Abstract
Amyloid beta (Aβ) is implicated in Alzheimer’s disease (AD) as an integral component of both neural toxicity and plaque formation. Brains of the longest-lived rodents, naked mole-rats (NMRs) approximately 32 years of age, had levels of Aβ similar to those of the 3xTg-AD mouse model of AD. Interestingly, there was no evidence of extracellular plaques, nor was there an age-related increase in Aβ levels in the individuals examined (2–20+ years). The NMR Aβ peptide showed greater homology to the human sequence than to the mouse sequence, differing by only 1 amino acid from the former. This subtle difference led to interspecies differences in aggregation propensity but not neurotoxicity; NMR Aβ was less prone to aggregation than human Aβ. Nevertheless, both NMR and human Aβ were equally toxic to mouse hippocampal neurons, suggesting that Aβ neurotoxicity and aggregation properties were not coupled. Understanding how NMRs acquire and tolerate high levels of Aβ with no plaque formation could provide useful insights into AD, and may elucidate protective mechanisms that delay AD progression.
Keywords: Alzheimer’s disease, Amyloid beta, Naked mole-rat, Aggregation, Neuronal toxicity, 3xTg-AD mice, Heterocephalus glaber
1. Introduction
Naked mole-rats (Heterocephalus glaber; NMRs) are mouse-size rodents that live more than 30 years in captivity and maintain good health for most of their long lives (Buffenstein, 2008). NMRs naturally exhibit 2 known risk factors for neurodegeneration, namely vitamin D deficiency (Buffenstein and Yahav,1991) and high levels of oxidative stress (Andziak et al., 2006). Nevertheless, recent studies reveal unchanged gene expression (Kim et al., 2011) and sustained protein levels of key neurotrophic factors (Edrey et al., 2012) in the NMR brain during aging. Given the extreme longevity of NMRs, their naturally high levels of oxidative stress and low levels of vitamin D, we questioned whether NMRs accrue amyloid beta (Aβ), a cleaved small (38–43 amino acids) component of amyloid precursor protein (APP) that is causally implicated in Alzheimer’s disease (AD). This common neurodegenerative disease is characterized by neuronal loss and senile plaques containing Aβ.
Several forms of Aβ exist, with the longer Aβ1–42 peptide regarded as more aggregation-prone and harmful than the shorter forms (LaFerla et al., 2007). Indeed, increased production of Aβ1–42 has been strongly implicated in AD (Citron et al., 1992). The precise role of Aβ in AD remains controversial; although Aβ is a key component of senile plaques, there is no correlation between plaques and impaired cognition (Arriagada et al., 1992; Giannakopoulos et al., 2003). Several studies suggest that extracellular insoluble aggregations of Aβ are not the culprits of AD symptoms. Rather, these, may be benign or even protective, removing from within the cell the more toxic soluble oligomeric Aβ aggregates (Klein et al., 2001; LaFerla et al., 2007). In vitro cell culture studies confirm this premise, for these oligomers are more neurotoxic than fibrillar Aβ (Pimplikar, 2009; Shankar et al., 2008).
Species differences in Aβ peptide sequences have been previously reported (Gotz and Ittner, 2008), and these vary in their in vitro toxicity and aggregation properties (Boyd-Kimball et al., 2004; Cecchi et al., 2007; Liu et al., 1999). Mice and rats have the same Aβ1–42 sequence, which differs from human Aβ by 3 amino acids. This change in sequence causes a reduction in both the propensity of Aβ to self-aggregate and its degree of neurotoxicity (Boyd-Kimball et al., 2004), and may contribute to findings that mice/rats do not naturally develop AD-like symptoms. In this study, we hypothesized that extraordinarily long-lived NMRs would be useful models for human AD. Specifically, we asked whether NMRs showed an age-associated accrual of Aβ with concomitant plaque presence and asked whether the properties of the NMR Aβ peptide were similar to those of the human form.
