Abstract
Virtually all humans are exposed to bisphenol A (BPA). Since BPA can act as a ligand for estrogen receptors, potential hazardous effects of BPA should be evaluated in the context of endogenous estrogenic hormones. Because estrogen is metabolized in the placenta, developing fetuses are normally exposed to very low endogenous estrogen levels. BPA, on the other hand, passes through the placenta and might have distinct adverse consequences during the sensitive stages of fetal development. Testicular gametogenesis and steroidogenesis begin early during fetal development. These processes are sensitive to estrogens and play a role in determining the number of germ stem cells, sperm count, and male hormone levels in adulthood. Although studies have shown a correlation between BPA exposure and perturbed reproduction, a clear consensus has yet to be established as to whether current human gestational BPA exposure results in direct adverse effects on male genital development and reproduction. However, studies in animals and in vitro have provided direct evidence for the ability of BPA exposure to influence male reproductive development. This review discusses the current knowledge of potential effects of BPA exposure on male reproductive health and whether gestational exposure adversely affects testis development.
Keywords: bisphenol A, estrogen receptors, reproductive toxicology, testis development
Introduction
Testicular dysgenesis syndrome (TDS) includes hypospadias, combined with cryptorchidism, impaired spermatogenesis, and an increased risk of testicular germ cell cancer. TDS is often present in males with rare genetic abnormalities such as 45 X0 or 46 XY karyotypes. However, over the past 50 y the incidence of individuals with symptoms of TDS has been reported to be rapidly increasing in the general population (for a review, see ref. 1). One hypothesis that accounts for the increasing incidence of TDS is exposure to environmental compounds that disturb estrogen or androgen signaling during critical developmental periods.
It is known from work in experimental animals that exposure to high concentrations of estrogen during fetal development can result in phenotypes resembling TDS. In humans, gestational exposure to the estrogenic compound diethylstilbestrol (DES) results in abnormalities of the male and female reproductive tract along with infertility in women and possibly in men (for a review, see ref. 2). Estrogenic compounds are present in the environment and are likely to result in human exposure. In water and river sediments, biologically relevant concentrations of both natural and synthetic estrogens, such as bisphenol A (BPA), are readily detectable.3 This review discusses the current knowledge of the potential effects of BPA exposure on human health, in particular, on male reproductive health and whether gestational exposure adversely affects testis development.
BPA Exposure
BPA is one of the most highly produced chemicals worldwide. It was first synthesized in 1891 and its estrogenic capacity was defined by experiments in rats in 1938.4 It was proposed for clinical use as an estrogen, however, the more potent estrogenic compound, DES, was synthesized that same year and BPA was never used clinically. Instead, polymerized BPA was found to be useful for manufacturing polycarbonate plastics and epoxy resins. BPA has been in commercial use as such since 1957, and its production was estimated to be in the range of 4 million metric tons/year in 2006.5 BPA is used in numerous products such as reusable food and beverage plastic containers, lining of cans, dental sealants, computers, and thermal paper.
While polymeric BPA lacks estrogenic activity, the ester bond that links BPA monomers together is not stable and monomers are released with time. Monomeric BPA can then be absorbed from the plastic containers or cans into foods and drinks consumed by humans.6 Another major exposure route is dermal exposure to BPA from thermal papers used for receipts.7 Daily adult exposure of BPA is estimated to be between 0.05–10 μg/kg body weight per day, and BPA can be consistently detected in the blood, serum, urine, saliva, and other bodily fluids of virtually all humans.8–11 In addition, BPA is spread into the atmosphere through the burning of plastics, and is present at detectable concentrations throughout the world, including the polar regions.12
Exposure to BPA starts before birth, as BPA can pass through the placenta.13 As BPA can be accumulated in fat tissues,14 the developing fetus may be subjected to elevated levels of BPA as maternal lipid stores are metabolized during pregnancy releasing deposited BPA. BPA is also secreted into breast milk, and formula-fed babies and young children are exposed through food, water, and plastic containers.15,16 Based on estimates obtained from food consumption and concentration data, infants have the highest BPA exposure in the general population.17 Thus, an urgent question is whether this early estrogenic exposure at sensitive developmental stages has adverse consequences.
BPA Acts as an Estrogen
The structure of BPA makes it a ligand for estrogen receptor α (ESR1/ERα) and estrogen receptor β (ESR2/ERβ).18,19 ERs regulate gene expression by forming hetero- or homodimers that interact with their cognate DNA estrogen-responsive elements (EREs), or by tethering to other DNA-binding proteins. In addition, BPA like other estrogens elicits “nongenomic” or “membrane-initiated” effects through ERs located in the cytoplasm or membrane, or through activation of the membrane-bound G protein-coupled estrogen receptor 1 (GPER1/GPR30) and subsequent activation of downstream cytoplasmic signaling cascades.20 BPA at higher concentrations can also modulate the activity of several other receptors, including the arylhydrocarbon receptor (AHR), all of the peroxisome proliferator-activated receptors (PPARs), the androgen receptor (AR), both thyroid hormone receptors (THRs/TRs), the pregnane X receptor (NR1I2/PXR), and the estrogen-related receptor gamma (ESRRG/ERRg).21–23 Because of BPA’s capacity to interfere with hormone signaling, it is categorized as an endocrine disrupting chemical (EDC).
Tissue- and Cell-Specific Effects of BPA
Tissue-specific effects of BPA primarily depend on its uptake and expression of the two ERs. The ERs have both cell-specific and receptor-specific actions, and their tissue distribution differs. For example, based on their respective mRNA levels in adult human tissue, ERα is readily detected in the uterus, ovary, muscle, mammary gland, pituitary gland, spleen, thymus, prostate, adrenal gland, heart, and kidney; while ERβ is primarily detected in the testis and lung.24 The effect of BPA on a specific tissue is also dependent on its concentration within that tissue. In the zebrafish model, general estrogen-induced activation during early development, based on transgenic ERE-luciferase reporter, is detected in the liver, pancreas, brain, and kidney.25 Subsequently, BPA exposure in zebrafish disrupted axonal growth of primary and secondary motoneurons, and activated expression of the liver-specific and estrogen-regulated vitellogenin 1 (vtg1) gene.26,27 In rat uterus and vagina, both ERα-expressing tissues, BPA produces physiologic effects identical to those elicited by estradiol.28 It is clear that BPA can activate the ERs at relatively low concentrations and that exposure can impact target organs that express either ER.
