Abstract
BACKGROUND
Although inhibitors of the renin–angiotensin–aldosterone system can slow the progression of diabetic kidney disease, the residual risk is high. Whether nuclear 1 factor (erythroid-derived 2)–related factor 2 activators further reduce this risk is unknown.
METHODS
We randomly assigned 2185 patients with type 2 diabetes mellitus and stage 4 chronic kidney disease (estimated glomerular filtration rate [GFR], 15 to <30 ml per minute per 1.73 m2 of body-surface area) to bardoxolone methyl, at a daily dose of 20 mg, or placebo. The primary composite outcome was end-stage renal disease (ESRD) or death from cardiovascular causes.
RESULTS
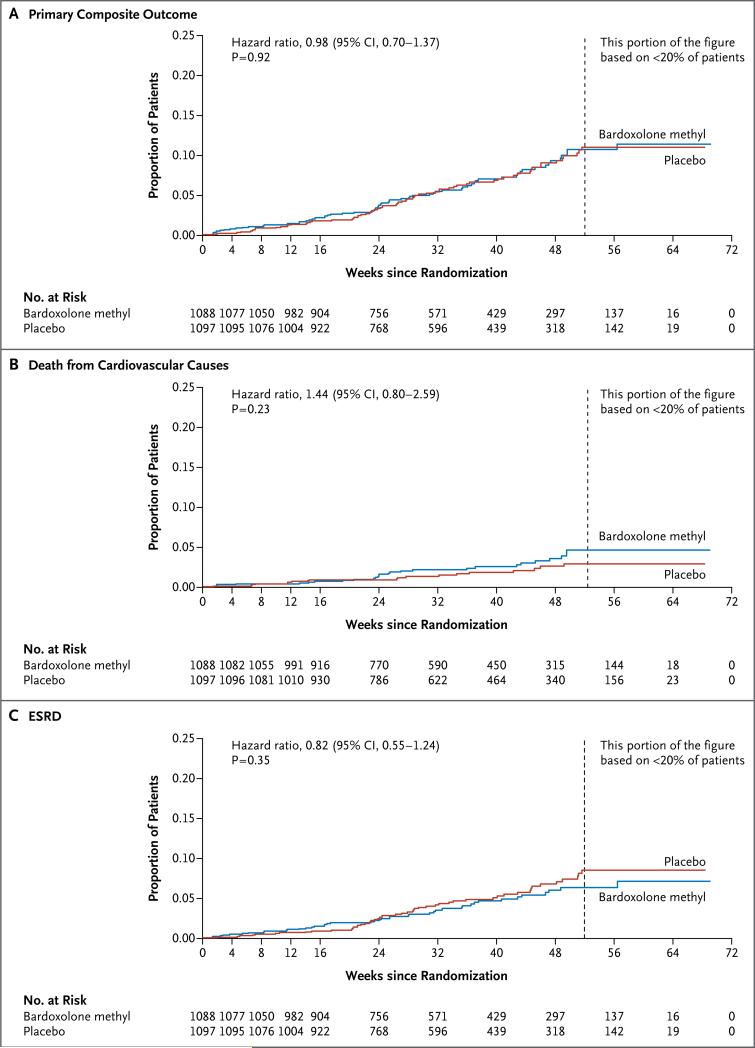
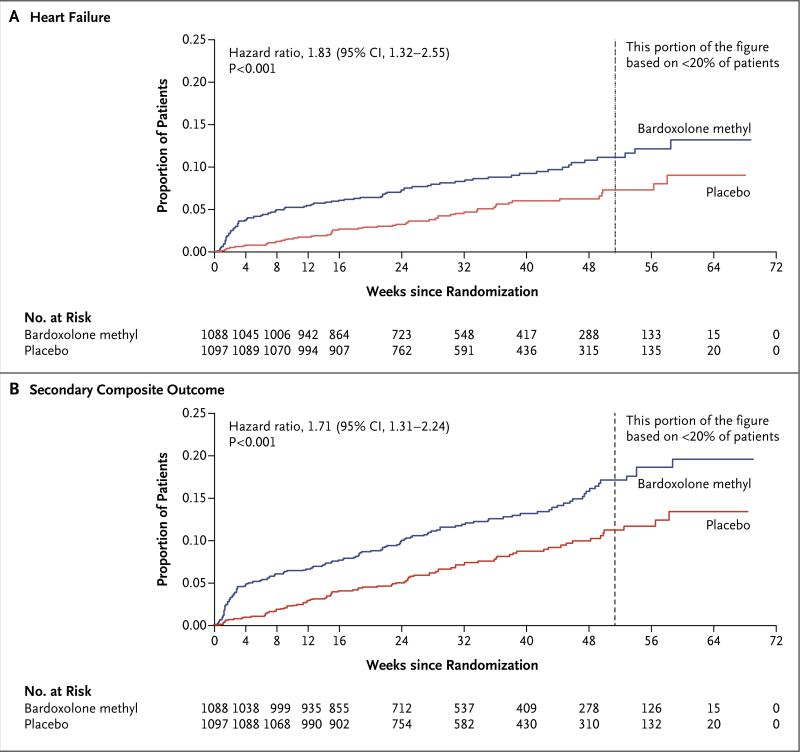
The sponsor and the steering committee terminated the trial on the recommendation of the independent data and safety monitoring committee; the median follow-up was 9 months. A total of 69 of 1088 patients (6%) randomly assigned to bardoxolone methyl and 69 of 1097 (6%) randomly assigned to placebo had a primary composite outcome (hazard ratio in the bardoxolone methyl group vs. the placebo group, 0.98; 95% confidence interval [CI], 0.70 to 1.37; P = 0.92). In the bardoxolone methyl group, ESRD developed in 43 patients, and 27 patients died from cardiovascular causes; in the placebo group, ESRD developed in 51 patients, and 19 patients died from cardiovascular causes. A total of 96 patients in the bardoxolone methyl group were hospitalized for heart failure or died from heart failure, as compared with 55 in the placebo group (hazard ratio, 1.83; 95% CI, 1.32 to 2.55; P<0.001). Estimated GFR, blood pressure, and the urinary albumin-to-creatinine ratio increased significantly and body weight decreased significantly in the bardoxolone methyl group, as compared with the placebo group.
CONCLUSIONS
Among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease, bardoxolone methyl did not reduce the risk of ESRD or death from cardiovascular causes. A higher rate of cardiovascular events with bardoxolone methyl than with placebo prompted termination of the trial. (Funded by Reata Pharmaceuticals; BEACON ClinicalTrials.gov number, NCT01351675.)
Type 2 diabetes mellitus is the most important cause of progressive chronic kidney disease in the developed and developing worlds. Various therapeutic approaches to slow progression, including restriction of dietary protein, glycemic control, and control of hypertension, have yielded mixed results.1-3 Several randomized clinical trials have shown that inhibitors of the renin–angiotensin–aldosterone system significantly reduce the risk of progression,4-6 although the residual risk remains high.7 None of the new agents tested during the past decade have proved effective in late-stage clinical trials.8-12
