Abstract
This article provides a brief overview of recent progress in the synthesis and functionalization of magnetic nanoparticles and their applications in the early detection of malignant tumors by magnetic resonance imaging (MRI). The intrinsic low sensitivity of MRI necessitates the use of large quantities of exogenous contrast agents in many imaging studies. Magnetic nanoparticles have recently emerged as highly efficient MRI contrast agents because these nanometer-scale materials can carry high payloads while maintaining the ability to move through physiological systems. Superparamagnetic ferrite nanoparticles (such as iron oxide) provide excellent negative contrast enhancement. Recent refinement of synthetic methodologies has led to ferrite nanoparticles with narrow size distributions and high crystallinity. Target-specific tumor imaging becomes possible through functionalization of ferrite nanoparticles with targeting agents to allow for site-specific accumulation. Nanoparticulate contrast agents capable of positive contrast enhancement have recently been developed in order to overcome the drawbacks of negative contrast enhancement afforded by ferrite nanoparticles. These newly developed magnetic nanoparticles have the potential to enable physicians to diagnose cancer at the earliest stage possible and thus can have an enormous impact on more effective cancer treatment.
Introduction
Magnetic resonance imaging (MRI) is a noninvasive imaging technique whereby images are generated based on the nuclear magnetic resonance signals of the water proton (1H) nuclei in the specimen.1 The spin-lattice (longitudinal) relaxation time (T1) and the spin-spin (transverse) relaxation time (T2) of the proton spins along with the proton density determine the MR signal intensity from a particular tissue. T1-weighted MR images are generated based on the rate of longitudinal relaxation (proportional to 1/T1) of the water 1H nuclei. When the protons resonating at an equilibrium frequency are excited with a radio frequency pulse, a change in their net magnetization will occur as a result of the overpopulation of the higher energy states of the nuclear spins. Protons having more rapid longitudinal relaxation (i.e., shorter T1) will relax back to their equilibrium state faster, yielding higher net electromagnetic signals to afford positive contrast enhancement in the MR image. T2-weighted MR images, on the other hand, are generated based on the rate of transverse relaxation (proportional to 1/T2) of the water 1H nuclei. A faster transverse relaxation leads to more rapid dephasing of individual spins, resulting in reduced signal intensity (negative contrast enhancement) in the MR image. Owing to its incredibly high spatial resolution, excellent soft tissue contrast, and superior depth of penetration, MRI has become a very powerful diagnostic tool in medicine.
The main drawback of MRI, however, is its intrinsically low sensitivity. As a result, large doses of contrast agents are typically administered to alter the water 1H relaxation times in order to enhance image contrast of specific tissues. Approximately half of clinical MR images are performed with the aid of contrast agents to detect many diseases, including cancer lesions.2 Most of the current MR contrast agents are highly paramagnetic gadolinium (Gd) chelates, which can reduce water proton T1 values to result in increased (hyperintense) signal intensity. The efficiency of a contrast agent to increase the rate of relaxation is expressed by the relaxivity value (r1), which is defined as the slope of a plot of 1/T1 versus concentration in the units of mM−1 s−1. Gd chelates typically have modest r1 values of ∼4–5 mM−1 s−1, and discernible contrast in the MR image can be achieved with sub-mM concentrations of Gd chelates in the tissue.3 Alternatively, a contrast agent such as iron oxide nanoparticles can be used to enhance water transverse relaxation (r2 relaxivity), leading to reduced (hypo-intense) MR signals.
A straightforward strategy is to selectively and specifically deliver imaging contrast agents by conjugation to affinity molecules that target the biomarkers overexpressed by diseased cells. Such an approach has been successfully used to design target-specific positron emission tomography and single photon emission com puted tomography contrast agents by tagging radionuclides or their complexes with antibodies or other cell-targeting molecules.4,5 Superparamagnetic iron oxide and related ferrite nanoparticles have an extraordinary ability to shorten T2 relaxation times due to their large magnetization and result in large enhancements of signal void (darkening of images). They can be readily conjugated to affinity molecules to provide target-specific T2 contrast agents for MRI. The first part of this article will provide an overview of the latest advances in the applications of superparamagnetic iron oxide and related ferrite nanoparticles in target-specific T2-weighted MRI of malignant lesions.
Because of the need for high concentrations of paramagnetic Gd chelates, it remains a great challenge to develop T1 contrast agents that specifically target the diseased tissues and overcome the drawbacks of negative contrast enhancement afforded by ferrite nanoparticles. Sufficient T1-weighted MR image contrasts are typically achieved in the presence of sub-mM concentrations of the Gd chelates,3 which are several orders of magnitude higher than that of overexpressed biomarkers. There is thus an urgent need for synthetic strategies that allow for the delivery of a very large payload of Gd chelates with each cell-targeting molecule. Nanometer-scale materials have the ability to carry high payloads while maintaining the ability to move through physiological systems. For T1-weighted MRI contrast enhancement, these materials continue to require paramagnetic metals ions, such as Gd3+ and Mn2+, necessitating the development of hybrid cargo platforms. This article also will review the advances in the design of nanoparticulate T1 contrast agents, including microemulsions, liposomes, hybrid silica nanoparticles, and nanoscale metalorganic frameworks, and their applications in target-specific T1-weighted MRI of cancers.