It has been suggested that BPA may induce BPA-specific target gene expression via ERs, and that its effect in this way would differ from that of other estrogens. In a recent study, we compared BPA-induced gene regulation with that of 17β-estradiol and phytoestrogens in human ERα-positive MCF-7 breast cancer cells. We found that identical genes were regulated by BPA, phytoestrogens, and 17β-estradiol.29 Although MCF-7 cells also express the AR, PPARα/β/γ, TRα/β, GPER1, and AHR,30–32 we found that the expression of all genes regulated at 24 h BPA treatment were inhibited by co-treatment with the ERα antagonist ICI.29 This suggests that the transcriptional activity elicited by BPA in these cells was mediated through ERα, and that there were no unique BPA target genes that estrogen did not activate. We also demonstrated that BPA and phytoestrogens acted in an additive manner.29 The additive effect of BPA and phytoestrogens raises concerns that the feeding of phytoestrogen-rich soy-based formula to infants may enhance the effect of BPA exposure.
Health Consequences of BPA Exposure
The levels of BPA that humans are exposed to are relatively low, and BPA binds to ERs 100 to 1000-fold less efficiently than 17β-estradiol.19,29 Thus, in theory, BPA should have negligible human health effects at the current exposure levels. Multiple studies have, however, shown that low level BPA exposure can alter many physiological processes, and epidemiologic evidence supports that BPA is related to altered neuronal and brain development, immune, ovarian, and metabolic diseases (for a recent review, see ref. 33).
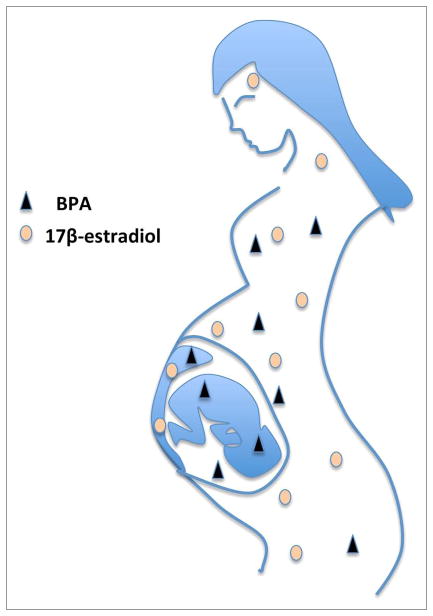
One reason why BPA exposure appears to elicit more severe health consequences than predicted from its exposure levels may be due to gestational exposure, as illustrated in Figure 1. Both male and female fetuses have very low endogenous levels of estrogens: maternal 17-β-estradiol is metabolized by the placenta, and 17-β-estradiol in the fetus is sequestered by fetal α-fetoprotein and not active. BPA, on the other hand, passes through the placenta and binds only weakly to serum proteins.13,34 As a result, even low-level BPA exposure can readily exceed the normal estrogen levels in utero, thereby exposing the developing fetus to greater estrogenic activity than expected. Fetal tissues express both ERα and/or ERβ and in utero exposure to DES, which has a three or four times higher affinity for both ERs than 17-β-estradiol itself,35 results in adverse effects (reviewed in ref. 2). DES was given to many pregnant women during the 1940s to the 1970s in an effort to reduce the risk of habitual and spontaneous abortion, and to make babies stronger at birth. This caused a 40-fold increased risk of rare vaginal/cervical clear cell adenocarcinoma in girls born to these women, making DES one of the first transplacental carcinogens discovered in humans.36 Later studies showed that gestational DES exposure also increased the risk of cervical squamous cell dysplasia and breast cancer in adulthood, along with abnormalities of the reproductive tract and infertility.37,38
Figure 1.
BPA from the mother’s exposure can pass through the placenta and expose the developing fetus. Maternal 17β-estradiol, on the other hand, is metabolized in the placenta and does not reach the fetus. Therefore, the levels of estrogenic active BPA can readily exceed endogenous levels of 17β-estradiol in the developing fetus, also at low-level exposure.
Abnormal activation of ERs by BPA during pre-natal and early development may have similar adverse consequences that, even if small, are potentially problematic given the large population numbers that are exposed. In animals, maternal exposure to BPA (2.4–20 μg/kg) accelerates weight gain and puberty in females along with altered development of the mammary gland and uterus.39–41 Developmental BPA exposure has also been shown to result in stable epigenetic changes in offspring.42 The increased level of BPA exposure in industrial countries coincides with a lowering of semen quality and an increase in male reproductive disorders.43 Moreover, three reports describe a significant correlation between BPA levels in men and lower sperm quality measures (for a recent review, see ref. 33), and one study has linked parental BPA exposure to shorter anogenital distance in sons.44 These associations have led to concerns that BPA exposure may affect male reproductive health.