Oxidative stress and impaired antioxidant capacity intensify with the progression of chronic kidney disease.13 In animals with chronic kidney disease, oxidative stress and inflammation are associated with impaired activity of the nuclear 1 factor (erythroid-derived 2)–related factor 2 (Nrf2) transcription factor. The synthetic triterpenoid bardoxolone methyl and its analogues are the most potent known activators of the Nrf2 pathway. Studies involving humans,14 including persons with type 2 diabetes mellitus and stage 3b or 4 chronic kidney disease, have shown that bardoxolone methyl can reduce the serum creatinine concentration for up to 52 weeks.15
We designed the Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events (BEACON) trial to test the hypothesis that treatment with bardoxolone methyl reduces the risk of end-stage renal disease (ESRD) or death from cardiovascular causes among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease.
METHODS
STUDY DESIGN AND OVERSIGHT
The BEACON trial was a phase 3, randomized, double-blind, parallel-group, international, multi-center trial of once-daily administration of bardoxolone methyl (at a dose of 20 mg in an amorphous spray-dried dispersion formulation), as compared with placebo. Participants were receiving background conventional therapy that included inhibitors of the renin–angiotensin–aldosterone system, insulin or other hypoglycemic agents, and, when appropriate, other cardiovascular medications. The trial design and the characteristics of the trial participants at baseline have been described previously.16,17
Reata Pharmaceuticals sponsored the trial. The trial was jointly designed by employees of the sponsor and the academic investigators who were members of the steering committee. The steering committee, which was led by the academic investigators and included members who were employees of the sponsor, supervised the trial design and operation. An independent data and safety monitoring committee reviewed interim safety data every 90 days or on an ad hoc basis on request. The sponsor collected the trial data and transferred them to independent statisticians at Statistics Collaborative. The sponsor also contracted a second independent statistical group (Axio Research) to support the independent data and safety monitoring committee. The trial protocol was approved by the institutional review board at each participating study site. The protocol and amendments are available with the full text of this article at NEJM.org. The steering committee takes full responsibility for the integrity of the data and the interpretation of the trial results and for the fidelity of the study to the protocol. The first and last authors wrote the first draft of the manuscript. All the members of the steering committee made the decision to submit the manuscript for publication.
STUDY POPULATION
Briefly, we included adults with type 2 diabetes mellitus and an estimated glomerular filtration rate (GFR) of 15 to <30 ml per minute per 1.73 m2 of body-surface area. Persons with poor glycemic control, uncontrolled hypertension, or a recent cardiovascular event (≤12 weeks before randomization) or New York Heart Association class III or IV heart failure were excluded. Additional inclusion and exclusion criteria are listed in Table S1 in the Supplementary Appendix, available at NEJM.org. All the patients provided written informed consent.