2. Methods
2.1. Tissue collection and processing
Whole brains (minus cerebellum and brain stem) were harvested from NMRs (n = 39) of both sexes and various ages (2–29 years). These were flash-frozen in liquid nitrogen and stored in −80 °C until used in ELISAs and immunoblotting. Brains of 3xTg-AD mice served both as positive experimental controls and as a gauge with which to compare NMR Aβ1–40 and Aβ1–42 levels. Eight-month-old 3xTg-AD mice, albeit still young, show behavioral deficits as well as high levels of the soluble forms of Aβ (Billings et al., 2005; Oddo et al., 2003; Oddo et al., 2005; Oddo et al., 2008). Additional NMR brains were perfused and/or post-fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemical analyses. In this case, 18 month-old 3xTg-AD (n = 4) mice were used in tandem as positive controls. Animal procurement and euthanasia were carried out in adherence to NIH, Federal, State, and Institutional guidelines at University of Texas Health Science Center–San Antonio (University of Texas Health Science Center at San Antonio UTHSCSA; protocol #07123).
2.2. Sequencing of NMR Aβ
RNA was extracted from NMR brains (n = 5) by homogenization in TRI reagent (Sigma; St. Louis, MO), and RNA integrity was verified by agarose gel electrophoresis. cDNA was synthesized using the Masterscript kit (5 PRIME, Gaithersburg, MD). Following the manufacturer’s instructions, 2 µg of RNA was used per reaction. Primers were designed based on the availability of sequences for other rodents as well as humans in GenBank, and encompassed the region 276 to 317 of the equivalent of human APP.
Forward: 5′-TCGACCAGGTTCTGGGTTGACAAA-3′;
Reverse: 5′-TTCGTAGCCGTTCTGCTGCATCTT-3′.
PCR reactions were subject to the following conditions: 1 minute denaturation cycle at 95 °C, 35 cycles each consisting of 1 minute at 95 °C, 30 seconds at 55.5 °C, and 1 minute at 72 °C, and a final extension step at 72 °C. The amplicons were cloned using One Shot TOPO10 chemically potent Escherichia coli cells (Invitrogen, Grand Island, NY). Plasmids were prepared by QIAprep Miniprep kits (Qiagen, Valencia, CA) and sequenced (Genewiz, South Plainfield, NJ). PCR fragments from each sample were sequenced for both strands, and these were compared to one another. In cases where mismatches occurred, the entire process was repeated until 100% homology was reached.
2.3. Aβ1–42 aggregation
2.3.1. Kinetic assay
Human Aβ1–42 (AnaSpec, Fremont, CA, catalog no. 20276), rodent Aβ (catalog no. 25381) and customized NMR Aβ (AnaSpec run no. 86396) consisting of 42 amino acids at the highest purity available (−95%) were solubilized with 1.5% NH4OH and diluted with phosphate-buffered saline (PBS) to a stock solution of 226 µmol/L. Aliquots were stored at −20 °C until use. The propensity to aggregate was based on a kinetic assay (Cecchi et al., 2007). Aβ1–42 peptides representing all 3 species were incubated at a concentration of 226 µmol/L in PBS, pH 7.2 at 37 °C, and each sample was measured in triplicate in this kinetic assay. At regular time intervals, 10-µL aliquots of each sample were added to 490 µL of a solution containing 25 µmol/L thioflavin-T (ThT), 25 mmol/L phosphate buffer, pH 6.0. The steady-state fluorescence values of the resulting samples were measured at 25 °C using a spectrophotometer (Molecular Devices, Sunnyvale, CA). The excitation and emission wavelengths were 440 and 485 nm, respectively. All measured fluorescence values are given after subtracting the ThT fluorescence intensity measured in the absence of protein and normalized so that the final fluorescence intensity at the endpoint of the kinetic trace was 100% using the following equation: (initial/final) −100/max.
