Abstract
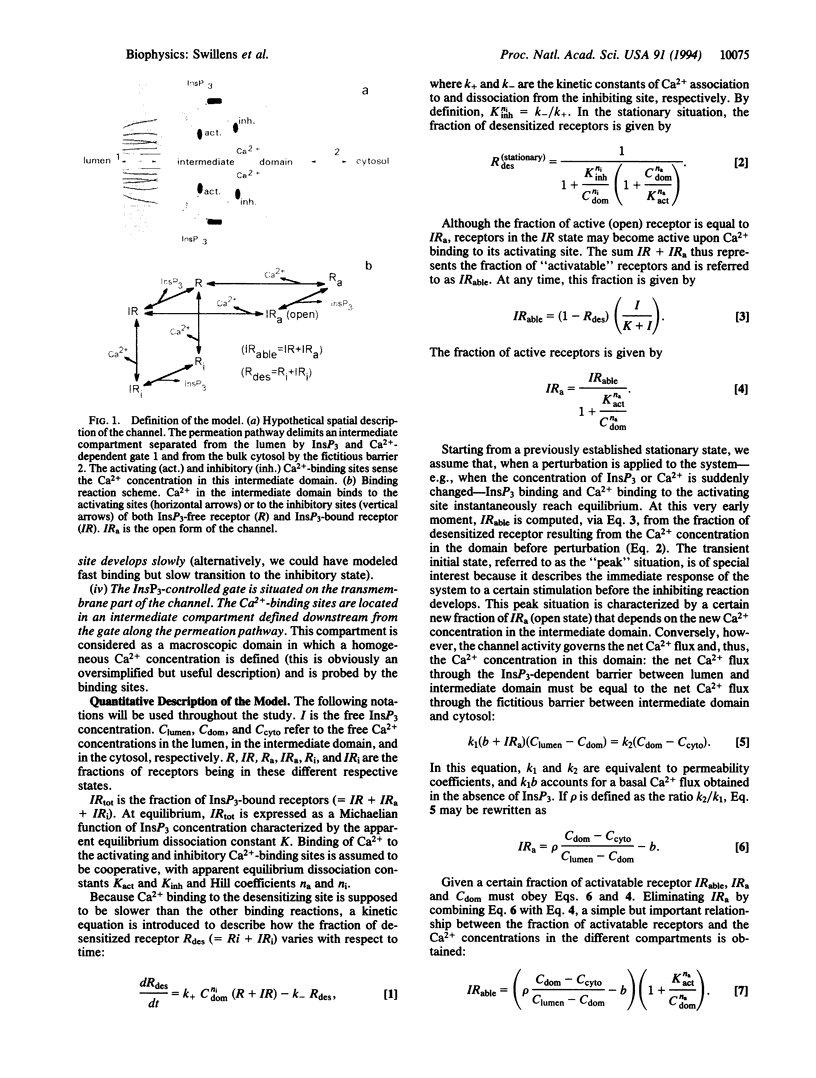
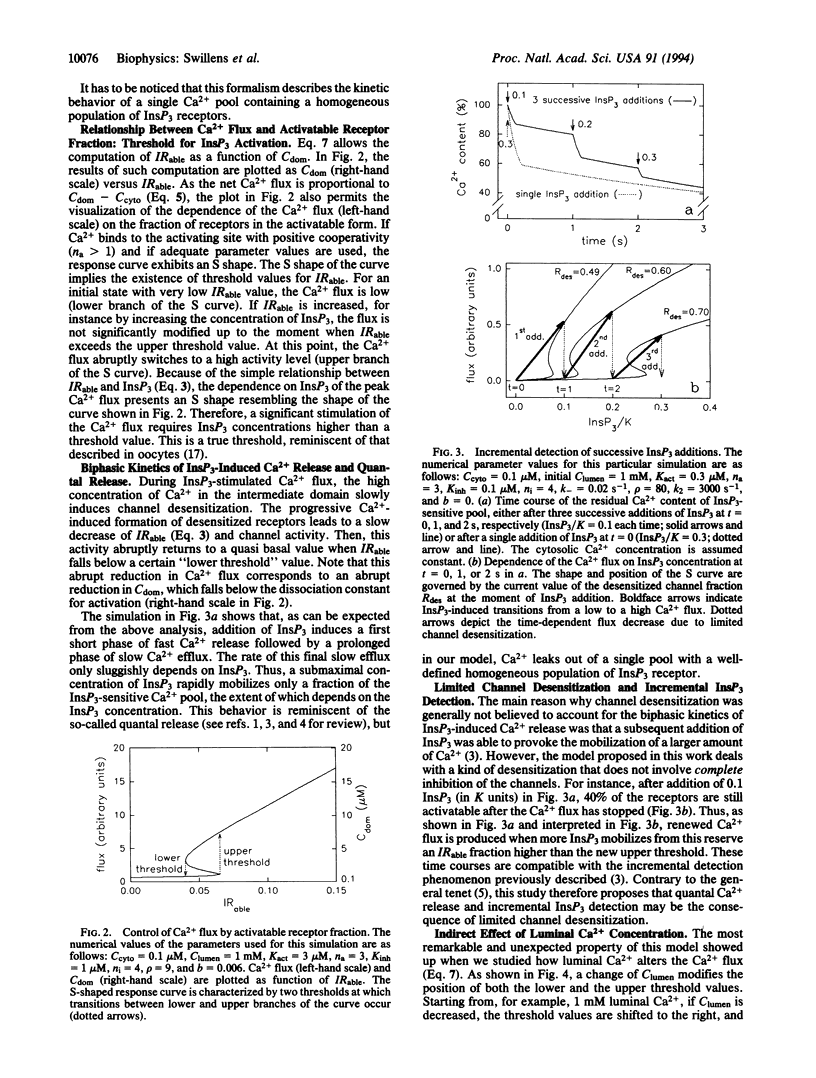
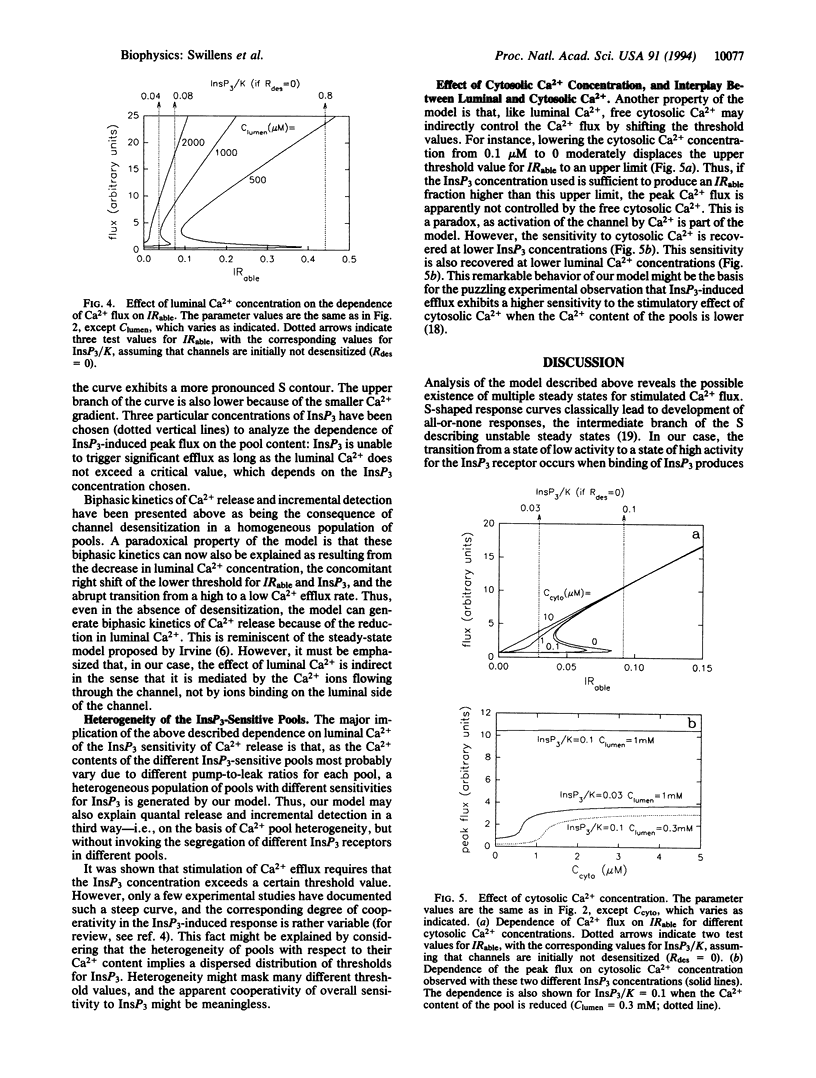
A remarkable property of Ca2+ fluxes through the inositol 1,4,5-trisphosphate (InsP3)-gated Ca2+ channel is that successive increments of InsP3 induce repeated transient release of accumulated Ca2+. The initial aim of this study was to propose a model, based on hypotheses compatible with the current description of this Ca2+ channel, which could account for such experimental observations. The key feature of the model was the assumption that the Ca(2+)-binding sites on the receptor, whose occupancy leads to immediate channel activation but to subsequent slow channel desensitization, were located somewhere along the permeation pathway and were therefore sensitive to the flux of Ca2+ rather than the cytosolic or luminal Ca2+ concentration per se. Simulation showed that, provided Ca2+ bound to both activating and inhibitory sites with adequate cooperativity, addition of submaximal concentrations InsP3 resulted in transient opening well above the stationary state. The model also rationalized the documented existence of a threshold for InsP3 action, the puzzling control of channel sensitivity to InsP3 by luminal and cytosolic Ca2+, as well as the functional heterogeneity of the Ca2+ pools.