Estrogen Signaling Impacts Testis Development
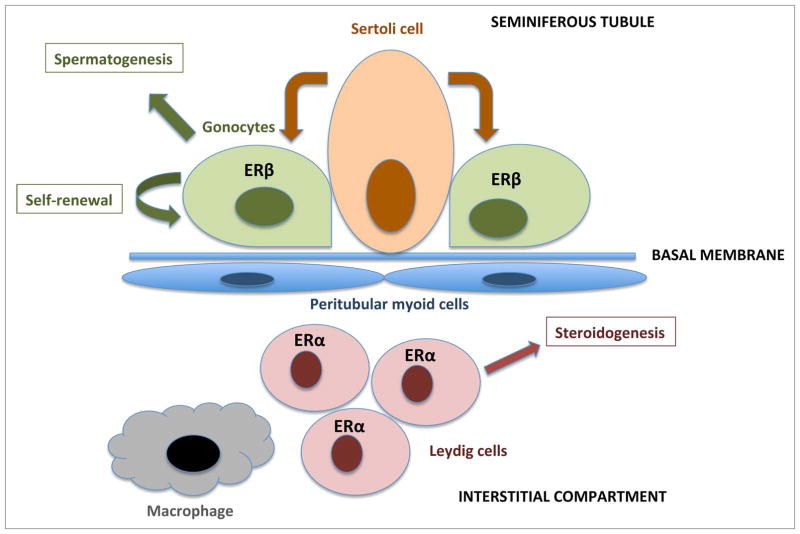
Normal development of the testis is critical for male reproduction. The testis begins gametogenesis and steroidogenesis during early fetal development, and these processes play a role in determining the number of germ stem cells, sperm count, and male hormone levels in adulthood. Testis development is in fact very sensitive to estrogens: ERβ mRNA is detected in mouse gonocytes as early as 14 d post-conception and disappears by post-natal day 26, while ERα is expressed in the interstitial compartment of the testis,45 as illustrated in Figure 2. Inactivation of ERβ in the mouse leads to a 50% increase in the numbers of gonocytes at 2 and 6 d after birth, with no change in Sertoli cell number or Leydig cell number.46 Inactivation of the ERα increases testosterone production as early as 13.5 dpc.46
Figure 2.
A schematic view of the developing testis: cells and functions, including expressions of the ERs. Sertoli cells in the seminiferous tubules comprise a stem cell niche and support the gonocytes (germ cells). The gonocytes express ERβ during fetal and early neonatal development, and are capable of differentiation into spermatids through several stages of spermatogonia/spermatocytes. Leydig cells are located in the interstitial compartment, they express ERα and are responsible for hormone synthesis.
Men exposed to DES before birth have an increased risk for non-cancerous epididymal cysts, fibrotic testis, and undescended testicles (cryptorchidism), along with an increased risk of hypogonadism and low testosterone levels.2 Early studies found decreased fertility and lower sperm count in men exposed to DES in utero, but a 40 y follow-up study found no increased risk of infertility.47,48 A later analysis, however, indicated that DES may have a negative effect on sperm count only if administered during the first trimester of pregnancy.49 While gestational exposure to potent estrogens has major disadvantages for male reproductive development, BPA is far less potent than DES, and exposure to BPA occurs at significantly lower doses. Although studies have shown a correlation, a consensus does not yet exist as to whether or not current human BPA exposure results in adverse effects on male genital development and reproduction. However, studies in animals have provided direct evidence for the ability of BPA exposure to influence male reproductive development.
BPA Exposure and Testis Development
BPA exposure appears to affect male development over a wide range of species and models. In the wild, EDC-induced feminization of male fish has been demonstrated for several species.50 In animal experiments, male goldfish exposed to BPA exhibit modulation of AR, ER-regulated genes, and testicular responses.51 In carp, BPA exposure (from 1 μg/L) caused testicular changes, including loss of typical lobular structure, reduction of spermatogenic cysts, and lobule diameter.52 In mice, high-dose BPA exposure (480 and 960 mg/kg/day at postnatal days 31–44) resulted in underdeveloped testes, apoptotic Leydig and germ cells, and disruption of spermatogenesis.53 Meanwhile, postnatal exposure to lower BPA doses relevant to human exposure (20 and 40 μg/kg/day from day 3) resulted in slowed meiotic progression of germ cells after 5 wk treatment along with decreased quality and quantity of spermatozoa in 7-wk-old mice.54
Gestational BPA exposure at higher concentrations have also been shown to affect testis development in rodents: Exposed mice (5 or 50 mg/kg, days 7 and 14 of pregnancy) exhibited lower epididymal sperm counts, sperm with abnormal morphology and reduced mobility, disruption of sperm differentiation, and decreased numbers of Sertoli cells.55 Lower exposure levels (2 and 20 μg/kg at gestational days 11–17) were found to result in longer anogenital distances.40 In rats, the testis effects of a mixture of plastic-derived compounds (including 25 mg/kg BPA per day, embryonic days 8 to 14) exhibited transgenerational epigenetic inheritance.56
In our previous study, we explored the effects of gestational BPA exposure on testis. The dose, 10 μg/ml in 1% ethanol in the mothers’ drinking water, results in BPA accumulation in mouse tissues comparable with concentrations found in humans.57 Dams from two mouse strains, C57BL/6 (B6) and SJL/JCrHsd (SJL), were treated with BPA or control from preconception through weaning.58 We found no significant changes in weight-corrected anogenital distance or in testis wet weight. However, degeneration of the testes, determined by greater levels of germ cell apoptosis compared with baseline, along with a notable loss of germ cells and general disruption of normal maturation, was observed more often in BPA-exposed mice, and was most common in the later VIII–XII stages of spermatogenesis.58 Our data suggest potential developmentally induced alterations in germ cell maturation, and support the findings from multiple groups that gestational BPA exposure can induce changes in the testis.
