of arm, latency before entering an open arm were measured. Animal’s grooming, stretching, and rearing behaviors as well as the exploration outside the maze (head-dipping) were measured. The floor of the EPM was washed after each testing with 70% ethanol solution to remove odors left by previous subjects.
Marble Burying Assay
The marble burying test is used to assess anxiety-like behavior in a less vision-dependent manner than other available tests such as the elevated plus maze and light/dark transfer tests. Mice were placed individually in a standard mouse cage containing bedding that is 5 cm in depth, with 20 small marbles arranged in 4 evenly spaced rows of 5 on top of the bedding material as previously described58. After 30 min mice was removed and the number of marbles buried (at least 2/3 covered by bedding) is determined. Increased marble burying is associated with increased anxiety-like behavior in tests of anxiety medications.
Light Dark Box Assay
The light/dark transfer procedure has been used to assess anxiety-like behavior in mice by capitalizing on the conflict between exploration of a novel environment and the avoidance of a brightly lit open field58. The apparatus is a rectangular box made of Plexiglas divided by a partition into two environments. One compartment (14.5x27x26.5 cm) is dark (8–16 lux) and the other compartment (28.5x27x26.5 cm) is highly illuminated (600–800 lux) by a 60 W light source located above it. The compartments are connected by an opening (7.5x7.5 cm) located at floor level in the center of the partition. The time spent in the light compartment was used as a predictor of anxiety-like behavior, i.e. a greater amount of time in the light compartment was indicative of decreased anxiety-like behavior. Mice were placed in the dark compartment to start the 5-minute test.
Conditioned Place Preference Assay
Studies were conducted using a three-compartment apparatus with two equal-sized chambers (17x12.7x12.3 cm) separated by a neutral gray chamber (8.5x12.7x12.3 cm) (ENV–3013; Med Associates, St. Albans, VT). The large compartments differed in the wall color (black or white) and flooring (wire bars or wire mesh) and were separated from the center gray compartment by sliding doors. Mice were first assessed for baseline preference across two 30-min sessions. Each animal was placed in the center gray chamber and permitted to freely move through the chambers. Time spent in each chamber was recorded utilizing Med PC software. Based on the time spent in either the white or black chamber during the baseline sessions, the non-preferred chamber was assigned as the drug-paired chamber, while the preferred chamber was paired with saline. Mice were subsequently trained across six consecutive days. For each session, animals were injected with either the drug or vehicle 15 min prior to being confined to the assigned white or black chamber for 30 min. Injection and chamber pairings were counterbalanced across training sessions. On the final test day, mice were placed in the center gray compartment and permitted to freely move throughout the apparatus for 30 min. The time spent in each chamber was recorded as above, and the data from the test day was compared to the baseline level to obtain a preference score (post-training/pre-training).
Electroencephalogram (EEG)
EEG analysis was performed as previously described64–67. EEG data are recorded from stainless steel screw electrodes implanted on the frontal and parietal bone over the hippocampus (coordinates: 2.0 mm posterior and 2.0 mm lateral to bregma according to The Mouse Brain in Stereotaxic Coordinates from Franklin and Paxinos, 1977), and under general anesthesia (1–1.5% isoflurane). A fourth EEG electrode is implanted over the cerebellum, used to ground the animal to reduce signal artifacts. Insulated leads from the EEG electrodes are crimped to male pins (220-P02) and then cemented to the skull with dental acrylic. Following surgical implantation mice are allowed 1–2 weeks to recover prior to the study. To record EEG, mice are connected to commutators (PlasticOne) with flexible recording cables allowing their unrestricted movements within the cage and habituated to the recording cages for 48h. Compounds were administered and recording was continued for another 48h. The EEG and EMG signals were amplified in a Grass Model 7D polygraph in a frequency range of 0.3 to 10 KHz. The EEG and EMG are displayed on a computer monitor and stored with a resolution of 128 Hz in the hard drive of a computer for the off-line analysis of the vigilance states and spectral analysis using software supplied by Kissei Comptec. The polygraphic results are analyzed semiautomatically by 15-second epochs and classified as W, SWS and REM sleep. The total time of these vigilance states is calculated in periods of 1 hour. The number and duration of the individual W, SWS and REM sleep episodes are evaluated. In addition to standard sleep analysis, EEG spectral analysis in the different states of vigilance is performed by Fourier fast transformer (FFT) analysis using 4-second epochs instead of the 15-second epochs.