Dextran-Coated Iron Oxide Nanoparticles as T2 Contrast Agents
Superparamagnetic iron oxide (SPIO) nanoparticles were initially shown to improve the detection of focal liver lesions over the Gd-diethylenetriaminepenta-acetic acid (Gd-DTPA)-enhanced MRI scans, leading to their approval by the U.S. Food and Drug Administration (FDA) for clinical use in 1996.6,7 SPIOs are composed of either maghemite (γ-Fe2O3) or magnetite (Fe3O4) phases that can be prepared by a variety of methods, most commonly by a coprecipitation reaction of a mixture of ferrous and ferric salts in aqueous media in the presence of stabilizers such as hydrophilic polymers.8 They have the extraordinary ability to shorten T2 relaxation times due to their large magnetization and result in large enhancements of signal void (darkening of images). The early clinical targets of SPIOs were liver diseases because of their selective uptake by the liver Kupffer cells, which are specialized cells in the liver that destroy bacteria, foreign proteins, and worn-out blood cells.9,10 If the normal liver architecture is destructed by a hepatic disease, such as hepatocellular carcinoma or liver metastasis, the region will have a reduced density of Kupffer cells. Due to the low uptake by the abnormal liver, the SPIO provides a strong contrast between normal and abnormal tissue, thereby enabling the detection of focal liver lesions.
Weissleder and co-workers first developed SPIO/biomolecule conjugates for target-specific MRI of cancers and other diseases.11,12 In one formulation, SPIOs were coated with cross-linked dextran, a naturally occurring glucose-based polysaccharide capable of complexing iron to achieve longer blood circulation times. Several variations of the formulation exist and are called cross-linked iron oxide (CLIO) and ultrasmall superparamagnetic iron oxide (< 50 nm). CLIO is composed of materials on the generally regarded as safe list and has been approved by the FDA. CLIO has been extensively used to image diseases in animals and humans, such as cancers, as well as to delineate biological processes in vivo.
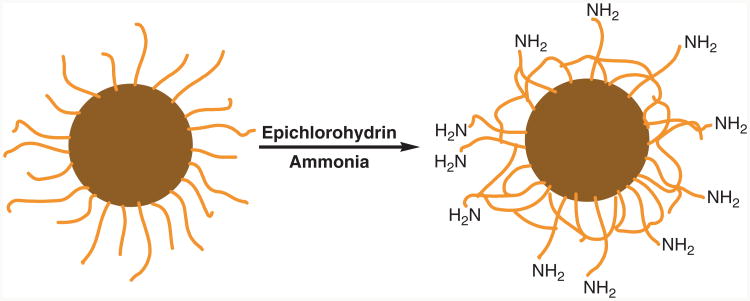
As shown in Scheme 1, the cross-linked dextran can be chemically modified for conjugation of targeting molecules.11 Dextran-coated iron oxide nanoparticles can be used without any further functionalization to show image enhancement of disease states. Due to dextran's ability to evade the reticuloendothelial system (RES), which is a group of cells with the ability to take up and sequester inert particles, signal void in the circulation can be detected due to the T2 relaxation of the water protons. Upon capture, the CLIO contrast enhancement allows the delineation of the RES abnormalities. Harisinghani et al. showed such imaging capabilities in a clinical study of 80 patients with prostate cancers12 as well as other cancers.13,14 The CLIO was shown to be lymphotrophic and could be effectively used to image nodal abnormalities that were otherwise undetectable.
Scheme 1.
Preparation of iron oxide nanoparticles with cross-linked dextran surface coating (light brown strands) and amino functional groups for bioconjugation.
The dextran coating can be chemically modified to allow the conjugation of affinity molecules to endow the SPIOs' targetspecificity. Peptides and proteins have been used as functional conjugates with these nanoparticles to image prostatic, hepatic, splenic, pancreatic, and glial neoplasia, a subtype of brain tumor; the use of CLIO in imaging rodent pancreatic cancer is shown in Figure 1.12,14–16 When conjugated to E-selectin or VCAM-1, CLIO was used to detect endothelium-specific proteins to monitor tumor angiogenesis.17 Apoptotic cells also can be detected when the CLIO particles are functionalized with Annexin V, a calcium-dependent protein that has high affinity for the phosphatidylserine apoptosis marker in the cell membrane.18 Although studied extensively and considered relatively mature, the SPIO contrast agents continue to be the trailblazer in biomedical imaging.
Figure 1.

Cross-linked iron oxide (CLIO) nanoparticles for T2-weighted images of rodent pancreatic cancer: (a) preinjection of CLIO, (b) postinjection of CLIO, and (c) higher magnification of postinjection image with the arrow indicating tumor. L, liver; P, pancreas; K, kidney; B, bowel.16
Superparamagnetic Nanoparticles With Enhanced Magnetization and Surface Coatings
As shown in the model developed by Koenig and Keller, spin-spin relaxation is dependent on the magnetic moment of the nanoparticles (μ):19
| (1) |
In Equation 1, a is a constant, dNP the diameter of the nanoparticle, D the diffusion coefficient, μ the magnetic moment of the nanoparticles, γ the gyromagnetic ratio of the water proton, CNP the concentration of the nanoparticles, and J(ω,τD) the spectral density function.
Equation 1 shows the quadratic dependence of r2 on the magnetization of the nanoparticle. It is also established that the magnetic properties of nanoparticles are strongly dependent on their size, shape, and surface properties. The magnetization of a nanoparticle can be significantly reduced as a result of the surface defects as shown in Equation 2:20
| (2) |
where mS is the saturation magnetization of the nanoparticle, MS the saturation magnetization of the bulk material, r the size of the nanoparticle, and d the thickness of the disordered surface layer.