Other studies, however, have found no effects of BPA exposure in rodents over a wide range of concentrations and endpoints.59,60 The outcome appears to depend on both the experimental design and potential genetic effects. With regard to the latter, neonatal exposure to potent and environmental estrogens, including tamoxifen, has been shown to lead to strain-specific differences in male reproductive organ abnormalities.61
Direct BPA Exposure Affects the Function of Testicular Cells
In testicular cells, high-dose BPA exposure (100 μM) can inhibit the activity of 11β-hydroxysteroid dehydrogenases (HSD11b1/11β-HSD) in both humans and rats.62 In cultured Leydig cells from the mouse fetal testis, BPA exposure (10 μM) had a negative effect on testosterone production and this effect was maintained in the absence of ERα.63 While BPA concentrations as low as 10 nM affected the function of cultured human fetal Leydig cells, concentrations of 10 μM BPA were required in rodent cells, suggesting that the human testis may be more sensitive to BPA exposure than the testis of mice and rats.63 In the murine spermatogonial GC-1 cell line (ERβ-negative), indications that low-dose BPA exposure (0.1–10 nM) induce proliferation through ERα and GPER1-signaling have been presented,64 while another study reported that BPA did not activate the GPER1 pathway in spermatozoa.65 BPA has also been found to inhibit the activity of ATP-binding cassette, sub-family G (WHITE), member 2, also known as breast cancer-resistance protein (ABCG2/BCRP), a transporter expressed in the blood-testis barrier that transports e.g., steroids.66
Direct and Gestational BPA Exposure Affects Gene Expression in the Testis
BPA exposure can lead to transcriptional changes in rodent male reproductive organs. Direct BPA exposure of adult rat testes (50 mg/kg, 6 d per week for 8 wk) led to decreased expression of testicular steroidogenic enzymes, along with altered n-6 fatty acid composition and antioxidant enzyme levels.67 Similar results were observed in another study of BPA-exposed adult rats (5 mg/kg/day for 8 wk) where Star and Cyp450scc (Cyp11a) expression was increased, and Ar, 3β-HSD (Hsd3b), 17β-HSD (Hsd17b), and Aromatase (Cyp19a1) expression was decreased.68 In mice, upregulation of Fas, Fasl, and Casp3 expression was noted in the testis after BPA exposure (480 and 960 mg/kg/day).53 Young male mice exposed to BPA in drinking water (50 μg/ml) exhibited changes of both ERα and ERβ expression in testes 8 wk after exposure, and in pooled testis samples of mice treated with higher doses of BPA (50 mg/kg, administered twice) downregulation of Msi1h, Ncoa1, Nid1, Hspb2, and Gata6 were detected using a testis-focused small microarray.55,69 However, the latter changes were not confirmed by qPCR, and variability between individuals was not assessed. Newborn mice exposed to a low concentration of BPA (20 and 40 μg/kg/day from postnatal day 3) showed increased testicular expression of ERα after 5 wk treatment,60 and mice exposed to a mixture containing BPA and phthalates during gestation (1–10 mg BPA/kg/day) exhibited decreased expression levels of anti-Mullerian hormone (Amh), Ar, Ccna1 (Cyclin A1), and Star in the testis.70 Gestational BPA-exposure of rats (0.02, 0.5, 400 mg/kg/day, from gestation day 11) resulted in dose-related decrease of Star in the testis at gestation day 20.71
We investigated the result of gestational and early BPA exposure on gene expression in the adult testis of B6 mice (as described above) using microarray analysis. We found subtle changes in gene expression suggesting dysregulation of cell proliferation, spermatogenesis, and apoptosis, consistent with histopathological analyses and increased levels of germ cell apoptosis.58 We further observed downregulation of platelet-derived growth factor α (Pdgfa), a gene well documented to be critical for proper testes development and function.72 This downregulation was confirmed by qPCR in individual mice, and it aligns with previous reports of BPA and other estrogens stimulating testicular gonocyte proliferation in a PDGF-dependent manner in vitro.73 Previous finding that BPA exposure can alter the expression of receptors for PDGF in neonatal testes indicates the dysregulation of the PDGF-signaling pathway as a general theme in BPA-mediated endocrine disruption of the testes.74
Discussion
Combined, current data and literature suggest that BPA exposure can induce histological changes in the testis along with dysregulated proliferation and apoptosis, repression of steroidogenesis, and reduce the number of germ cells, at least in rodents. These effects may involve different molecular pathways including ERα-, ERβ-, and GPER1-mediated pathways, and affect Pdgfa expression and steroidogenesis enzymes.
It’s possible that systemic effects of BPA through the neuroendocrine system also contribute to testicular degeneration and function. In adult rare minnow Gobiocypris rarus, a freshwater teleost, BPA disrupted the gonadotropin-releasing hormone (GnRH) system in the brain.75 Mice exposed to BPA in early life (postnatal day) exhibited altered ERα and ERβ expression in the hypothalamus-pituitary-ovary axis, and BPA exposure during perinatal and postnatal periods resulted in the upregulation of Kiss1, Gnrh1, and Fshb (FSH) mRNA in pups.76 It is not clear whether these changes are induced through interaction with ERs in the testes or is a systemic effect originating from BPA affecting the hypothalamus/pituitary axis. Experiments using tissue-specific ERα- and ERβ-knockout animals are required to address these possibilities.
Multiple reports have implicated BPA exposure in a wide variety of physiological abnormalities, and many countries have banned the use of BPA in food packaging.77 Yet, compared with other xenoestrogens, such as DES, there is often a lack of consensus regarding the effects of BPA. This may be in part due to the fact that (1) many different exposure protocols and animal models are used, (2) the exposure concentrations of BPA and time points vary widely from study to study, (3) that some physiological responses to BPA do not always show classical dose dependence, and (4) that responsiveness to both potent and environmental estrogens is genetically controlled.78–83 Further, monomeric form of BPA can react with other molecules and form a number of derivatives. For example, BPA in drinking water can react with chlorine resulting in chlorinated aqueous BPA which has other molecular properties.84 Chlorinated BPA has been detected in human adipose and placental tissues, dichlorinated BPA being the most abundant form.85,86 Also, halogenated analogs of BPA, such as brominated and chlorinated BPAs, are produced today as flame retardants. The highly produced tetrabromobisphenol A (TBBPA) can be dehalogenated by microorganisms in contaminated sediments from streambeds to form BPA.87 The relevance for human exposure of modified BPA molecules is currently unknown. Toxicology testing today is largely performed on one compound at a time. However, the effect of a compound needs to be examined in its environmental context, e.g., in combination with other man-made compounds or natural compounds, and genetic context. There is a concern that different EDCs act in synergy and that the effects seen in humans are a result of the so-called cocktail effect. There is a lack of studies analyzing the specific effects and combined exposure to environmental estrogenic ligands and the genetic control of responsiveness to individual and combined exposures. We recently investigated the impact of combined BPA and phytoestrogens exposure and detected additive activation of ER signaling.29 Other studies have shown that mixtures of EDCs produce large effects, even when each chemical is present at doses below when they individually have any measurable effects (reviewed in ref. 88). Furthermore, comparatively little is known about mixtures composed of chemicals from different classes of EDCs acting through different molecular pathways. A recent report demonstrated that the combination of a variety of common chemicals with endocrine-disruptor properties is directly interfering with human semen quality89 emphasizing that these knowledge gaps need to be filled to enable a reliable risk assessment of environmental exposure for male reproductive health.