Tail Suspension Test (TST)
The TST was carried out essentially as previously described by Steru et al.68. Mice were allowed to acclimatize to the holding room for 30 min before the behavioral procedure. One hour after final administration of vehicle or SR9011 mice were individually suspended by the tail from each of the hooks of the TST apparatus using adhesive tape placed about 1/3rd the way from the tip of the tail. Immobility duration was recorded for 6 min after 1 min of acclimatization. Mice were considered immobile only when they hung passively and completely motionless. The duration of immobility during the 6-min test period was automatically recorded by a camera and stored on a computer equipped with EthoVision XT software.
Telemetric assessment of locomotor activity and core body temperature
The Physiotel® TA-F10 transmitters (Data Sciences International, USA) were surgically implanted to the peritoneal cavity of mice under constant general isofluorane anesthesia (1–1.5%). The implanted mice were allowed to recover for seven days before the telemetric recordings started. Post-surgical care included antibiotic treatment (enrofloxacin 2.5–5 mg kg−1, s.c.) for 5 days and management of pain with Rimadyl tablets (2mg). The locomotor and temperature signals were wirelessly collected by a radio receiver (PhysioTel RPC-1, Data Sciences International), placed on the bottom of each mouse cage. The signals were transferred to data matrixes (PhysioTel Multiplus Analog Adaptor Model DL-10, Data Sciences International) that amplified and converted the analogical signals to digital data that was acquired continuously in real-time at 250Hz, using the computer-based Dataquest ART acquisition software (Data Sciences International, USA). The acquired data was analyzed using Neuroscore (Data Sciences International, USA) and the sum of arbitrary counts of locomotor activity was obtained for the 2 hours following vehicle or compound administration was presented. Core body temperature was monitored over 24h.
Social Interaction Assay
C57BL/6J mice were used in this experiment. They were administered 100 mg kg−1SR9011 or vehicle i.p. B.I.D. for 5 days and then one hour following the final injection were tested in the social interaction test. This test was originally developed to model in mice aspects of autism spectrum disorders in humans69,70 and has been used widely by behavioral neuroscientists71. Additionally, the social interaction assay has been utilized as a model for social anxiety disorder52. The social interaction apparatus is a rectangular, three chambered Plexiglas box, with each chamber measuring 20 cm x 40.5 cm x 22 cm (L x W x H). Dividing walls are clear with small semicircular openings (3.5 cm radius) allowing access into each chamber. The middle chamber is empty, and the two outer chambers contain small, round wire cages (Galaxy Cup, Spectrum Diversified Designs, Inc., Streetsboro, OH) during testing. The mice were habituated to the entire apparatus with the round wire cages removed for 5 min. Mice were familiarized with a stranger mouse (B6 of the same sex being tested, habituated to the wire cage) in one of the wire cages in an outer compartment and another identical wire cage in the opposite compartment for 5 minutes followed by removal for 5 minutes before initiating the test. For the assay, mice were returned to the middle chamber, this time with the original familiar mouse in its chamber and a new unfamiliar mouse (novel mouse) in the previously empty wire cage. Time spent in each chamber was recorded for 5 min. Twelve mice were assessed per group.
Statistical Analysis
All data are expressed as the mean ± s.e.m. (N as indicated in the figure legends). Statistical test utilized to determine significant differences between treatment groups are indicated in the figure legends. Variance within groups was assessed by calculation of s.d. and variance was similar between groups that were compared. For all in vivo experiments, the treatment groups were blinded to the investigator. Mice were randomized to treatment groups to control of potential effects of cage mates and litter effects. Group sizes for particular experiments were based on potential levels of efficacy compared to known positive controls (e.g. CDP or cocaine) and power analysis. Normal distribution was confirmed for all experimental groups.