2.3.2. Visualization by atomic force microscopy
Aliquots of synthetic NMR or human Aβ1–42 (AnaSpec) were diluted to 1.13 µmol/L in ddH2O. A 3-µL quantity of the samples was deposited on freshly cleaved mica, washed with ddH2O, dried under a stream of nitrogen, and mounted in a Nanoscope IIIa microscope (Bruker/Veeco, Irvine, CA) equipped with an E scanning head-tapping mode. Imaging was performed in tapping mode in air, with TESP probes (Bruker/Veeco). Resonant frequency of the probes was 280 to 320 kHz, with 50 to 100 mV amplitude and a setpoint of 1.2 and 1.8V. Trace and retrace images were acquired for fields ranging from 0.49 µm2 to 4 µm2, with rates of 2.6 to 3 Hz. Standard flattening and plain-fit, and occasional removal of single aberrant scan lines were the only processing applied to raw images (Nanoscope III software, Bristol, UK). Grain analysis was performed with SPIP 5.1.11 (Image Metrology, Horsholm, Denmark), with automatically set baseline. The threshold of 0.4 nm was automatically set for the human peptide samples incubated for 48 hours. Grain analysis data collected from 3 0.49 µm2 or 1 µm2 fields in each case was further examined with OriginPro 8.6 (OriginLab Corp, Northampton, MA). The number of particles analyzed was as follows: human 770 (time 0); 529 (1 hour); 960 (48 hours); NMR 782 (0); 382 (1); 729 (48). The numerical values of areas covered by particles at the 0.4-nm threshold (“particle footprint” in nm2) were delivered by the SPIP grain analysis. The areas are approximated by the closest polygon covering contour of the particle at a given height (threshold).
2.4. Neurotoxicity assessment on primary hippocampal neuron culture
Primary neurons were prepared from hippocampi of postnatal day 0 C57BL/6 mice. Cells were dispersed by incubation for 7 minutes at 37 °C in papain (Worthington Biochemical Corp., Lakewood, NJ) followed by trituration. Cell suspension was diluted in glial-conditioned neurobasal media supplemented with 1% B-27, 0.5 mmol/L glutamine, and 1× Insulin-Transferrin-Selenium-A supplement (Invitrogen). Neurons were plated on poly-d-lysine–coated 48-well plates at a density of 1.5 × 105 per well and used after culturing for 5 days. Neurons from each individual were equally dispersed among 3 wells that were treated with only media (control) or 10 µmol/L of either NMR or human Aβ1–42 for 24 hours at 37 °C. Wells were treated with 1 mg/mL MTT in media for 2 hours, after, treatment with DMSO and read using a spectrophotometer (Molecular Devices) at 550 nm. Samples originating from the same individuals were compared to the controls from the same origin, and average fold decrease in cell viability was calculated.
2.5. Quantification of soluble and insoluble Aβ
Hemibrains not containing cerebellum or brain stem were dissected from 39 non-littermate NMRs (2–29 years old) and 3xTg-AD mice (n = 3). Samples were sonicated and homogenized and Aβ levels were measured by a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) (Oddo et al., 2005). Brains were homogenized in protein extraction reagent (T-PER buffer, Pierce, Rockford, IL) containing protease (Roche Complete Mini) and phosphatase inhibitors (Calbiochem, Billerica, MA, 1:100). The homogenized mixes were sonicated to shear the DNA and centrifuged at 4 °C for 1 hour at 100,000 × g. The supernatant was collected and stored in −80 °C until use as the soluble fraction. The pellet was rehomogenized in 70% formic acid and centrifuged as above. The supernatant was similarly stored, and is referred to as the insoluble fraction. Proteins from the soluble fraction were loaded directly onto microplates, and formic acid fractions were diluted 1:20 in neutralization buffer (1 mol/L Tris base/0.5 mol/L NaH2PO dibasic) before loading. Maxisorp immunoplates (Nalge Nunc) were coated with monoclonal antibody 20.1, a specific antibody against Aβ1–16 (a kind gift from F. LaFerla, University of California–Irvine) in coating buffer (0.1 mol/L NaCO3, pH 9.6), and blocked with 3% bovine serum albumin. Synthetic Aβ standards (Bachem, Torrance, CA) were defibrillated by dissolving in hexafluoroisopropanol (HFIP) at 1 mg/mL, and the HFIP evaporated with a stream of N2. The defibrillated Aβ was dissolved in DMSO at 1 mg/mL. Standards of both Aβ1–40 and Aβ1–42 were made in antigen capture buffer (20 mmol/L NAH2PO4/2 mmol/L ethylenediaminetetraacetic acid (EDTA)/0.4 mol/L NaCl/0.5 g of CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate)/1% bovine serum albumin, pH 7.0) and loaded onto microplates in duplicate. Samples were loaded in duplicate and incubated overnight at 4 °C. Plates were washed and probed with either horseradish peroxidase–conjugated anti–Aβ 35–40 (MM32–13.1.1 for Aβ40) or anti–Aβ 35–42 (MM40–21.3.4 for Aβ42) overnight at 4 °C. 3,3′,5,5′-tetramethylbenzidine was used as the chromagen, and the reaction was stopped with the addition of 30% O-phosphoric acid and read at 450 nm on a plate reader (Molecular Devices). Values were normalized by protein content.