The r2 relaxivities of SPIO and related ferrite nanoparticles can be optimized by enhancing their magnetic properties through (1) the intrinsic material properties such as material composition and crystal structure and (2) the extrinsic factors such as crystallinity size, and shape. Over the past decade, many synthetic protocols have been developed for monodisperse and highly crystalline iron oxide and related ferrite nanoparticles, most notably through the thermal decomposition of metal precursors in organic surfactant solutions at a high temperature of >300°C.21 The resulting hydrophobic nanoparticles had uniform sizes (σ < 10%), and their sizes (from 4 to 25 nm) could be finely controlled by modifying synthetic parameters. In particular, Park et al. reported the large-scale production of various metal oxide nanoparticles from the thermal decomposition of metalsurfactant complexes.22 Jun et al. systematically studied the relationships among size, magnetism, and relaxivity of uniform-sized iron oxide nanoparticles.23 Large iron oxide nanoparticles have a large magnetization and high r2 relaxivity, which make it possible to increase the contrast in T2-weighted images. Various bimetallic ferrite nanoparticles, including CoFe2O4, MnFe2O4, and NiFe2O4, have been tested as T2 contrast agents. The MnFe2O4 nanoparticles (MnMEIO) have been found to have very high magnetization and large relaxivity, resulting in higher signal enhancement as compared with CLIO (Figure 2).24
Figure 2.
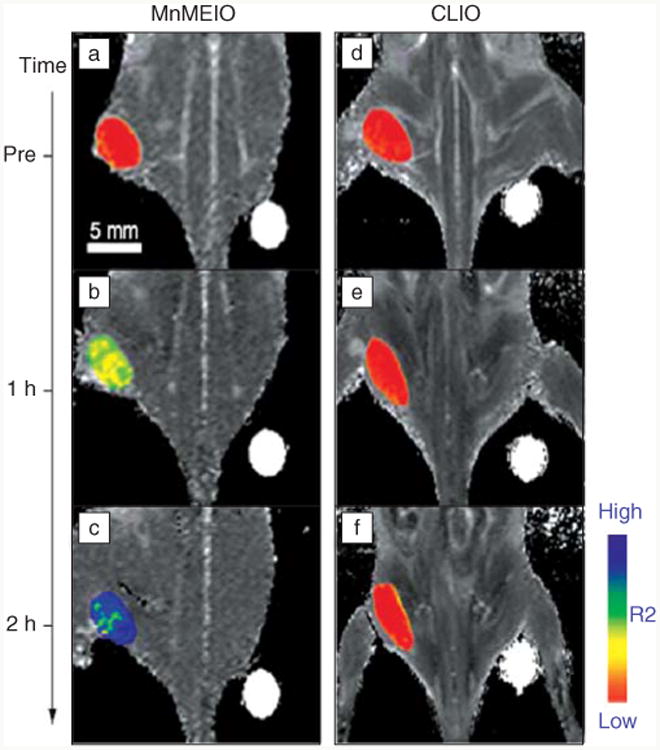
In vivo magnetic resonance detection of cancer after administration of magnetic nanoparticles Herceptin conjugates. MnFe2O4 nanoparticles (MnMEIO) (a–c) show higher signal enhancement than cross-linked iron oxide (CLIO) (d–f).24 R2, inverse of transverse relaxation time.
Since the uniform and crystalline superparamagnetic nanoparticles were synthesized in organic media, their hydrophobic surfaces have to be modified with hydrophilic coatings to ensure their aqueous dispersibility and biocompatibility as well as their reactivity with targeting molecules (such as antibodies). Several new coating strategies have recently been developed for ferrite nanoparticles, in particular, iron oxide nanoparticles. For example, several functional groups were immobilized on iron oxide nanoparticles with dopamine,25,26 hydroxamic acid,27 and 2,3-dimercaptosuccinic acid (DMSA).24 The DMSA-coated iron oxide nanoparticles were dispersible in water and used for in vivo MRI.24 Several strategies also have been developed to coat SPIOs with biocompatible polymers such as poly-(ethylene glycol) (PEG).28
Superparamagnetic nanoparticles with suitable surface coatings have been conjugated with affinity molecules such as antibodies, peptides, and oligonucleotides to afford target-specific contrast agents for efficient lesion detection. Lee et al., for example, demonstrated that Herceptin conjugated MnFe2O4 nanoparticles showed more sensitive in vivo cancer imaging with large r2 relaxivity (Figure 2).24 Shells of the nanoparticles can provide novel functions in addition to MRI. Unique shell structures, such as porous silica, can conserve not only hydrophilicity and biocompatibility but also load functional molecules in their pores. For example, Kim et al. synthesized discrete and monodisperse core-shell nanostructures with the magnetite nanocrystal core and the mesoporous silica shell loaded with a fluorescent dye for optical imaging and an anticancer drug inside nano-sized pores.29 The resulting composite core/shell nanoparticles were applied to simultaneous MR and optical imaging and drug delivery (Figure 3).
Figure 3.

The synthesis of magnetite nanoparticle/mesoporous silica core (Fe3O4@mSiO2)-shell nanostructures and its in vivo dual modal imaging (magnetic resonance imaging [MRI] and optical imaging).29 PEG, poly(ethylene glycol); CTAB, cetyl trimethyl ammonium bromide.
Nanoparticulate Microemulsion and Liposomal T1 Contrast Agents
Superparamagnetic nanoparticles typically act as negative contrast agents that decrease the proton MR signal from the tissue. The resulting dark signal can be confused with other pathogenic conditions and cause undesired false positives. Moreover, the high susceptibility of the T2 contrast agents induces distortion of the magnetic field on neighboring normal tissues. This distortion of the background is called the susceptibility artifact or “blooming effect,” which generates obscure images and distorts the background around the lesions.30 There is thus very strong interest in creating nanoparticles for efficient T1-weighted MRIs.
Lanza et al. developed perfluorocarbon-based microemulsion contrast agents and extensively examined their utility in target-specific MRI in vivo.31 The nanoparticles of ∼250 nm in diameter are composed of a perfluorocarbon core and a monolayer lipid shell. The particles can be synthesized by emulsification of the perfluorooctylbromide, surfactants, and glycerin to form a stable nanoparticle with a few hundred homing ligands and extraordinarily high payloads of paramagnetic metal centers, about 100,000 or more Gd chelates per nanoparticle (Figure 4).32 These Gd-containing nanoparticles possess extremely large MR relaxivities on a per particle basis.33
Figure 4.