RANDOMIZATION AND INTERVENTION
Randomization was stratified according to study site with the use of variable-sized blocks. The steering committee, sponsor, investigators, and trial participants were unaware of the group assignments. After randomization, patients received either bardoxolone methyl or placebo. The prescription of all other medications was at the discretion of treating physicians, who were encouraged to adhere to published clinical-practice guidelines. Patients underwent event ascertainment and laboratory testing according to the study schema shown in Figure S1 in the Supplementary Appendix. Ambulatory blood-pressure monitoring was performed in a substudy that included 174 patients (8%).
The statistical analysis plan defined the study period as the number of days from randomization to a common study-termination date. In the case of patients who were still receiving the study drug on the termination date, data on vital events were collected for an additional 30 days.
OUTCOMES
The primary composite outcome was ESRD or death from cardiovascular causes. We defined ESRD as the need for maintenance dialysis for 12 weeks or more or kidney transplantation. If a patient died before undergoing dialysis for 12 weeks, the independent events-adjudication committee adjudicated whether the need for dialysis represented ESRD or acute renal failure. Patients who declined dialysis and who subsequently died were categorized as having had ESRD. All ESRD events were adjudicated. Death from cardiovascular causes was defined as death due to either cardiovascular or unknown causes.
The trial had three prespecified secondary outcomes — first, the change in estimated GFR as calculated with the use of the four-variable Modification of Diet in Renal Disease study equation, with serum creatinine levels calibrated to an isotope-dilution standard for mass spectrometry; second, hospitalization for heart failure or death due to heart failure; and third, a composite outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death from cardiovascular causes. The events-adjudication committee, whose members were unaware of the study assignments, evaluated whether ESRD events, cardiovascular events, neurologic events, and deaths met the prespecified criteria for primary and secondary outcomes (described in detail in the Supplementary Appendix).
STATISTICAL ANALYSIS
We calculated that we needed to enroll 2000 patients on the basis of the following assumptions: a two-sided type I error rate of 5%, an event rate of 24% for the primary composite outcome in the placebo group during the first 2 years of the study, a hazard ratio of 0.68 (bardoxolone methyl vs. placebo) for the primary composite outcome, discontinuation of the study drug by 13.5% of the patients in the bardoxolone methyl group each year, and a 2.5% annual loss to follow-up in each group. Under these assumptions, if 300 patients had a primary composite outcome, the statistical power would be 85%.
We collected and analyzed all outcome data in accordance with the intention-to-treat principle. We calculated Kaplan–Meier product-limit estimates of the cumulative incidence of the primary composite outcome. We computed hazard ratios and 95% confidence intervals with the use of Cox proportional-hazards regression models with adjustment for the baseline estimated GFR and urinary albumin-to-creatinine ratio. We performed analogous analyses for secondary time-to-event outcomes. Given the abundance of early adverse events, we also report discrete cumulative incidences at 4 weeks and 52 weeks.
For longitudinal analyses of estimated GFR, we performed mixed-effects regression analyses using study group, time, the interaction of study group with time, estimated GFR at baseline, the interaction of baseline estimated GFR with time, and urinary albumin-to-creatinine ratio as covariates, and we compared the means between the bardoxolone methyl group and the placebo group. We adopted similar approaches when examining the effects of treatment on other continuous measures assessed at multiple visits. Since the between-group difference in the primary composite outcome was not significant, secondary and other outcomes with P values of less than 0.05 were considered to be nominally significant. Statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute). Additional details of the statistical analysis are provided in the Supplementary Appendix.
RESULTS
PATIENTS
From June 2011 through September 2012, a total of 2185 patients underwent randomization, including 1545 (71%) in the United States, 334 (15%) in the European Union, 133 (6%) in Australia, 87 (4%) in Canada, 46 (2%) in Israel, and 40 (2%) in Mexico. Figure S2 in the Supplementary Appendix shows the disposition of the study participants.
As shown in Table 1, the patients were diverse with respect to age, sex, race or ethnic group, and region of origin; diabetic retinopathy and neuropathy were common conditions among the patients, as was overt cardiovascular disease. See Table S2 in the Supplementary Appendix for a more detailed description of the characteristics of the patients at baseline; Figure S3 in the Supplementary Appendix shows the distribution of baseline estimated GFR and urinary albumin-to-creatinine ratio.
Table 1.