2.6. C-terminal fragments
Four brain homogenates were used for immunoblots. Gels were transferred to nitrocellulose membranes and these were treated with antibodies for CT-20 (1:2500, Calbiochem #17160) and actin (1:5000; mouse, Calbiochem) for normalization purposes. Both C-terminal fragments were observed and quantified from the same membranes, and therefore the ratio between the 2 was compared. Data were analyzed using ImageJ (National Institutes of Health) and appear as mean ± SEM.
2.7. Aβ plaques
Immunohistochemical assessments of Aβ distribution and analysis included paraffin-embedded sagittal sections (5-µm thick; n = 28) from brains of 2- to 29-year-old breeding (n = 16) and nonbreeding (n = 10) NMR that were either perfused with 4% paraformaldehyde or were not perfused. In addition, free-floating coronal sections of the oldest individuals (20+ years) were used (50 µmthick; n = 3). Sections were treated with the antibodies 4G8 and 6E10 (Covance, Princeton, NJ, 1:1000) with or without formic acid as an antigen retrieval step (reviewed in LaFerla et al., 2007). Some free-floating sections were also treated with a 1% w/v solution of Thioflavin-S alongside 18-month-old 3xTg-AD mice to visualize mature plaques.
Negative controls were treated identically except that the primary antibody was not included. Free-floating sections of 18-month 3xTg-AD mice, known to acquire AD pathology served as positive controls. Visualization was performed with the Axio A1 Microscope (Zeiss) and images taken at various magnifications using AxioVision 4.7.2 software (Zeiss). Thioflavin-S samples were visualized with Leica Microsystems.
3. Results
3.1. Unique sequence of NMR Aβ
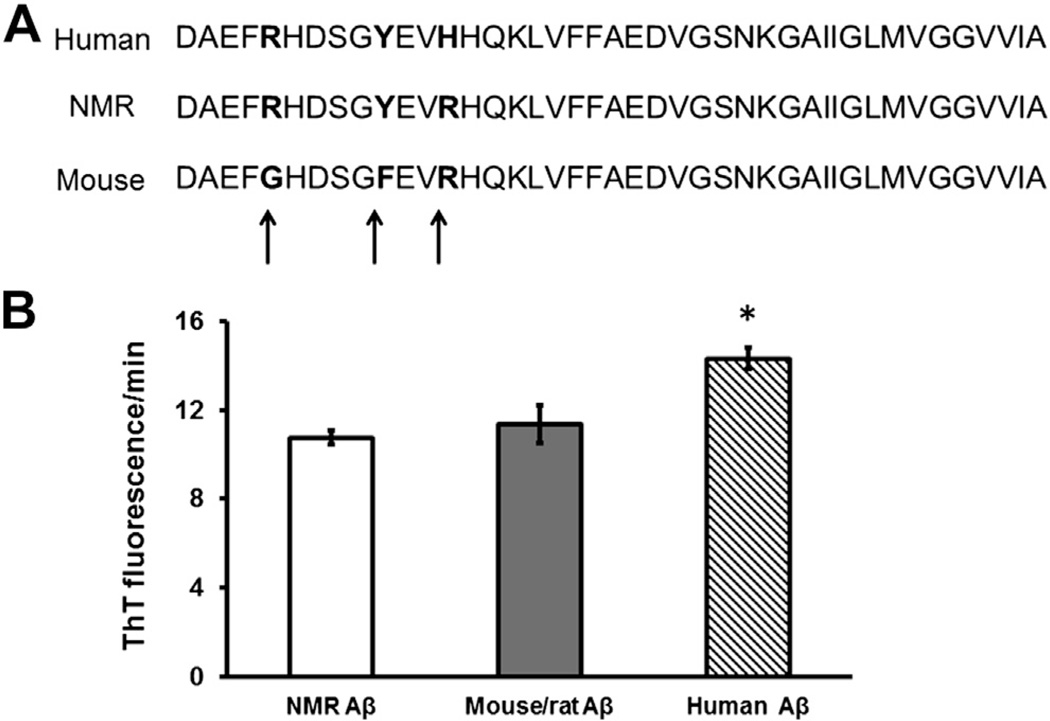
NMR Aβ differs from humans by only 1 amino acid: at position 13, human Aβ contains histidine, whereas in NMRs it is arginine (H13R; Fig. 1A). NMR Aβ differs from mouse/rat Aβ by 2 amino acids (R5G, Y10F), whereas human Aβ sequence differs from the mouse/rat sequence by 3 amino acids (R5G, Y10F, H13R). Nucleotide and protein sequences were submitted to GenBank (GenBank IDs: HM446016.1 and ADL59579.1, respectively).