Scanning electron microscope image of porcine aortic smooth muscle cells known to overexpress cell surface tissue factor (TF) in culture. (a) Cells exposed to TF-targeted microemulsion nanoparticles. (b) Cells pretreated with anti-tissue factor antibody and subsequently exposed to TF-targeted microemulsion nanoparticles.32
Targeting molecules can be incorporated into the stabilizing surface lipids to allow for their selective delivery to specific sites. Ligands for overexpressed disease biomarkers that have been used for this material include small molecules, integrins, fibrin, and antibodies. The targeted derivatives of the perfluorocarbon-lipid nanoparticles were used extensively to image tumors and angiogenesis34,35 as well as neovascularization (the formation of new blood vessels in tissues) in atherosclerosis (Figure 5).36
Figure 5.
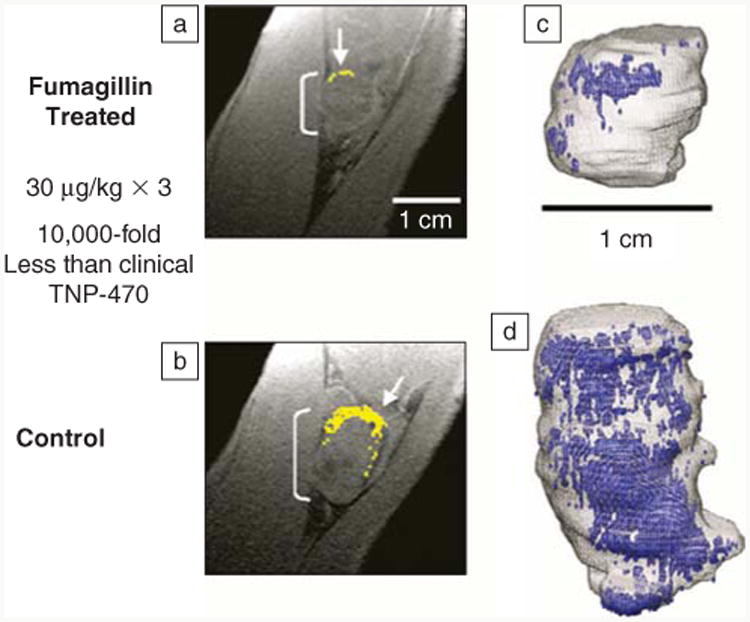
(a) Diminished αVβ3-integrin contrast enhancement in a T1-weighted 3D gradient echo magnetic resonance (1.5 T) single slice image of a VX2 rabbit tumor following αVβ3-targeted fumagillin nanoparticles versus (b) those given αVβ3-targeted nanoparticles without drug. (Enhancing pixels are color coded in yellow.) (c) 3D angiogenesis maps in VX2 tumors following αVβ3-targeted fumagillin nanoparticles versus (d) αVβ3-nanoparticles without drug.36
The perfluorocarbon core also allows for the unique capability of an additional MRI probe using the 19F signal. 19F MRI is an advantageous imaging tool because the MR sensitivity is nearly as high as 1H (83%), the low occurrence of endogenous 19F in biological systems and the 100% natural abundance of the 19F isotope. Perfluorocarbon emulsions encapsulated by lipid-surfactant monolayers have been used to prepare MRI contrast agents with high 19F payloads. Perfluorocarbon-based microemulsion nanoparticles are thus multimodal and prove to be versatile in the early detection of cancers.
Oil-in-water microemulsions have been used as templates to engineer stable wax nanoparticles containing Gd chelates for potential use as MRI contrast agents.37 Gd was incorporated into the lipid layer by forming chelates with dimyristoyl phosphoethanolamine diethylene triamine pentaacetate. The wax nanoparticles have a payload of approximately 105 Gd per nanoparticle. In a 4.7 T MR scanner, relaxivities were determined to be r1 = 7.1 and r2 = 13.0 mM−1 s−1 Gd. These core-shell nanoparticles were also PEGylated to increase blood circulation times, and the PEGylated wax nanoparticles were used in MRI of nude mice bearing A549 lung carcinoma xenografts by taking advantage of the enhanced permeability and retention effect.38
Liposomes are lipid vesicles consisting of an aqueous core enclosed in a lipid bilayer membrane. Gd-labeled liposomes have been prepared and extensively examined for in vivo MRI.39 The first Gd-containing liposomes were confirmed to be viable as MRI contrast agents for imaging the liver and spleen of normal Balb/c mice.39 Later formulations were reported to show contrast enhancement of hepatic metastases in rats.40 Liposomes also can be rendered target-specific by adding cell-targeting moieties to the bilayer surface. A number of target-specific liposome-based contrast agents already have been explored for imaging IGROV-1 (human ovary carcinoma) xenograft tumors and hepatic metastases in rodents.33,41
Inorganic Nanoparticles as T1 Contrast Agents
Inorganic nanoparticles composed of highly paramagnetic metal ions such as Gd3+ and Mn2+ also have been developed as new T1 contrast agents. Large surface-to-volume ratios of such ultrasmall nanoparticles should allow facile interactions between the metal ions at or near the surface with surrounding water molecules, leading to fast longitudinal relaxation of the water protons. Nanoparticles of Gd2O3,42 GdF3,43 and GdPO444 have been investigated as MRI contrast agents. They exhibited signal-enhancing contrast in T1-weighted images. The Gd2O3 nanoparticle-based agents are composed of small cores of <5 nm and stabilizing shells of dextran, PEG, and silica.42 Water dispersible GdF3 nanoparticles were prepared with either a positively charged surface by conjugation with 2-aminoethyl phosphate groups or a negatively charged surface by coating with citrate groups.43 It is important to control the charge of nanoparticles to avoid aggregation or precipitation of nanoparticles in biological media and to control the interaction between proteins and nanoparticles. Dextran-coated GdPO4 nanoparticles were synthesized by a hydrothermal process in the presence of dextran.44 The clinical utility of such Gd-based nanoparticles, however, is uncertain because of the potential leaching of free Gd3+ ions, which are the culprit of the recently discovered nephrogenic systemic fibrosis syndrome.