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Combettes L., Claret M., Champeil P. Calcium control on InsP3-induced discharge of calcium from permeabilised hepatocyte pools. Cell Calcium. 1993 Apr;14(4):279–292. doi: 10.1016/0143-4160(93)90049-c. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Fryer M. W., Zucker R. S. Ca(2+)-dependent inactivation of Ca2+ current in Aplysia neurons: kinetic studies using photolabile Ca2+ chelators. J Physiol. 1993 May;464:501–528. doi: 10.1113/jphysiol.1993.sp019648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Györke S., Palade P. Role of local Ca2+ domains in activation of Ca(2+)-induced Ca2+ release in crayfish muscle fibers. Am J Physiol. 1993 Jun;264(6 Pt 1):C1505–C1512. doi: 10.1152/ajpcell.1993.264.6.C1505. [DOI] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Kindman L. A., Meyer T. Use of intracellular Ca2+ stores from rat basophilic leukemia cells to study the molecular mechanism leading to quantal Ca2+ release by inositol 1,4,5-trisphosphate. Biochemistry. 1993 Feb 9;32(5):1270–1277. doi: 10.1021/bi00056a011. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci U S A. 1990 May;87(10):3841–3845. doi: 10.1073/pnas.87.10.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Mikoshiba K. Inositol 1,4,5-trisphosphate receptor. Trends Pharmacol Sci. 1993 Mar;14(3):86–89. doi: 10.1016/0165-6147(93)90069-v. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992 Jun 18;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Parys J. B., Casteels R. Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem. 1994 Mar 11;269(10):7238–7242. [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn D. L., Taylor C. W. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol. 1992 Jan;41(1):115–119. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+]. J Biol Chem. 1992 Feb 25;267(6):3573–3576. [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Swillens S. Dynamic control of inositol 1,4,5-trisphosphate-induced Ca2+ release: a theoretical explanation for the quantal release of Ca2+. Mol Pharmacol. 1992 Jan;41(1):110–114. [PubMed] [Google Scholar]
- Taylor C. W., Potter B. V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J. 1990 Feb 15;266(1):189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]