Baseline Characteristics of the Patients in the Intention-to-Treat Population.*
| Characteristic | Placebo (N = 1097) | Bardoxolone Methyl (N = 1088) | All Patients (N = 2185) |
|---|---|---|---|
| Age — yr | 68.2±9.4 | 68.9±9.7 | 68.5±9.6 |
| Female sex — no. (%) | 472 (43) | 462 (42) | 934 (43) |
| Race — no. (%)† | |||
| White | 848 (77) | 846 (78) | 1694 (78) |
| Black | 176 (16) | 185 (17) | 361 (17) |
| Other | 73 (7) | 57 (5) | 130 (6) |
| Hispanic ethnic group — no. (%)† | 184 (17) | 186 (17) | 370 (17) |
| Region — no. (%) | |||
| United States | 772 (70) | 773 (71) | 1545 (71) |
| European Union | 169 (15) | 165 (15) | 334 (15) |
| Australia | 67 (6) | 66 (6) | 133 (6) |
| Canada | 46 (4) | 41 (4) | 87 (4) |
| Israel | 23 (2) | 23 (2) | 46 (2) |
| Mexico | 20 (2) | 20 (2) | 40 (2) |
| Weight — kg | 95.3±21.1 | 95.1±22.0 | 95.2±21.5 |
| Glycated hemoglobin — % | 7.1±1.2 | 7.2±1.3 | 7.1±1.2 |
| Serum creatinine — mg/dl | 2.7±0.6 | 2.7±0.6 | 2.7±0.6 |
| Estimated GFR — ml/min/1.73 m2 | 22.5±4.6 | 22.4±4.3 | 22.5±4.5 |
| Urinary albumin-to-creatinine ratio‡ | |||
| Median | 351 | 292 | 320 |
| Interquartile range | 60–1136 | 53–1151 | 57–1140 |
| Diabetes history | |||
| Time since diabetes diagnosis — yr | 18.1±9.7 | 18.9±9.7 | 18.5±9.7 |
| Retinopathy — no. (%) | 445 (41) | 446 (41) | 891 (41) |
| Neuropathy — no. (%) | 500 (46) | 517 (48) | 1017 (47) |
| Amputation — no. (%) | 49 (4) | 57 (5) | 106 (5) |
| Foot ulcer — no./total no. (%) | 37/428 (9) | 36/433 (8) | 73/861 (8) |
| Cardiovascular history — no. (%) | |||
| Any history of cardiovascular disease | 619 (56) | 609 (56) | 1228 (56) |
| Hospitalization for heart failure | 118 (11) | 120 (11) | 238 (11) |
Plus–minus values are means ±SD. There were no significant between-group differences at baseline. To convert the values for creatinine to micromoles per liter, multiply by 88.4. GFR denotes glomerular filtration rate.
Race or ethnic group was self-reported. Patients of any race could identify themselves as Hispanic.
The ratio is based on measurement of albumin in milligrams and creatinine in grams.
DRUG EXPOSURE
The median duration of exposure to the study drug was 7 months (interquartile range, 3 to 11) among patients randomly assigned to bardoxolone methyl and 8 months (interquartile range, 5 to 11) among those randomly assigned to placebo. Figure S4 in the Supplementary Appendix shows the time to discontinuation of the study drug. Table S3 in the Supplementary Appendix shows the reasons that patients discontinued the study drug and the reasons that patients discontinued the study. The median duration of follow-up was 9 months in both groups.
OUTCOMES
Primary Composite Outcome
A total of 69 of 1088 patients (6%) randomly assigned to bardoxolone methyl and 69 of 1097 (6%) randomly assigned to placebo had a primary composite outcome (hazard ratio in the bardoxolone methyl group vs. the placebo group, 0.98; 95% confidence interval [CI], 0.70 to 1.37; P = 0.92) (Fig. 1A). Death from cardiovascular causes occurred in 27 patients randomly assigned to bardoxolone methyl and in 19 randomly assigned to placebo (hazard ratio, 1.44; 95% CI, 0.80 to 2.59; P = 0.23) (Fig. 1B). ESRD developed in 43 patients randomly assigned to bardoxolone methyl and in 51 randomly assigned to placebo (hazard ratio, 0.82; 95% CI, 0.55 to 1.24; P = 0.35) (Fig. 1C). One patient in each group died from cardiovascular causes after the development of ESRD. The mean (±SD) estimated GFR before the development of ESRD was 18.1±8.3 ml per minute per 1.73 m2 in the bardoxolone methyl group and 14.9±4.0 ml per minute per 1.73 m2 in the placebo group.
Figure 1. Kaplan–Meier Plots of the Time to the First Event of the Primary Outcome and Its Components.
Panel A shows the time to the first occurrence of the primary composite outcome (end-stage renal disease [ESRD] or death from cardiovascular causes) among patients in the bardoxolone methyl group and those in the placebo group. Panel B shows the time to death from cardiovascular causes in the two study groups, and Panel C the time to the development of ESRD in the two groups.