MnO nanoparticles were recently examined as T1 contrast agents for in vivo MRI.45 MnO nanoparticles conjugated with a tumor-specific antibody were used for the selective imaging of breast cancer cells in a metastatic brain tumor with signal-enhanced contrast (positive contrast) in T1-weighted images; maximum intensity occurred three hours after the injection (Figure 6).46 Metallic nanoparticles also have recently been shown to be promising T1 contrast agents. Seo et al. synthesized FeCo nanoparticles stabilized with single graphitic carbon shells (FeCo/GC).47 After noncovalent functionalization with phospholipid–poly(ethylene glycol) (PL-PEG) molecules, the FeCo/GC nanoparticles were long-circulating and provided positive contrast enhancement in depicting the artery in a rabbit model. Despite their promise as T1 contrast agents, the detailed contrast enhancement mechanisms in both MnO and FeCo/GC nanoparticles have yet to be elucidated.
Figure 6.
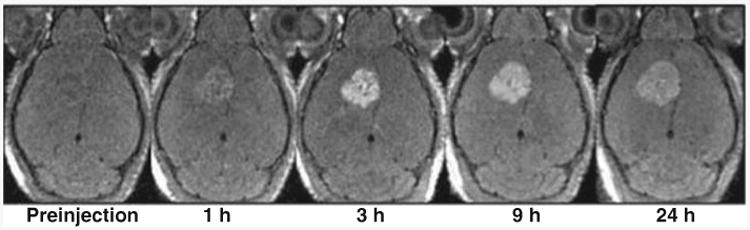
Breast cancer cells were selectively enhanced in T1-weighted magnetic resonance imaging by Herceptin-functionalized MnO nanoparticles.46
Hybrid Silica Nanoparticles as T1 Contrast Agents
Inspired by the microemulsion and liposomal contrast agents, Rieter et al. prepared 37-nm hybrid silica nanoparticles containing embedded [Ru(bpy)3]Cl2 (where bpy is 2,2′-bipyridine) luminophore and a shell of Gd chelates.48 Unlike the microemulsion and liposomal systems, the hybrid silica particles have a tunable size from 20–100 nm. The large Stokes shift of the [Ru(bpy)3]Cl2 fluorophore (excitation: 525 nm; emission: 610 nm) in the core significantly reduces the background signal from the biological systems. Furthermore, [Ru(bpy)3]Cl2 is much more photostable than organic fluorophores, such as fluorescein. The luminescence of the hybrid silica nanoparticles is thus highly compatible with biological imaging applications.
The Gd chelates were immobilized onto the surface of the silica nanoparticles in either a monolayer or multilayer fashion.48 The surface-immobilized Gd chelates effectively relax water protons. The resulting nanoparticle has relaxivities five orders of magnitude higher than the constituent Gd chelate due not only to the greatly increased Gd payload but also to much enhanced per Gd relaxivity as a result of the reduced tumbling rates. Notably, the particles with multilayers of Gd chelates had reduced relaxivities (on per Gd basis) compared to those with the monolayer coating, likely due to reduced water accessibility to the Gd-chelates. Murine monocyte cells were successfully labeled with the hybrid silica nanoparticles, and T1-weighted signal enhancement of the cell pellet was observed in a 3 T MR scanner.48
Kim et al. recently devised a poly-electrolyte layer-by-layer self-assembly approach to increase the Gd payload while maintaining the relaxivity on a per Gd basis.49 Positively charged MR-enhancing Gd-chelate polymers were deposited onto negatively charged silica nanoparticles with a Gd-(trimethoxysilylpropyl)diethy-lenetriamine tetraacetate (Gd-Si-DTTA) monolayer by electrostatic interactions. The resulting particle was further treated with negatively charged polystyrenesulfonate (PSS) to provide a net negatively charged layer. These steps were repeated to form the multilayer architecture as shown in Scheme 2. TEM and fluorescence studies indicated the consecutive deposition of polymeric electrolytes onto the particle, whereas MR phantom studies demonstrated that nanoparticles with more layers of the Gd-tetra-azacyclodode-canetetraacetic acid (Gd-DOTA) polymers had the expected higher relaxivities on a per particle basis. The layer-by-layer self-assembly thus increases the Gd payload without deleteriously affecting the MR relaxivity on a per Gd basis, presumably as a result of the flexible and disordered nature of the polyelectrolyte layer-by-layer assembly, which allows ready water access to the Gd centers.
Scheme 2.
Layer-by-layer self-assembly of multifunctional hybrid nanoparticles for an increased Gd payload. PSS, polystyrene sulfonate; DTTA, diethylenetriamine tetraacetate.
A peptide sequence containing arginine-glycine-aspartate (RGD) and seven consecutive lysine (K) residues (K7RGD) was adsorbed onto the particles terminated with a negatively charged PSS layer by electrostatic interactions.50 The RGD peptide is known to bind strongly to the integrin cell surface receptors that are upregulated in angiogenic cancer cells. The RGD-terminated nanoparticles allowed target-specific optical imaging and MRIs of HT-29 human colon cancer cells (Figure 7).
Figure 7.