Acknowledgments
This work was supported by grants from National Institutes of Health’s National Cancer Institute under award number R01CA172437 (Williams C); National Institute of Environmental Health Sciences under award number R21ES020036 (Bondesson M); National Institute of Health under award numbers NS076200, NS069628, NS076200, NS036526, and NS060901 (Teuscher C); the National Multiple Sclerosis Society Pilot Research Award PP1728 (Teuscher C) and National MS Society postdoctoral fellowship FG 1911-A-1 (Krementsov DN). The views expressed in this article reflect the views of the authors and not necessarily of the funders.
Abbreviations
- BPA
bisphenol A
- DES
diethylstilbestrol
- EDC
endocrine disrupting chemical
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- ERE
estrogen-response element
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Virtanen HE, Rajpert-De Meyts E, Main KM, Skakkebaek NE, Toppari J. Testicular dysgenesis syndrome and the development and occurrence of male reproductive disorders. Toxicol Appl Pharmacol. 2005;207(Suppl):501–5. doi: 10.1016/j.taap.2005.01.058. http://dx.doi.org/10.1016/j.taap.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Palmlund I. Exposure to a xenoestrogen before birth: the diethylstilbestrol experience. J Psychosom Obstet Gynaecol. 1996;17:71–84. doi: 10.3109/01674829609025667. http://dx.doi.org/10.3109/01674829609025667. [DOI] [PubMed] [Google Scholar]
- 3.Kinani S, Bouchonnet S, Creusot N, Bourcier S, Balaguer P, Porcher JM, Aït-Aïssa S. Bioanalytical characterisation of multiple endocrine- and dioxin-like activities in sediments from reference and impacted small rivers. Environ Pollut. 2010;158:74–83. doi: 10.1016/j.envpol.2009.07.041. http://dx.doi.org/10.1016/j.envpol.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Dodds EC, Lawson W. Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc R Soc Lond, B. 1938;125:222–32. http://dx.doi.org/10.1098/rspb.1938.0023. [Google Scholar]
- 5.Bailin PD, Byrne M, Lewis S, Liroff R. Public awareness drives market for safer alternatives: bisphenol A market analysis report. Investor Environmental Health Network. 2008:1–37. [Google Scholar]
- 6.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–12. doi: 10.1289/ehp.95103608. http://dx.doi.org/10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424–30. doi: 10.1016/j.chemosphere.2010.09.058. http://dx.doi.org/10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–41. doi: 10.1093/humrep/17.11.2839. http://dx.doi.org/10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi K, Watanabe S. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:365–70. doi: 10.1016/s1570-0232(02)00547-0. http://dx.doi.org/10.1016/S1570-0232(02)00547-0. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, Tachino H, Tuchida K, Kubono K. Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med. 2005;16:297–300. doi: 10.1007/s10856-005-0627-8. http://dx.doi.org/10.1007/s10856-005-0627-8. [DOI] [PubMed] [Google Scholar]
- 11.Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C. Lead and bisphenol A concentrations in the Canadian population. Health Rep. 2010;21:7–18. [PubMed] [Google Scholar]
- 12.Fu P, Kawamura K. Ubiquity of bisphenol A in the atmosphere. Environ Pollut. 2010;158:3138–43. doi: 10.1016/j.envpol.2010.06.040. http://dx.doi.org/10.1016/j.envpol.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 2010;202:e1–7. doi: 10.1016/j.ajog.2010.01.025. http://dx.doi.org/10.1016/j.ajog.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez MF, Arrebola JP, Taoufiki J, Navalón A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;24:259–64. doi: 10.1016/j.reprotox.2007.06.007. http://dx.doi.org/10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol Lett. 2010;199:372–6. doi: 10.1016/j.toxlet.2010.09.022. http://dx.doi.org/10.1016/j.toxlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Bailey A, Hatwell K, Mihalov K. Exposure to bisphenol A (BPA) for infants, toddlers and adults from the consumption of infant formula, toddler food and adult (canned) food. Department of Health & Human Services, Food and Drug Administration; 2009. [Google Scholar]
- 17.Beronius A, Rudén C, Håkansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of Bisphenol. A Reprod Toxicol. 2010;29:132–46. doi: 10.1016/j.reprotox.2009.11.007. http://dx.doi.org/10.1016/j.reprotox.2009.11. [DOI] [PubMed] [Google Scholar]
- 18.Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A. 2012;109:14930–5. doi: 10.1073/pnas.1203574109. http://dx.doi.org/10.1073/pnas.1203574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson J-Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 20.Ordóñez-Morán P, Muñoz A. Nuclear receptors: genomic and non-genomic effects converge. Cell Cycle. 2009;8:1675–80. doi: 10.4161/cc.8.11.8579. http://dx.doi.org/10.4161/cc.8.11.8579. [DOI] [PubMed] [Google Scholar]
- 21.Matsushima A, Teramoto T, Okada H, Liu X, Tokunaga T, Kakuta Y, Shimohigashi Y. ERRgamma tethers strongly bisphenol A and 4-alpha-cumylphenol in an induced-fit manner. Biochem Biophys Res Commun. 2008;373:408–13. doi: 10.1016/j.bbrc.2008.06.050. http://dx.doi.org/10.1016/j.bbrc.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 22.Molina-Molina JM, Amaya E, Grimaldi M, Sáenz JM, Real M, Fernández MF, Balaguer P, Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol. 2013;272:127–36. doi: 10.1016/j.taap.2013.05.015. http://dx.doi.org/10.1016/j.taap.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90. doi: 10.1210/jc.2002-020209. http://dx.doi.org/10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- 24.Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, Chetvernin V, Church DM, Dicuccio M, Federhen S, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2012;40:D13–25. doi: 10.1093/nar/gkr1184. http://dx.doi.org/10.1093/nar/gkr1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao R, Bondesson M, Singh AV, Riu A, McCollum CW, Knudsen TB, Gorelick DA, Gustafsson J-Å. Identification of estrogen target genes during zebrafish embryonic development through transcriptomic analysis. PLoS One. 2013;8:e79020. doi: 10.1371/journal.pone.0079020. http://dx.doi.org/10.1371/journal.pone.0079020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Dong Q, Chen Y, Jiang H, Xiao Q, Wang Y, Li W, Bai C, Huang C, Yang D. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat Toxicol. 2013;142–143:104–13. doi: 10.1016/j.aquatox.2013.07.011. http://dx.doi.org/10.1016/j.aquatox.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Shi X, Du Y, Zhou B. Effects of xenoestrogens on the expression of vitellogenin (vtg) and cytochrome P450 aromatase (cyp19a and b) genes in zebrafish (Danio rerio) larvae. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2011;46:960–7. doi: 10.1080/10934529.2011.586253. http://dx.doi.org/10.1080/10934529.2011.586253. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139:2741–7. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- 29.Katchy A, Pinto C, Jonsson J, Nguyen-Vu T, Pandelova M, Riu A, Schramm KW, Samarov D, Gustafsson J-Å, Bondesson M, et al. Coexposure to phytoestrogens and bisphenol A mimics estrogenic effects in an additive manner. Toxicol Sci. 2014;138:21–35. doi: 10.1093/toxsci/kft271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holbeck S, Chang J, Best AM, Bookout AL, Mangelsdorf DJ, Martinez ED. Expression profiling of nuclear receptors in the NCI60 cancer cell panel reveals receptor-drug and receptor-gene interactions. Mol Endocrinol. 2010;24:1287–96. doi: 10.1210/me.2010-0040. http://dx.doi.org/10.1210/me.2010-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo R, Matthews J. High-resolution genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq. Toxicol Sci. 2012;130:349–61. doi: 10.1093/toxsci/kfs253. http://dx.doi.org/10.1093/toxsci/kfs253. [DOI] [PubMed] [Google Scholar]
- 32.Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, et al. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 2010;70:1184–94. doi: 10.1158/0008-5472.CAN-09-3068. http://dx.doi.org/10.1158/0008-5472.CAN-09-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–55. doi: 10.1016/j.reprotox.2013.08.008. http://dx.doi.org/10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Milligan SR, Khan O, Nash M. Competitive binding of xenobiotic oestrogens to rat alpha-fetoprotein and to sex steroid binding proteins in human and rainbow trout (Oncorhynchus mykiss) plasma. Gen Comp Endocrinol. 1998;112:89–95. doi: 10.1006/gcen.1998.7146. http://dx.doi.org/10.1006/gcen.1998.7146. [DOI] [PubMed] [Google Scholar]
- 35.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-Å. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 36.Herbst AL, Kurman RJ, Scully RE, Poskanzer DC. Clear-cell adenocarcinoma of the genital tract in young females. Registry report N Engl J Med. 1972;287:1259–64. doi: 10.1056/NEJM197212212872501. http://dx.doi.org/10.1056/NEJM197212212872501. [DOI] [PubMed] [Google Scholar]
- 37.Troisi R, Hatch EE, Titus-Ernstoff L, Hyer M, Palmer JR, Robboy SJ, Strohsnitter WC, Kaufman R, Herbst AL, Hoover RN. Cancer risk in women prenatally exposed to diethylstilbestrol. Int J Cancer. 2007;121:356–60. doi: 10.1002/ijc.22631. http://dx.doi.org/10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- 38.Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–14. doi: 10.1056/NEJMoa1013961. http://dx.doi.org/10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 39.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. http://dx.doi.org/10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 40.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–22. doi: 10.1016/s0890-6238(02)00006-0. http://dx.doi.org/10.1016/S0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 41.Markey CM, Luque EH, Munoz De Toro M, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–23. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 42.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. http://dx.doi.org/10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. http://dx.doi.org/10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 44.Miao M, Yuan W, He Y, Zhou Z, Wang J, Gao E, Li G, Li DK. In utero exposure to bisphenol-A and ano-genital distance of male offspring. Birth Defects Res A Clin Mol Teratol. 2011;91:867–72. doi: 10.1002/bdra.22845. http://dx.doi.org/10.1002/bdra.22845. [DOI] [PubMed] [Google Scholar]
- 45.Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR. Expression of estrogen receptor beta is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod. 2000;62:310–7. doi: 10.1095/biolreprod62.2.310. http://dx.doi.org/10.1095/biolreprod62.2.310. [DOI] [PubMed] [Google Scholar]
- 46.Delbès G, Levacher C, Habert R. Estrogen effects on fetal and neonatal testicular development. Reproduction. 2006;132:527–38. doi: 10.1530/rep.1.01231. http://dx.doi.org/10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- 47.Gill WB, Schumacher GF, Bibbo M, Straus FH, 2nd, Schoenberg HW. Association of diethylstilbestrol exposure in utero with cryptorchidism, testicular hypoplasia and semen abnormalities. J Urol. 1979;122:36–9. doi: 10.1016/s0022-5347(17)56240-0. [DOI] [PubMed] [Google Scholar]
- 48.Wilcox AJ, Baird DD, Weinberg CR, Hornsby PP, Herbst AL. Fertility in men exposed prenatally to diethylstilbestrol. N Engl J Med. 1995;332:1411–6. doi: 10.1056/NEJM199505253322104. http://dx.doi.org/10.1056/NEJM199505253322104. [DOI] [PubMed] [Google Scholar]