Fig. 1.
Slight species differences in Aβ sequence lead to marked differences in Aβ aggregation properties. Note positions 5, 10, and 13 of the Aβ sequence, where species differences between human and mouse Aβ are evident. Only position 13 differs between human and NMR (histidine and arginine, respectively) (A). Nevertheless, NMR and mouse/rat Aβ have a lower propensity to aggregate than the human form (ANOVA, p = 0.011), as measured by the change in kinetic florescence of ThT (B). Data are presented as mean ± SEM for triplicates of each sample.
3.2. Low aggregation propensity for NMR Aβ1–42
Aggregation propensity was assessed using synthetic peptides with the same amino acid sequences as NMR Aβ1–42, mouse Aβ1–42, and human Aβ1–42 in a kinetic thioflavin-T (ThT) fluorescence assay. NMR Aβ, like mouse Aβ (Fig. 1B), showed a similar propensity to aggregate (10.76 ± 0.33 A.U, and 11.34 ± 0.84 A.U, respectively), which was significantly lower than that of human Aβ (14.33 ± 0.47 A.U; analysis of variance (ANOVA), F = 10.4, p = 0.011).
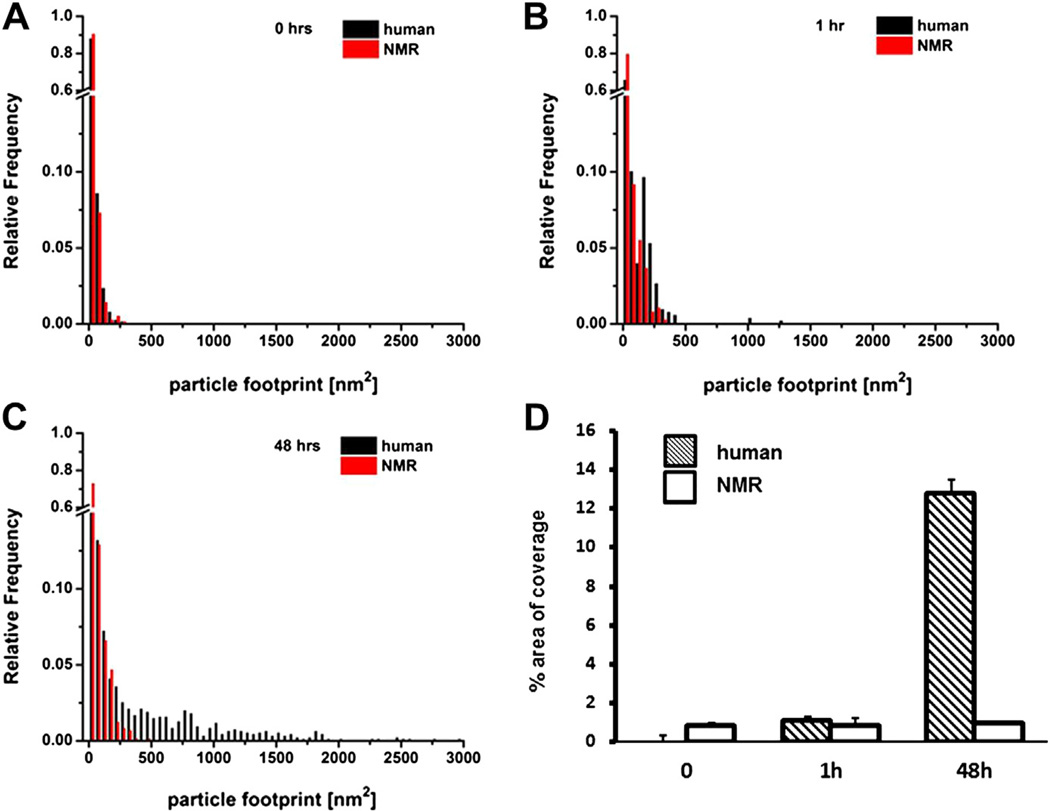
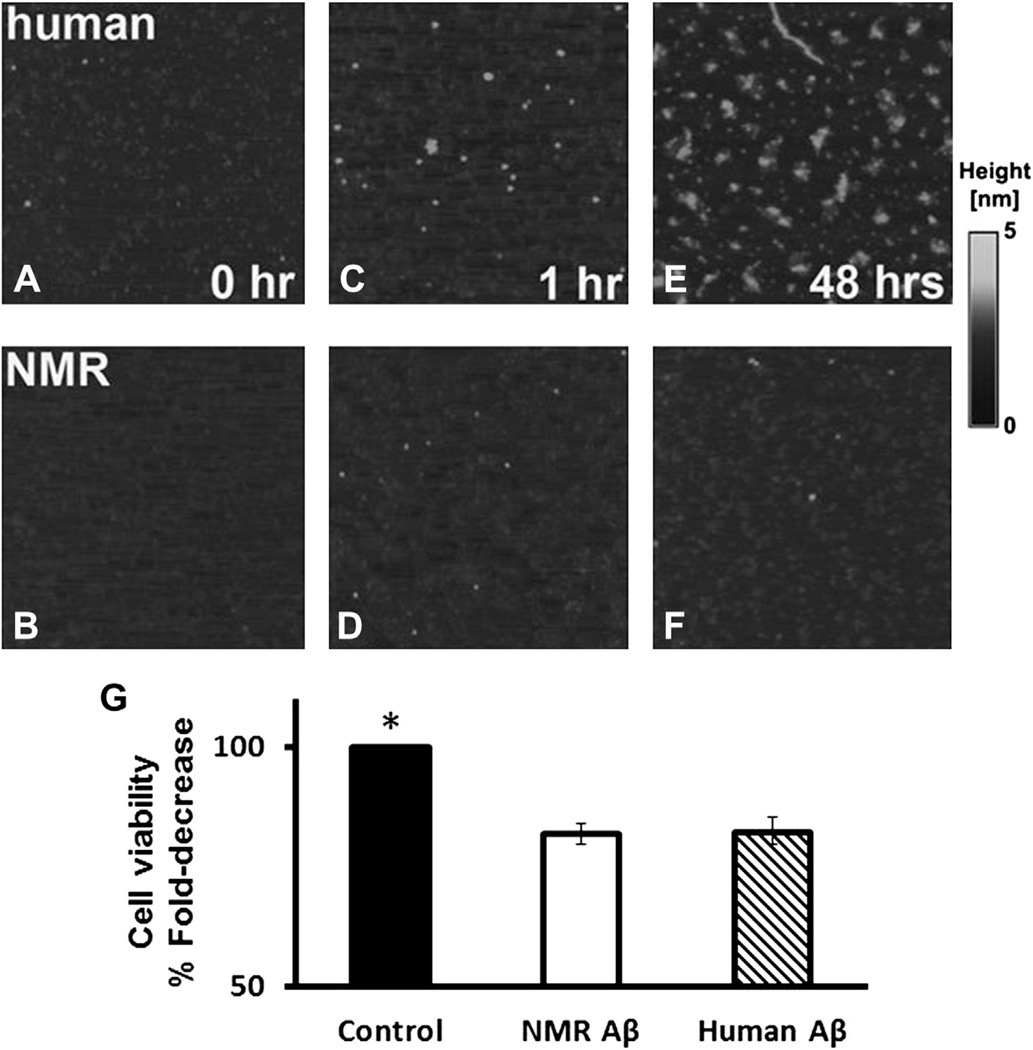
Distinct aggregation properties for both human and NMR Aβ1–42 were confirmed using atomic force microscopy (AFM) at different time points. At time 0, Aβ particles from both sources showed similar particle footprints; most particle areas were up to 15 nm2 with ~60% of the particles in that small size category (Fig. 2A, Fig. 3A and B). Human Aβ1–42 showed a clear tendency to form much bigger aggregates. After 1 hour of incubation. the fraction of human Aβ1–42 small particles declined (~43%), whereas NMR Aβ1–42 showed considerably more small particles (~55%; Fig. 2B, Fig. 3C and D), and these remained at that size even at 48 hours (~52%; Fig. 2C, Fig. 3E and F). After 48 hours, the