Figure 10.
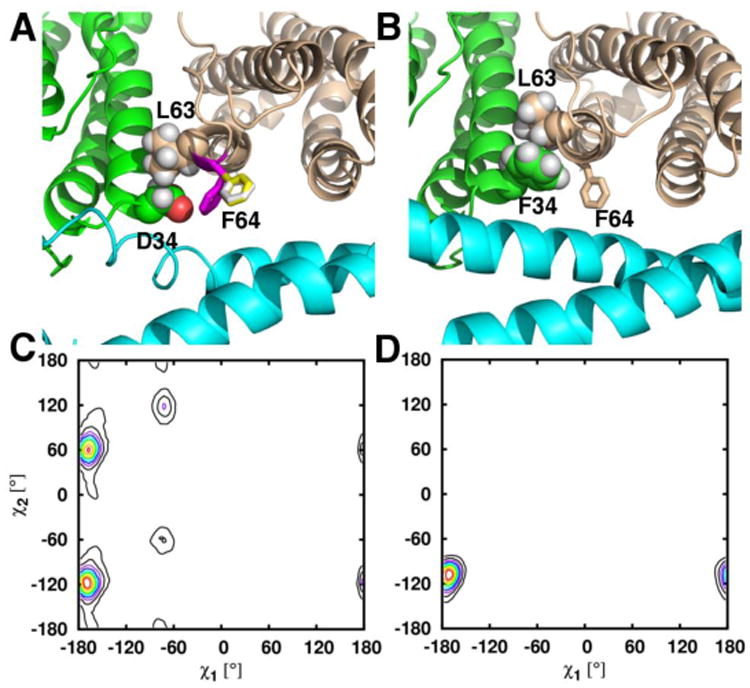
Close up view of a B-pore taken during the MD simulations of (A) wt and (B) D34F BfrB. In wt BfrB, the three rotameric states of the F64 side chain in wt BfrB are depicted by white, yellow and magenta sticks in (A), and the three rotameric states of D34 are indicated in the plot shown in (C). The rotameric exchange of D34 and F64 contributes to the breathing motions of the B-pores as well as ion traffic across B-pores in wt BfrB. In the D34F strcture packing of F34 against L63 (spheres) likely contributes to the lower flexibility of B-pores in the mutant, which is manifested in only one conformational rotamer of the F34 side chain (D), and only one conformation of the F64 side chain, wheat sticks in (B).