Secondary Outcomes
During the study period, 96 patients in the bardoxolone methyl group had heart-failure events (93 patients with at least one hospitalization due to heart failure and 3 patients who died from heart failure without hospitalization), as compared with 55 in the placebo group (55 patients with at least one hospitalization due to heart failure and no patients who died from heart failure without hospitalization) (hazard ratio, 1.83; 95% CI, 1.32 to 2.55; P<0.001) (Fig. 2A). A total of 139 patients in the bardoxolone methyl group, as compared with 86 in the placebo group, had a composite outcome event of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death from cardiovascular causes (hazard ratio, 1.71; 95% CI, 1.31 to 2.24; P<0.001) (Fig. 2B).
Figure 2. Kaplan–Meier Plots of the Time to the First Event of the Discrete Secondary Outcomes.
Panel A shows the time to the first event of heart failure, defined as death due to heart failure or hospitalization for heart failure, among patients in the bardoxolone methyl group and those in the placebo group. Panel B shows the time to the first event of the secondary composite outcome (nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death from cardiovascular causes) in the two study groups. The first event was nonfatal myocardial infarction in 17 patients in the bardoxolone methyl group and 11 in the placebo group, nonfatal stroke in 12 patients in the bardoxolone methyl group and 8 in the placebo group, hospitalization for heart failure in 91 patients in the bardoxolone methyl group and 54 in the placebo group, and death from cardiovascular causes in 19 patients in the bardoxolone methyl group and 13 in the placebo group.
Incidences of Composite Outcomes and Rates of Death from Any Cause
The cumulative incidences of the primary composite outcome and of the two secondary composite outcomes at 4 weeks and at 52 weeks are shown in Table S4 in the Supplementary Appendix. The rates of death from any cause are shown in Figure S5 in the Supplementary Appendix. From the time of randomization to the end of follow-up, 75 patients died: 44 patients in the bardoxolone methyl group and 31 in the placebo group (hazard ratio, 1.47; 95% CI, 0.93 to 2.32; P = 0.10). The causes of death are listed in Table S5 in the Supplementary Appendix.
Estimated GFR
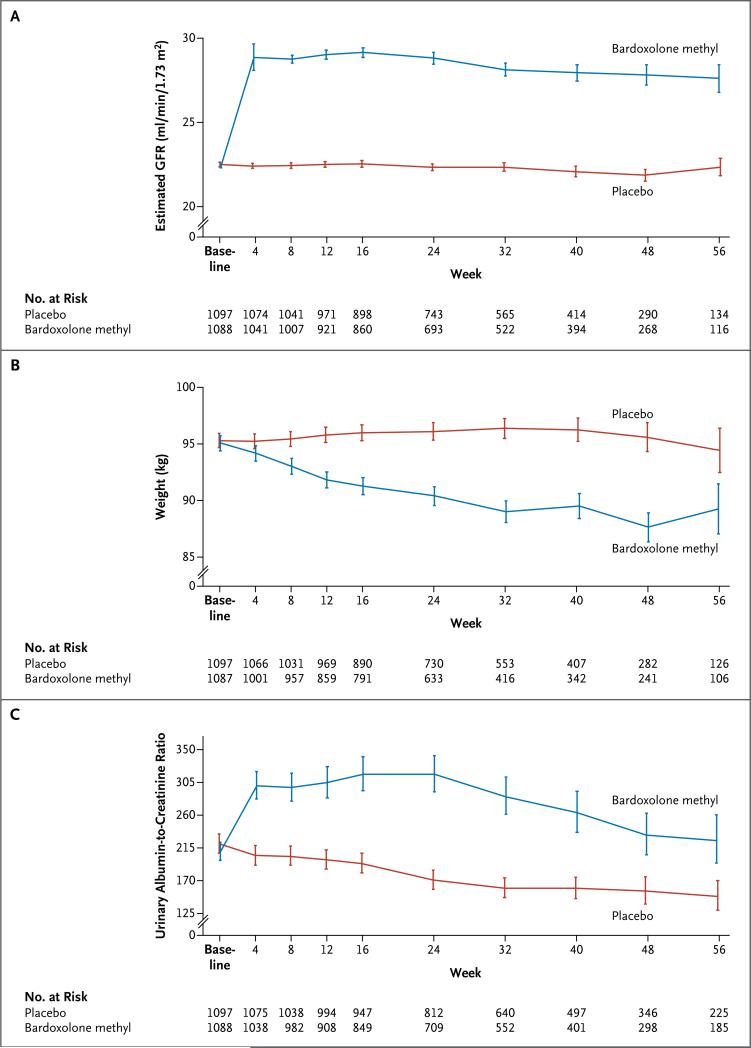
Patients randomly assigned to placebo had a significant mean decline in the estimated GFR from the baseline value (–0.9 ml per minute per 1.73 m2; 95% CI, –1.2 to –0.5), whereas those randomly assigned to bardoxolone methyl had a significant mean increase from the baseline value (5.5 ml per minute per 1.73 m2; 95% CI, 5.2 to 5.9). The difference between the two groups was 6.4 ml per minute per 1.73 m2 (95% CI, 5.9 to 6.9; P<0.001) (Fig. 3A).