- 49.Storgaard L, Bonde JP, Olsen J. Male reproductive disorders in humans and prenatal indicators of estrogen exposure. A review of published epidemiological studies. Reprod Toxicol. 2006;21:4–15. doi: 10.1016/j.reprotox.2005.05.006. http://dx.doi.org/10.1016/j.reprotox.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Harris CA, Hamilton PB, Runnalls TJ, Vinciotti V, Henshaw A, Hodgson D, Coe TS, Jobling S, Tyler CR, Sumpter JP. The consequences of feminization in breeding groups of wild fish. Environ Health Perspect. 2011;119:306–11. doi: 10.1289/ehp.1002555. http://dx.doi.org/10.1289/ehp.1002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatef A, Zare A, Alavi SM, Habibi HR, Linhart O. Modulations in androgen and estrogen mediating genes and testicular response in male goldfish exposed to bisphenol A. Environ Toxicol Chem. 2012;31:2069–77. doi: 10.1002/etc.1919. http://dx.doi.org/10.1002/etc.1919. [DOI] [PubMed] [Google Scholar]
- 52.Mandich A, Bottero S, Benfenati E, Cevasco A, Erratico C, Maggioni S, Massari A, Pedemonte F, Viganò L. In vivo exposure of carp to graded concentrations of bisphenol A. Gen Comp Endocrinol. 2007;153:15–24. doi: 10.1016/j.ygcen.2007.01.004. http://dx.doi.org/10.1016/j.ygcen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Li Y-J, Song T-B, Cai Y-Y, Zhou JS, Song X, Zhao X, Wu XL. Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci. 2009;108:427–36. doi: 10.1093/toxsci/kfp024. http://dx.doi.org/10.1093/toxsci/kfp024. [DOI] [PubMed] [Google Scholar]
- 54.Zhang GL, Zhang XF, Feng YM, Li L, Huynh E, Sun XF, Sun ZY, Shen W. Exposure to bisphenol A results in a decline in mouse spermatogenesis. Reprod Fertil Dev. 2013;25:847–59. doi: 10.1071/RD12159. http://dx.doi.org/10.1071/RD12159. [DOI] [PubMed] [Google Scholar]
- 55.Tainaka H, Takahashi H, Umezawa M, Tanaka H, Nishimune Y, Oshio S, Takeda K. Evaluation of the testicular toxicity of prenatal exposure to bisphenol A based on microarray analysis combined with MeSH annotation. J Toxicol Sci. 2012;37:539–48. doi: 10.2131/jts.37.539. http://dx.doi.org/10.2131/jts.37.539. [DOI] [PubMed] [Google Scholar]
- 56.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. http://dx.doi.org/10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–40. doi: 10.1016/j.lfs.2003.07.060. http://dx.doi.org/10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- 58.Krementsov DN, Katchy A, Case LK, Carr FE, Davis B, Williams C, Teuscher C. Studies in experimental autoimmune encephalomyelitis do not support developmental bisphenol a exposure as an environmental factor in increasing multiple sclerosis risk. Toxicol Sci. 2013;135:91–102. doi: 10.1093/toxsci/kft141. http://dx.doi.org/10.1093/toxsci/kft141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci. 2002;68:121–46. doi: 10.1093/toxsci/68.1.121. http://dx.doi.org/10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- 60.Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, Völkel W, Wollin KM, Gundert-Remy U. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–91. doi: 10.3109/10408444.2011.558487. http://dx.doi.org/10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakai M, Uchida K, Teuscher C. The development of male reproductive organ abnormalities after neonatal exposure to tamoxifen is genetically determined. J Androl. 1999;20:626–34. [PubMed] [Google Scholar]
- 62.Guo J, Yuan X, Qiu L, Zhu W, Wang C, Hu G, Chu Y, Ye L, Xu Y, Ge RS. Inhibition of human and rat 11β-hydroxysteroid dehydrogenases activities by bisphenol A. Toxicol Lett. 2012;215:126–30. doi: 10.1016/j.toxlet.2012.10.002. http://dx.doi.org/10.1016/j.toxlet.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 63.N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS One. 2012;7:e51579. doi: 10.1371/journal.pone.0051579. http://dx.doi.org/10.1371/journal.pone.0051579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheng ZG, Zhu BZ. Low concentrations of bisphenol A induce mouse spermatogonial cell proliferation by G protein-coupled receptor 30 and estrogen receptor-α. Environ Health Perspect. 2011;119:1775–80. doi: 10.1289/ehp.1103781. http://dx.doi.org/10.1289/ehp.1103781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luconi M, Bonaccorsi L, Forti G, Baldi E. Effects of estrogenic compounds on human spermatozoa: evidence for interaction with a nongenomic receptor for estrogen on human sperm membrane. Mol Cell Endocrinol. 2001;178:39–45. doi: 10.1016/s0303-7207(01)00416-6. http://dx.doi.org/10.1016/S0303-7207(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 66.Dankers AC, Roelofs MJ, Piersma AH, Sweep FC, Russel FG, van den Berg M, van Duursen MB, Masereeuw R. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro[Epub ahead of print] Toxicol Sci. 2013;136:382–91. doi: 10.1093/toxsci/kft198. http://dx.doi.org/10.1093/toxsci/kft198. [DOI] [PubMed] [Google Scholar]
- 67.Chen M, Xu B, Ji W, Qiao S, Hu N, Hu Y, Wu W, Qiu L, Zhang R, Wang Y, et al. Bisphenol A alters n-6 fatty acid composition and decreases antioxidant enzyme levels in rat testes: a LC-QTOF-based metabolomics study. PLoS One. 2012;7:e44754. doi: 10.1371/journal.pone.0044754. http://dx.doi.org/10.1371/journal.pone.0044754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu LL, Wang X, Zhang XH, Zhang Z, Gu J, Liu L, Wang Y, Wang X, Wang SL. Decreased androgen receptor expression may contribute to spermatogenesis failure in rats exposed to low concentration of bisphenol A. Toxicol Lett. 2013;219:116–24. doi: 10.1016/j.toxlet.2013.03.011. http://dx.doi.org/10.1016/j.toxlet.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Takao T, Nanamiya W, Nazarloo HP, Matsumoto R, Asaba K, Hashimoto K. Exposure to the environmental estrogen bisphenol A differentially modulated estrogen receptor-alpha and -beta immunoreactivity and mRNA in male mouse testis. Life Sci. 2003;72:1159–69. doi: 10.1016/s0024-3205(02)02364-0. http://dx.doi.org/10.1016/S0024-3205(02)02364-0. [DOI] [PubMed] [Google Scholar]