Supplementary Material
Acknowledgments
This research was supported by grants from the NIH to T.P.B. (MH092769 and MH093429) and L.A.S. (DK088499).
Footnotes
Author Contributions
All authors designed various components of the research. S.B., Y.W., L.A.S., K.G., M.K., S. H-R, and A.J.R performed the behavioral experiments and analyzed the results. S.B. performed the cell-based experiments and analyzed the results. B.M.E., Y.S. and T.M.K. performed the medicinal chemistry and chemical analysis. T.P.B. conceived the study and was responsible for the general design of the experiments and wrote the paper. All authors read and contributed to the editing of the manuscript during its preparation.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Crumbley C, Burris TP. Direct regulation of CLOCK expression by REVERB. PLoS One. 2011;6:e17290. doi: 10.1371/journal.pone.0017290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. Journal of Biological Chemistry. 2010;285:35386–35392. doi: 10.1074/jbc.M110.129288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preitner N, et al. The orphan nuclear receptor REV-ERB alpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 4.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton JD, Kyriacou CP, Reppert SM. Keeping time with the human genome. Nature. 2001;409:829–831. doi: 10.1038/35057006. [DOI] [PubMed] [Google Scholar]
- 6.Bass J. Physiology: On time metabolism. Nature. 2011;480:466–467. doi: 10.1038/480466a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass J, Takahashi JS. Circadian Integration of Metabolism and Energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solt LA, et al. Regulation of Circadian Behavior and Metabolism by Synthetic REV-ERB Agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nature reviews Drug discovery. 2014;13:197–216. doi: 10.1038/nrd4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woldt E, et al. Rev-erba modulates skeletal muscle oxidative capacity by regulated mitochondrial biogenesis and autophagy. Nature Medicine. 2013;19:1039–1046. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacology & Therapeutics. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClung CA. How might circadian rhythms control mood? Let me count the ways. Biol Psychiatry. 2013;74:242–249. doi: 10.1016/j.biopsych.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagannath A, Peirson SN, Foster RG. Sleep and circadian rhythm disruption in neuropsychiatric illness. Current opinion in neurobiology. 2013;23:888–894. doi: 10.1016/j.conb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 16.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 17.Sipila T, et al. An Association Analysis of Circadian Genes in Anxiety Disorders. Biological Psychiatry. 67:1163–1170. doi: 10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Lavebratt C, Sjoholm LK, Partonen T, Schalling M, Forsell Y. PER2 Variantion Is Associated With Depression Vulnerability. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2010;153B:570–581. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- 19.Nievergelt CM, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath CL, et al. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244x-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm. 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 22.Johansson C, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 23.Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavebratt C, et al. CRY2 is associated with depression. PLoS One. 2010;5:e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roybal K, et al. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain and Behavior. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- 27.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Human Molecular Genetics. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 28.Masana MI, Sumaya IC, Becker-Andre M, Dubocovich ML. Behavioral characterization and modulation of circadian rhythms by light and melatonin in C3H/HeN mice homozygous for the ROR beta knockout. Am J Physiol-Regul Integr Comp Physiol. 2007;292:R2357–R2367. doi: 10.1152/ajpregu.00687.2006. [DOI] [PubMed] [Google Scholar]
- 29.Gray A, Allison C, Pratt JA. A role for AMPA/kainate receptors in conditioned place preference induced by diazepam in the rat. Neurosci Lett. 1999;268:127–130. doi: 10.1016/s0304-3940(99)00371-7. [DOI] [PubMed] [Google Scholar]
- 30.Spyraki C, Kazandjian A, Varonos D. Diazepam-induced place preference conditioning: appetitive and antiaversive properties. Psychopharmacology (Berl) 1985;87:225–232. doi: 10.1007/BF00431813. [DOI] [PubMed] [Google Scholar]
- 31.Spyraki C, Fibiger HC. A role for the mesolimbic dopamine system in the reinforcing properties of diazepam. Psychopharmacology (Berl) 1988;94:133–137. doi: 10.1007/BF00735894. [DOI] [PubMed] [Google Scholar]