Figure 3. Estimated Glomerular Filtration Rate (GFR), Body Weight, and Urinary Albumin-to-Creatinine Ratio.
The urinary albumin-to-creatinine ratio is based on measurement of albumin in milligrams and creatinine in grams. For the estimated GFR and body weight, I bars indicate ±1 SE; for the urinary albumin-to-creatinine ratio, I bars indicate the antilog of the standard error for log urinary albumin-to-creatinine ratio.
Physiological Variables
Physiological variables are shown in Table S6 in the Supplementary Appendix. The mean body weight remained stable in the placebo group but declined steadily and substantially in the bardoxolone methyl group (Fig. 3B). There was a significantly smaller decrease from baseline in mean systolic blood pressure in the bardoxolone methyl group than in the placebo group (between-group difference, 1.5 mm Hg [95% CI, 0.5 to 2.5]), and the mean diastolic blood pressure increased from baseline in the bardoxolone methyl group whereas it decreased in the placebo group (between-group difference, 2.1 mm Hg [95% CI, 1.6 to 2.6]). Blood-pressure results from the substudy in which ambulatory blood-pressure monitoring was performed were similar in direction but were more pronounced (between-group difference of 7.9 mm Hg [95% CI, 3.8 to 12.0] in systolic blood pressure and 3.2 mm Hg [95% CI, 1.3 to 5.2] in diastolic blood pressure). Heart rate also increased significantly in the bardoxolone methyl group, as compared with the placebo group (between-group difference, 3.8 beats per minute; 95% CI, 3.2 to 4.4).
Other Laboratory Variables
Data on laboratory variables are shown in Table S7 in the Supplementary Appendix. The urinary albumin-to-creatinine ratio increased significantly in the bardoxolone methyl group, as compared with the placebo group (Fig. 3C). Serum magnesium, albumin, hemoglobin, and glycated hemoglobin levels decreased significantly in the bardoxolone methyl group, as compared with the placebo group. The level of B-type natriuretic peptide increased significantly by week 24 in the bardoxolone methyl group, as compared with the placebo group.
ADVERSE EVENTS
The rates of serious adverse events are summarized in Table 2. Serious adverse events occurred more frequently in the bardoxolone methyl group than in the placebo group (717 events in 363 patients vs. 557 events in 295 patients). There were 11 neoplastic events in the bardoxolone methyl group and 10 in placebo group. The most commonly reported adverse events are summarized in Table S8 in the Supplementary Appendix.
Table 2.
Most Commonly Reported Serious Adverse Events in the Intention-to-Treat Population.*
| Variable | Placebo (N = 1097) | Bardoxolone Methyl (N = 1088) |
|---|---|---|
| Total no. of events | 557 | 717 |
| Any serious adverse event — no. of patients (%) | 295 (27) | 363 (33) |
| Serious adverse event in >1% of patients in either group — no. of patients (%) | ||
| Heart failure† | 42 (4) | 68 (6) |
| Renal failure or impairment‡ | 66 (6) | 49 (5) |
| Coronary-artery disorder§ | 35 (3) | 43 (4) |
| Lower respiratory tract or lung infection | 16 (1) | 35 (3) |
| Respiratory complication¶ | 24 (2) | 21 (2) |
| Anemia | 7 (1) | 18 (2) |
| CNS hemorrhage, CNS vascular disorder, or CVA∥ | 18 (2) | 17 (2) |
| Bacterial infection** | 15 (1) | 13 (1) |
| Accelerated, malignant, or vascular hypertension†† | 9 (1) | 13 (1) |
| Hypoglycemic condition | 8 (1) | 13 (1) |
| Supraventricular arrhythmia | 6 (1) | 13 (1) |
| Potassium imbalance | 14 (1) | 12 (1) |
| Increase in total fluid volume | 0 | 12 (1) |
| Pain and discomfort | 8 (1) | 11 (1) |
| Pulmonary edema | 6 (1) | 10 (1) |
| Abdominal or gastrointestinal infection | 4 (<1) | 10 (1) |
| Non-site-specific gastrointestinal hemorrhage | 16 (1) | 9 (1) |
| Urinary tract infection | 10 (1) | 8 (1) |
| Sepsis, bacteremia, viremia, or fungemia | 9 (1) | 8 (1) |
| Disturbance in consciousness‡‡ | 7 (1) | 8 (1) |
| Nausea or vomiting | 2 (<1) | 8 (1) |
| Gastrointestinal or abdominal pain, excluding oral or throat pain | 2 (<1) | 7 (1) |
| Edema | 6 (1) | 6 (1) |
| Decrease in total fluid volume | 5 (<1) | 6 (1) |
| Ventricular arrhythmia or cardiac arrest | 5 (<1) | 6 (1) |
| Asthenic condition | 3 (<1) | 6 (1) |
| Pulmonary thrombotic or embolic condition | 0 | 6 (1) |
| Hyperglycemic condition | 7 (1) | 1 (<1) |
Adverse events were coded with the use of the Medical Dictionary for Regulatory Activities. Data are presented for patients who received at least one dose of a study drug. Four patients randomly assigned to the placebo group received at least one dose of bardoxolone methyl during the study. Patients could have had more than one event in each category of serious adverse events. CNS denotes central nervous system, and CVA cerebrovascular accident.