- 70.Xi W, Wan HT, Zhao YG, Wong MH, Giesy JP, Wong CK. Effects of perinatal exposure to bisphenol A and di(2-ethylhexyl)-phthalate on gonadal development of male mice. Environ Sci Pollut Res Int. 2011;19:2515–27. doi: 10.1007/s11356-012-0827-y. http://dx.doi.org/10.1007/s11356-012-0827-y. [DOI] [PubMed] [Google Scholar]
- 71.Horstman KA, Naciff JM, Overmann GJ, Foertsch LM, Richardson BD, Daston GP. Effects of transplacental 17-α-ethynyl estradiol or bisphenol A on the developmental profile of steroidogenic acute regulatory protein in the rat testis. Birth Defects Res B Dev Reprod Toxicol. 2012;95:318–25. doi: 10.1002/bdrb.21020. http://dx.doi.org/10.1002/bdrb.21020. [DOI] [PubMed] [Google Scholar]
- 72.Basciani S, Mariani S, Spera G, Gnessi L. Role of platelet-derived growth factors in the testis. Endocr Rev. 2010;31:916–39. doi: 10.1210/er.2010-0004. http://dx.doi.org/10.1210/er.2010-0004. [DOI] [PubMed] [Google Scholar]
- 73.Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod. 2010;82:825–36. doi: 10.1095/biolreprod.109.081729. http://dx.doi.org/10.1095/biolreprod.109.081729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thuillier R, Wang Y, Culty M. Prenatal exposure to estrogenic compounds alters the expression pattern of platelet-derived growth factor receptors alpha and beta in neonatal rat testis: identification of gonocytes as targets of estrogen exposure. Biol Reprod. 2003;68:867–80. doi: 10.1095/biolreprod.102.009605. http://dx.doi.org/10.1095/biolreprod.102.009605. [DOI] [PubMed] [Google Scholar]
- 75.Qin F, Wang L, Wang X, Liu S, Xu P, Wang H, Wu T, Zhang Y, Zheng Y, Li M, et al. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp Biochem Physiol C Toxicol Pharmacol. 2013;157:192–202. doi: 10.1016/j.cbpc.2012.11.002. http://dx.doi.org/10.1016/j.cbpc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Yu B, Chen QF, Liu ZP, Xu HF, Zhang XP, Xiang Q, Zhang WZ, Cui WM, Zhang X, Li N. Estrogen receptor α and β expressions in hypothalamus-pituitary-ovary axis in rats exposed lactationally to soy isoflavones and bisphenol A. Biomed Environ Sci. 2010;23:357–62. doi: 10.1016/S0895-3988(10)60076-1. http://dx.doi.org/10.1016/S0895-3988(10)60076-1. [DOI] [PubMed] [Google Scholar]
- 77.Erler C, Novak J. Bisphenol a exposure: human risk and health policy. J Pediatr Nurs. 2010;25:400–7. doi: 10.1016/j.pedn.2009.05.006. http://dx.doi.org/10.1016/j.pedn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. http://dx.doi.org/10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fagin D. Toxicology: The learning curve. Nature. 2012;490:462–5. doi: 10.1038/490462a. http://dx.doi.org/10.1038/490462a. [DOI] [PubMed] [Google Scholar]
- 80.Silberberg M, Silberberg R. Susceptibility to estrogen of breast, vagina, and endometrium of various strains of mice. Proc Soc Exp Biol Med. 1951;76:161–4. doi: 10.3181/00379727-76-18423. http://dx.doi.org/10.3181/00379727-76-18423. [DOI] [PubMed] [Google Scholar]
- 81.Griffith JS, Jensen SM, Lunceford JK, Kahn MW, Zheng Y, Falase EA, Lyttle CR, Teuscher C. Evidence for the genetic control of estradiol-regulated responses. Implications for variation in normal and pathological hormone-dependent phenotypes. Am J Pathol. 1997;150:2223–30. [PMC free article] [PubMed] [Google Scholar]
- 82.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–61. doi: 10.1126/science.285.5431.1259. http://dx.doi.org/10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 83.Wall EH, Hewitt SC, Liu L, del Rio R, Case LK, Lin CY, Korach KS, Teuscher C. Genetic control of estrogen-regulated transcriptional and cellular responses in mouse uterus. FASEB J. 2013;27:1874–86. doi: 10.1096/fj.12-213462. http://dx.doi.org/10.1096/fj.12-213462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu JY, Aizawa T, Ookubo S. Products of aqueous chlorination of bisphenol A and their estrogenic activity. Environ Sci Technol. 2002;36:1980–7. doi: 10.1021/es011177b. http://dx.doi.org/10.1021/es011177b. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez MF, Santa-Marina L, Ibarluzea JM, Exposito J, Aurrekoetxea JJ, Torne P, Laguna J, Rueda AI, Pedraza V, Olea N. Analysis of population characteristics related to the total effective xenoestrogen burden: a biomarker of xenoestrogen exposure in breast cancer. Eur J Cancer. 2007;43:1290–9. doi: 10.1016/j.ejca.2007.03.010. http://dx.doi.org/10.1016/j.ejca.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Jiménez-Díaz I, Zafra-Gómez A, Ballesteros O, Navea N, Navalón A, Fernández MF, Olea N, Vílchez JL. Determination of Bisphenol A and its chlorinated derivatives in placental tissue samples by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3363–9. doi: 10.1016/j.jchromb.2010.10.021. http://dx.doi.org/10.1016/j.jchromb.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 87.Arbeli Z, Ronen Z, Díaz-Báez MC. Reductive dehalogenation of tetrabromobisphenol-A by sediment from a contaminated ephemeral streambed and an enrichment culture. Chemosphere. 2006;64:1472–8. doi: 10.1016/j.chemosphere.2005.12.069. http://dx.doi.org/10.1016/j.chemosphere.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 88.Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(Suppl 1):106–14. doi: 10.1289/ehp.9358. http://dx.doi.org/10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schiffer C, Müller A, Egeberg DL, Brenker C, Rehfeld A, Frederiksen H, Wäschle B, Kaupp UB, Balbach M, Wachten D, et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Reports. 2014 doi: 10.15252/embr.201438869. http://dx.doi.org/10.15252/embr.201438869. [DOI] [PMC free article] [PubMed]