(a) Transmission electron microscopy image and (b) schematic representation of layer-by-layer self-assembled nanoparticles decorated with arginine-glycine-aspartate (RGD) peptides for integrin targeting. (c) T1-weighted magnetic resonance images of HT-29 (human colon cancer) cells that have been incubated with various nanoparticles. From left to right, control cells without any nanoparticle, cells with layer-by-layer nanoparticles, cells with layer-by-layer nanoparticles that were functionalized with RGD peptide, and cells with layer-by-layer nanoparticles that were functionalized with RGD peptide. (d) Phase contrast optical and (e) confocal fluorescence images of HT-29 cells incubated with layer-by-layer nanoparticles that were functionalized with RGD peptide.49
Taylor et al. also prepared mesoporous silica nanospheres (MSNs) with grafted Gd chelates as T1 contrast agents.51 The extremely high surface area of MSN allows a high payload of Gd chelates with enhanced water accessibility, which is the key to high r1 relaxivity. MSNs with a diameter of 75 nm and 2.4-nm open channels were first prepared by a surfactant-templated, base-catalyzed condensation of tetra(ethoxy)silane (Figure 8). Subsequently, a monolayer of Gd-chelate was grafted by refluxing in toluene. The resulting MSNs exhibited exceptionally high relaxivities both on a per Gd (∼28.8 mM−1 s−1 at 3 T) and per particle basis (∼7.0 × 105 mM−1 s−1 at 3 T). The efficacy of MSNs as T1 contrast agents was demonstrated both in vitro and in vivo, suggesting their potential in early disease diagnosis.
Figure 8.
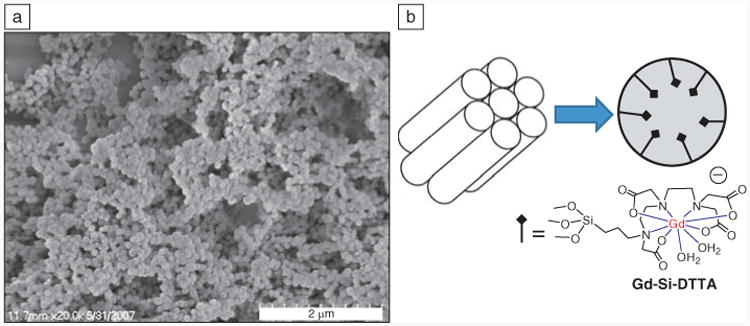
(a) Scanning electron microscope image of mesoporous silica nanospheres showing the formation of monodisperse, water-dispersable nanoparticles. (b) Schematic showing the Gd-(trimethoxysilylpropyl)diethylenetriamine tetraacetate (Gd-Si-DTTA) complexes residing in hexagonally ordered nanochannels of ∼2.4 nm in diameter.51
Nanoscale Metal-Organic Frameworks as T1 Contrast Agents
Metal-organic frameworks (MOFs) are a new class of isoreticular materials built from linking metal ions with well-defined coordination geometry with organic bridging ligands. Their scale-down to the nanometer regime can lead to a new class of tunable nanoscale metal-organic frameworks (NMOFs) with promising properties. Rieter et al. synthesized nanorods of Gd(BDC)1.5 (H2O)2 (BDC is 1,4-benzenedicarboxylate) using water-in-oil micro emulsions (Figure 9).52 The morphology and size of the crystalline Gd NMOFs could be controlled by the water to surfactant molar ratio (i.e., w-value).
Figure 9.

Scanning electron microscope images of Gd(BDC)1.5(H2O)2 nanorods synthesized with w = 5 at (a) lower magnficiation and (b) higher magnification; and w = 10 at (c) lower magnification and (d) higher magnification. BDC is 1,4-benzenedicarboxylate. w, water to surfactant molar ratio.52
The Gd NMOFs display a longitudinal relaxivity (r1) of 35.8 mM−1 s−1, a value almost an order of magnitude higher than that of any commercially available T1 contrast agent. The Gd NMOFs contain a large number of Gd metal centers and thus exhibit an r1 of 2.5 × 107 s−1 per mM nanorod. The Gd centers at or near the surface of the NMOF are likely responsible for the observed relaxivities. By doping the Gd NMOFs with 5% of Eu or Tb, the nanorods exhibited characteristic red or green luminescence, respectively. The Gd NMOFs, however, leached μM concentrations of Gd3+ ions in water, which precludes its in vivo applications as MRI contrast agents due to the toxicity of Gd3+ ions. Attempts were made to stabilize the NMOFs by coating them with a silica shell.53 The release of Gd3+ ions from the NMOF-silica core-shell nanostructures was significantly retarded but not entirely eliminated.
In order to eliminate the toxicity caused by Gd3+ ions, Taylor et al. recently synthesized Mn-based NMOFs as T1 contrast agents.54 NMOFs based on Mn2+ connectors and BDC and 1,3,5-benzenetricarboxylate (BTC) bridging ligands were synthesized in reverse microemulsions. Mn NMOF-silica core-shell nanostructures were further functionalized with rhodamine B and a targeting peptide for cancer imaging. Confocal fluorescence microscopy and MRI studies showed that the RGD-targeted core-shell nanoparticles exhibited enhanced uptake by angiogenic human colon carcinoma (HT-29) cells as compared to nontargeted particles.54 Mn NMOF-silica core-shell nanostructures thus provide a unique approach to delivering large doses of Mn2+ ions to targeted cells to allow for significant T1-weighted MR contrast enhancement.
Concluding Remarks
We have reviewed recent progress in the synthesis and functionalization of magnetic nanoparticles and their applications in early detection of malignant tumors by magnetic resonance imaging (MRI). The advancement in the synthesis of superparamagnetic ferrite nanoparticles with narrow size distributions and high cry stallinity has led to more efficient T2 contrast agents as a result of their enhanced magnetization. The new surface coatings also play an important role in the efficient conjugation of affinity molecules, which allows for tumor-specific accumulation of superparamagnetic nanoparticles. Superparamagnetic iron oxide and related superparamagnetic ferrite nanoparticles have thus enjoyed great success in early detection of malignant tumors by MRI.