Each of the following events was reported by at least one patient: heart failure, left ventricular failure, and right ventricular failure.
Each of the following events was reported by at least one patient: renal failure and impairment and renal failure complications.
Each of the following events was reported by at least one patient: ischemic coronary-artery disorder and coronary-artery disorder.
Each of the following events was reported by at least one patient: respiratory failure (excluding neonatal), breathing abnormality, and bronchospasm and obstruction.
Each of the following events was reported by at least one patient: CNS hemorrhage with CVA and CNS vascular disorder.
Each of the following events was reported by at least one patient: bacterial infection, clostridia infection, staphylococcal infection, klebsiella infection, and pasteurella infection.
Each of the following events was reported by at least one patient: vascular hypertensive disorder and accelerated or malignant hypertension.
Each of the following events was reported by at least one patient: disturbance in consciousness and coma state.
DISCUSSION
The current trial was designed to determine whether bardoxolone methyl, an activator of the cytoprotective Nrf2 pathway, would reduce the risk of ESRD among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease who were receiving guideline-based conventional therapy. The trial was terminated early because of safety concerns, driven primarily by an increase in cardiovascular events in the bardoxolone methyl group. Bardoxolone methyl did not lower the risk of ESRD or of death from cardiovascular causes, although too few events occurred during the trial to reliably determine the true effect of the drug on the primary composite outcome.
Given the truncated duration of the trial and the number of adjudicated events (46% of the events planned), and assuming no change in any of the original assumptions, we estimated the conditional power of the trial to be less than 40%. Although patients treated with bardoxolone methyl had a significant increase in the estimated GFR, as compared with those who received placebo, there was a significantly higher incidence of heart failure and of the composite outcome of nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death from cardiovascular causes in the bardoxolone methyl group. There were numerically more deaths from any cause among patients treated with bardoxolone methyl than among those in the placebo group.
Bardoxolone methyl is among the first orally available antioxidant Nrf2 activators. A small previous study showed that bardoxolone methyl reduced inflammation and oxidative stress13 and induced a decline in the serum creatinine level. In the 52-Week Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes (BEAM) trial,15 227 patients with type 2 diabetes mellitus and an estimated GFR of 20 to 45 ml per minute per 1.73 m2 had a significant increase in the estimated GFR (mean change, 8.2 to 11.4 ml per minute per 1.73 m2, depending on the dose group) that was sustained over the entire trial period. Muscle spasms and hypomagnesemia were the most common adverse events; there was no increase in the rate of heart failure or other cardiovascular events.
The current trial was designed to determine whether the change in estimated GFR that we anticipated on the basis of the results of the BEAM trial would translate into a slower progression toward ESRD. Although in the current trial ESRD developed in fewer patients in the bardoxolone methyl group than in the placebo group, the early termination of the trial precludes conclusion of the effect on ESRD events.
The mechanism linking bardoxolone methyl to heart failure is unknown. Since an excess in heart-failure events was unanticipated, echocardiography was not performed routinely before randomization. Although weight declined significantly in the bardoxolone methyl group, we were unable to determine whether there was loss of body fat, intracellular (skeletal muscle) water, or extracellular (interstitial) water. The fall in serum albumin and hemoglobin levels may reflect hemodilution caused by fluid retention.