fraction of small particles in human Aβ1–42 had further declined (26%) and several distinct footprint size classes corresponding to further aggregation stages were evident. At this time point, the largest particles found in human Aβ1–42 reached 3000 nm2 (Fig. 3E), whereas the largest particles in NMR samples never attained 500 nm2 (Fig. 3F). The total fraction of polymerized peptide was similar at time 0 (0.76% ± 0.003%) for the human and (0.81% ± 0.002%) for NMR particles (t test, p = 0.9; Fig. 2D). After 48 hours, pronounced species’ differences were evident with 12.75% ± 0.007% of the area covered by polymerized human Aβ1–42, and only 0.95% ± 0.001% by the NMR peptides (t test, p ≤ 0.00001). Interestingly, the NMR polymerized peptide showed no time-dependent increase in aggregation relative to earlier time points.
Fig. 2.
Grain analysis data reveal that aggregation properties depend on sequence and incubation conditions. Grain analysis data extracted from 3 fields for both human and NMR Aβ1–42 examined with AFM OriginPro 8.6 (OriginLab Corp., Northampton, MA) reveal significant differences between both samples as represented by distribution of footprint parameter (area covered by a particle). At time 0, no significant changes are evident between human and NMR Aβ1–42. (t test, p = 0.9) (A). After 1 hour incubation at room temperature, human Aβ1–42 aggregation is beginning to diverge from the NMR sample (t test, p = 0.69) (B). After 48 hours’ incubation at 37 °C, human and NMR Aβ1–42 show 2 discrete patterns of aggregation (C). Percentage of total area coverage by aggregates (particles) differs drastically (t test, p ≤ 0.00001) between human (hatched bars) and NMR (white bars) after 48 hours of incubation. The coverage after 0 or 1 hour does not differ significantly (D). Data presented as mean ± SEM.