T1 shortening nanoparticulate contrast agents can overcome the drawbacks of negative contrast enhancement afforded by ferrite nanoparticles, but their synthesis presents a much greater challenge. Perfluorocarbon-based microemulsions are the most mature nanoparticulate T1 contrast agents and have been used in the MRIs of tumors and angiogenesis. Several newer systems such as hybrid silica nanoparticles and nanoscale metal-organic frameworks have shown promise in target-specific MRI, but much more work needs to be carried out in order to assess their potential in clinical applications. The access to both nanoparticulate T1 and T2 contrast agents should allow physicians to diagnose cancer early and to more effectively treat patients with cancer.
References
- 1.Stark DD, Bradley WG., Jr . Magnetic Resonance Imaging. Mosby; St. Louis: 1999. [Google Scholar]
- 2.Aime S, Botta M, Fasano M, Terreno E. Chem Soc Rev. 1998;27:19. [Google Scholar]
- 3.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 4.Larson SM. Cancer. 1991;67(Suppl. 4):1253. doi: 10.1002/1097-0142(19910215)67:4+<1253::aid-cncr2820671523>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Yang DJ, Kim EE, Inoue T. Ann Nucl Med. 2006;20:1. doi: 10.1007/BF02985584. [DOI] [PubMed] [Google Scholar]
- 6.Gupta H, Weissleder R. Magn Reson Imaging Clin N Am. 1996;4:171. [PubMed] [Google Scholar]
- 7.Petersein J, Saini S, Weissleder R. Magn Reson Imaging Clin N Am. 1996;4:53. [PubMed] [Google Scholar]
- 8.Corot C, Robert P, Idee JM, Port M. Adv Drug Deliv Rev. 2006;58:1471. doi: 10.1016/j.addr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca Dias MH, Lauterbur PC. Magn Reson Med. 1986;3:328. doi: 10.1002/mrm.1910030218. [DOI] [PubMed] [Google Scholar]
- 10.Semelka RC, Helmberger TK. Radiology. 2001;218:27. doi: 10.1148/radiology.218.1.r01ja2427. [DOI] [PubMed] [Google Scholar]
- 11.Kang HW, Josephson L, Petrovsky A, Weissleder R, Bogdanov A., Jr Bioconjug Chem. 2002;13:22. doi: 10.1021/bc0155521. [DOI] [PubMed] [Google Scholar]
- 12.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. N Engl J Med. 2003;348:2491. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 13.Harisinghani MG, Jhaveri KS, Weissleder R, Schima W, Saini S, Hahn PF, Mueller PR. Clin Radiol. 2001;56:714. doi: 10.1053/crad.2001.0764. [DOI] [PubMed] [Google Scholar]
- 14.Harisinghani MG, Saini S, Weissleder R, Halpern EF, Schima W, Rubin DL, Stillman AE, Sica GT, Small WC, Hahn PF. Radiology. 1997;202:687. doi: 10.1148/radiology.202.3.9051017. [DOI] [PubMed] [Google Scholar]
- 15.Weissleder R, Elizondo G, Stark DD, Hahn PF, Marfil J, Gonzalez JF, Saini S, Todd LE, Ferrucci JT. Am J Roentgenol. 1989;152:175. doi: 10.2214/ajr.152.1.175. [DOI] [PubMed] [Google Scholar]
- 16.Montet X, Weissleder R, Josephson L. Bioconjug Chem. 2006;17:905. doi: 10.1021/bc060035+. [DOI] [PubMed] [Google Scholar]
- 17.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Circ Res. 2005;96:327. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 18.Schellenberger EA, Hogemann D, Josephson L, Weissleder R. Acad Radiol. 2002;9(Suppl. 2):S310. doi: 10.1016/s1076-6332(03)80212-x. [DOI] [PubMed] [Google Scholar]
- 19.Koenig SH, Keller KE. Magn Reson Med. 1995;34:227. doi: 10.1002/mrm.1910340214. [DOI] [PubMed] [Google Scholar]
- 20.Morales MP, Veintemillas-Verdaguer S, Montero MI, Serna CJ. Chem Mater. 1999;11:3058. [Google Scholar]
- 21.(a) Jeong U, Teng X, Wang Y, Yang H, Xia Y. Adv Mater. 2007;19:33. [Google Scholar]; (b) Cheon J, Lee JH. Acc Chem Res. 2008;41:1630. doi: 10.1021/ar800045c. [DOI] [PubMed] [Google Scholar]; (c) Aslam M, Schultz EA, Sun T, Meade TJ, Dravid VP. Cryst Growth Design. 2007;7:471. doi: 10.1021/cg060656p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Nat Mater. 2004;3:891. doi: 10.1038/nmat1251. [DOI] [PubMed] [Google Scholar]
- 23.Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. J Am Chem Soc. 2005;127:5732. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Huh YM, Jun YW, Seo JW, Jang JT, Song HT, Kim S, Cho EJ, Yoon HG, Suh JS, Cheon J. Nat Med. 2007;13:95. doi: 10.1038/nm1467. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Xu K, Gu H, Zheng R, Liu H, Zhang X, Guo Z, Xu B. J Am Chem Soc. 2004;126:9938. doi: 10.1021/ja0464802. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Valencia CA, Liu R, Lin W. Bioconjug Chem. 2007;18:333. doi: 10.1021/bc060195l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M, Chen Y, Liu Y, Peng X. Adv Mater. 2005;17:1429. doi: 10.1002/adma.200401991. [DOI] [PubMed] [Google Scholar]