Bardoxolone methyl also increased blood pressure. An increase in preload due to volume expansion and an increase in afterload (as reflected by increased blood pressure), coupled with an increase in heart rate, constitute a potentially potent combination of factors that are likely to precipitate heart failure in an at-risk population. The rise in the level of B-type natriuretic peptide with bardoxolone methyl is consistent with an increase in left ventricular wall stress owing to one or more of these mediators or to unrecognized factors such as impaired diastolic filling of the left ventricle. After recognizing the initial increase in heart-failure events, the independent data and safety monitoring committee tried to identify clinical characteristics that were associated with patients who were at elevated risk for heart failure after the initiation of bardoxolone methyl therapy (with the possibility of modifying eligibility criteria or otherwise altering the trial), but the committee was unable to do so. Other, noncardiovascular adverse events were also observed more frequently among patients exposed to bardoxolone methyl than among those who received placebo. Whether the effects of Nrf2 activation, or one or more counterregulatory responses, rendered this particular population vulnerable, is unknown. Zoja et al.18 found an increase in albuminuria and blood pressure along with weight loss in Zucker diabetic fatty rats treated with an analogue of bardoxolone methyl; these effects were not observed in other studies in Zucker diabetic fatty rats or other rodent models or in 1-year toxicologic studies in monkeys.19-21
Why were these adverse effects identified in the current trial and not in the BEAM trial? First, the number of patient-months of drug exposure in the current trial was roughly 10 times that in the BEAM trial. Second, the population in the present trial had more severe chronic kidney disease than did the population in the BEAM trial. Observational studies have shown significantly higher rates of death and cardiovascular events, including heart failure, among patients with stage 4 chronic kidney disease than among patients with stage 3 chronic kidney disease.22 Finally, our trial used an amorphous spray-dried dispersion formulation of bardoxolone methyl at a fixed dose rather than at an adjusted dose. We chose the 20-mg dose and the specific formulation used in the BEACON trial on the basis of four phase 2 studies of chronic kidney disease (three studies used the crystalline formulation, and one used the amorphous formulation), a crossover pharmacokinetics study involving humans that used both formulations, and several studies in animals that used both formulations (Meyer C: personal communication), to provide an activity and safety profile that was similar to that observed with 75 mg of the crystalline formulation, which was one of the dose levels tested in the BEAM trial.
In conclusion, among patients with type 2 diabetes mellitus and stage 4 chronic kidney disease, bardoxolone methyl did not reduce the risk of the primary composite outcome of ESRD or death from cardiovascular causes. Significantly increased risks of heart failure and of the composite cardiovascular outcome (nonfatal myocardial infarction, nonfatal stroke, hospitalization for heart failure, or death from cardiovascular causes) prompted termination of the trial.
Acknowledgments
Supported by Reata Pharmaceuticals.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–84. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 2.The ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 3.Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet. 1983;1:1175–9. doi: 10.1016/s0140-6736(83)92462-5. [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 7.Heerspink HJ, de Zeeuw D. The kidney in type 2 diabetes therapy. Rev Diabet Stud. 2011;8:392–402. doi: 10.1900/RDS.2011.8.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 9.Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–13. doi: 10.1056/NEJMoa1208799. [DOI] [PubMed] [Google Scholar]
- 10.Packham DK, Wolfe R, Reutens AT, et al. Sulodexide fails to demonstrate reno-protection in overt type 2 diabetic nephropathy. J Am Soc Nephrol. 2012;23:123–30. doi: 10.1681/ASN.2011040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol. 2010;21:527–35. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013;83:1029–41. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pergola PE, Krauth M, Huff JW, et al. Effect of bardoxolone methyl on kidney function in patients with T2D and stage 3b-4 CKD. Am J Nephrol. 2011;33:469–76. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 15.Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 16.de Zeeuw D, Akizawa T, Agarwal R, et al. Rationale and trial design of Bardoxo-lone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes: the Occurrence of Renal Events (BEACON). Am J Nephrol. 2013;37:212–22. doi: 10.1159/000346948. [DOI] [PubMed] [Google Scholar]
- 17.Lambers Heerspink HJ, Chertow GM, Akizawa T, et al. Baseline characteristics in the Bardoxolone methyl EvAluation in patients with Chronic kidney disease and type 2 diabetes mellitus: the Occurrence of renal eveNts (BEACON) trial. Nephrol Dial Transplant. 2013;28:2841–50. doi: 10.1093/ndt/gft445. [DOI] [PubMed] [Google Scholar]
- 18.Zoja C, Corna D, Nava V, et al. Analogs of bardoxolone methyl worsen diabetic nephropathy in rats with additional adverse effects. Am J Physiol Renal Physiol. 2013;304:F808–F819. doi: 10.1152/ajprenal.00376.2012. [DOI] [PubMed] [Google Scholar]
- 19.Chin M, Lee CY, Chuang JC, et al. Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of type 2 diabetes and obesity. Am J Physiol Renal Physiol. 2013;304:F1438–F1446. doi: 10.1152/ajprenal.00387.2012. [DOI] [PubMed] [Google Scholar]
- 20.Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice. J Biol Chem. 2010;285:40581–92. doi: 10.1074/jbc.M110.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reisman SA, Chertow GM, Hebbar S, Vaziri ND, Ward KW, Meyer CJ. Bardoxo-lone methyl decreases megalin and activates Nrf2 in the kidney. J Am Soc Nephrol. 2012;23:1663–73. doi: 10.1681/ASN.2012050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]