- 32.Nomikos GG, Spyraki C. Effects of ritanserin on the rewarding properties of d-amphetamine, morphine and diazepam revealed by conditioned place preference in rats. Pharmacol Biochem Behav. 1988;30:853–858. doi: 10.1016/0091-3057(88)90110-4. [DOI] [PubMed] [Google Scholar]
- 33.Brene S, et al. Running is rewarding and antidepressive. Physiol Behav. 2007;92:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris MJ, Na ES, Johnson AK. Voluntary running-wheel exercise decreases the threshold for rewarding intracranial self-stimulation. Behav Neurosci. 2012;126:582–587. doi: 10.1037/a0029149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwood BN, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behavioural Brain Research. 2011;217:354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erba is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 37.Noel R, et al. Synthesis and SAR of tetrahydroisoquinolines as Rev-erbalpha agonists. Bioorganic & medicinal chemistry letters. 2012;22:3739–3742. doi: 10.1016/j.bmcl.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin Y, et al. Small molecule tertiary amines as agonists of the nuclear hormone receptor Rev-erbalpha. Bioorganic & medicinal chemistry letters. 2012;22:4413–4417. doi: 10.1016/j.bmcl.2012.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albrecht U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 2012;74:246–260. doi: 10.1016/j.neuron.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cellular and molecular life sciences : CMLS. 2013;70:2985–2998. doi: 10.1007/s00018-012-1207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun SK, et al. Identification and validation of cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol. 2014;9:703–710. doi: 10.1021/cb400752k. [DOI] [PubMed] [Google Scholar]
- 43.Hirota T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337:1094–1097. doi: 10.1126/science.1223710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laposky A, et al. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- 45.Wisor JP, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naylor E, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopp C, Albrecht U, Zheng B, Tobler I. Homeostatic sleep regulation is preserved in mPer1 and mPer2 mutant mice. The European journal of neuroscience. 2002;16:1099–1106. doi: 10.1046/j.1460-9568.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 48.Shiromani PJ, et al. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am J Physiol Regul Integr Comp Physiol. 2004;287:R47–57. doi: 10.1152/ajpregu.00138.2004. [DOI] [PubMed] [Google Scholar]
- 49.Franken P, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: Genotype and sex interactions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerhart-Hines Z, et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature. 2013;503:410–413. doi: 10.1038/nature12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wisor JP. A metabolic-transcriptional network links sleep and cellular energetics in the brain. Pflugers Archiv : European journal of physiology. 2012;463:15–22. doi: 10.1007/s00424-011-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nature reviews Drug discovery. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kessler RC, et al. Lifetime 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- 55.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 56.Koob GF, Greenwell TN. Neuropeptide S: a novel activating anxiolytic? Neuron. 2004;43:441–442. doi: 10.1016/j.neuron.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Nesbitt PD. Smoking, physiological arousal, and emotional response. J Pers Soc Psychol. 1973;25:137–144. doi: 10.1037/h0034256. [DOI] [PubMed] [Google Scholar]
- 58.Xu YL, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U S A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, et al. The role of mPer1 in morphine dependence in mice. Neuroscience. 2005;130:383–388. doi: 10.1016/j.neuroscience.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spanagel R, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 63.Muoio DM, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabolism. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biological Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 65.Huitron-Resendiz S, et al. Urotensin II modulates rapid eye movement sleep through activation of brainstem cholinergic neurons. J Neurosci. 2005;25:5465–5474. doi: 10.1523/JNEUROSCI.4501-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huitron-Resendiz S, Sanchez-Alavez M, Wills DN, Cravatt BF, Henriksen SJ. Characterization of the sleep-wake patterns in mice lacking fatty acid amide hydrolase. Sleep. 2004;27:857–865. doi: 10.1093/sleep/27.5.857. [DOI] [PubMed] [Google Scholar]
- 67.Bourgin P, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 69.Moy SS, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 70.Moy SS, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nature reviews Neuroscience. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.