- 28.Kohler N, Fryxell GE, Zhang M. J Am Chem Soc. 2004;126:7206. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Angew Chem Int Ed. 2008;47:8438. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 30.Bulte JWM, Kraitchman DL. NMR Biomed. 2004;17:484. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 31.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Cyrus T, Marsh JN, Neubauer AM, Partlow KC, Wickline SA. Nanomed. 2006;1(3):321. doi: 10.2217/17435889.1.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Morawski AW, Winter PM, Crowder KC, Caruthers SD, Fuhrhop RW, Scott MJ, Robertson JD, Abendschein DR, Lanza GM, Wickline SA. Magn Reson Med. 2004;51:480. doi: 10.1002/mrm.20010. [DOI] [PubMed] [Google Scholar]
- 33.Caruthers SD, Wickline SA, Lanza GM. Curr Opin Biotechnol. 2007;18:26. doi: 10.1016/j.copbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Winter PM, Caruthers SD, Kassner A, Harris TD, Chinen LK, Allen JS, Lacy EK, Zhang H, Robertson JD, Wickline SA, Lanza GM. Cancer Res. 2003;63:5838. [PubMed] [Google Scholar]
- 35.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Circulation. 2003;108:2270. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 36.Lanza GM, Abendschein DR, Hall CS, Marsh JN, Scott MJ, Scherrer DE, Wickline SA. Invest Radiol. 2000;35:227. doi: 10.1097/00004424-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Zhu D, White RD, Hardy PA, Weerapreeyakul N, Sutthanut K, Jay M. J Nanosci Nanotechnol. 2006;6:996. doi: 10.1166/jnn.2006.169. [DOI] [PubMed] [Google Scholar]
- 38.Zhu D, Lu X, Hardy PA, Leggas M, Jay M. Invest Radiol. 2008;43:129. doi: 10.1097/RLI.0b013e31815878dd. [DOI] [PubMed] [Google Scholar]
- 39.Kabalka G, Buonocore E, Hubner K, Moss T, Norley N, Huang L. Radiology. 1987;163:255. doi: 10.1148/radiology.163.1.3454163. [DOI] [PubMed] [Google Scholar]; (b) Kabalka GW, Buonocore E, Hubner K, Davis M, Huang L. Magn Reson Med. 1988;8:89. doi: 10.1002/mrm.1910080111. [DOI] [PubMed] [Google Scholar]; (c) Kabalka GW, Davis MA, Moss TH, Buonocore E, Hubner K, Holmberg E, Maruyama K, Huang L. Magn Reson Med. 1991;19:406. doi: 10.1002/mrm.1910190231. [DOI] [PubMed] [Google Scholar]
- 40.(a) Unger EC, MacDougall P, Cullis P, Tilcock C. Magn Reson Imaging. 1989;7:417. doi: 10.1016/0730-725x(89)90491-8. [DOI] [PubMed] [Google Scholar]; (b) Unger EC, Winokur T, MacDougall P, Rosenblum J, Clair M, Gatenby R, Tilcock C. Radiology. 1989;171(1):81. doi: 10.1148/radiology.171.1.2928550. [DOI] [PubMed] [Google Scholar]; (c) Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Nat Med. 1998;4:623. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 41.Kamaly N, Kalber T, Ahmad A, Oliver MH, So PW, Herlihy AH, Bell JD, Jorgensen MR, Miller AD. Bioconjug Chem. 2008;19:118. doi: 10.1021/bc7001715. [DOI] [PubMed] [Google Scholar]
- 42.Bridot JL, Faure AC, Laurent S, Rivière C, Billotey C, Hiba B, Janier M, Josserand V, Coll JL, Elst LV, Muller R, Roux S, Perriat P, Tillement O. J Am Chem Soc. 2007;129:5076. doi: 10.1021/ja068356j. [DOI] [PubMed] [Google Scholar]
- 43.Evanics F, Diamente PR, van Veggel FCJM, Stanisz GJ, Prosser RS. Chem Mater. 2006;18:2499. [Google Scholar]
- 44.Hifumi H, Yamaoka S, Tanimoto A, Citterio D, Suzuki K. J Am Chem Soc. 2006;128:15090. doi: 10.1021/ja066442d. [DOI] [PubMed] [Google Scholar]
- 45.Gilad AA, Walczak P, McMahon MT, Na HB, Lee JH, An K, Hyeon T, van Zijl PCM, Bulte JWM. Magn Reson Med. 2008;60:1. doi: 10.1002/mrm.21622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Soo Kim K, Kim SO, Hyeon T. Angew Chem Int Ed. 2007;46:5397. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 47.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai H. Nat Mater. 2006;5:971. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 48.Rieter WJ, Kim JS, Taylor KML, An H, Lin W, Tarrant T, Lin W. Angew Chem Int Ed Engl. 2007;46:3680. doi: 10.1002/anie.200604738. [DOI] [PubMed] [Google Scholar]
- 49.Kim JS, Rieter WJ, Taylor KML, An H, Lin W, Lin W. J Am Chem Soc. 2007;129:8962. doi: 10.1021/ja073062z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Folkman J. Nature Med. 1995;1:27. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 51.Taylor KML, Kim JS, Rieter WJ, An H, Lin W, Lin W. J Am Chem Soc. 2008;130:2154. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 52.Rieter WJ, Taylor KML, An H, Lin W, Lin W. J Am Chem Soc. 2006;128:9024. doi: 10.1021/ja0627444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rieter WJ, Taylor KML, Lin W. J Am Chem Soc. 2007;129:9852. doi: 10.1021/ja073506r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor KML, Rieter WJ, Lin W. J Am Chem Soc. 2008;130:14358. doi: 10.1021/ja803777x. [DOI] [PubMed] [Google Scholar]