Fig. 3.
Aggregation and neurotoxicity properties of NMR Aβ are uncoupled. AFM images reveal differences in polymerization of human and NMR Aβ1–42. The presented raw topography images of 750 × 750-nm fields were zoomed in from 1-µm2 fields. Human Aβ1–42 (A) and NMR Aβ1–42 (B) at time 0 show a similar pattern of aggregation. After 1 hour at 37 °C, a divergent pattern begins to emerge (C and D). After 48 hours’ incubation at 37 °C, a striking difference is evident between both samples with greater aggregation evident in the human Aβ1–42 (E) compared to the NMR Aβ1–42 (F). The gray scale represents the height of particles, with black representing the background (lowest areas) and light shades representing Aβ polymers. Neurotoxicity of NMR Aβ is similar to that of human Aβ (G). Mouse primary neurons were pretreated with or without (control) 10 µmol/L of either form of Aβ, and cell viability was assessed with MTT assay 24 hours after treatment. A decline of 18% cell viability was apparent when Aβ was used, regardless of the sequence used (NMR or human). Data presented are mean ± SEM, as normalized to controls.
3.3. NMR Aβ1–42 has similar neurotoxicity to human Aβ1–42
Aβ1–42 exposure is reportedly more toxic to neurons than Aβ1–40. Therefore, we compared the neurotoxicity properties of both human and NMR Aβ1–42 by measuring the fraction of mouse primary hippocampal neurons surviving exposure to a supraphysiological dose (10 µmol/L) of either NMR or human Aβ1–42. Both forms of Aβ1–42 induced very similar levels of cell death, with 18% of all neurons in cell culture dying 24 hours post-treatment (Fig. 3G). Clearly both NMR and human Aβ1–42 have similar toxic properties of Aβ1–42 irrespective of the 1–amino acid difference in sequence.
3.4. High endogenous levels of Aβ in NMRs
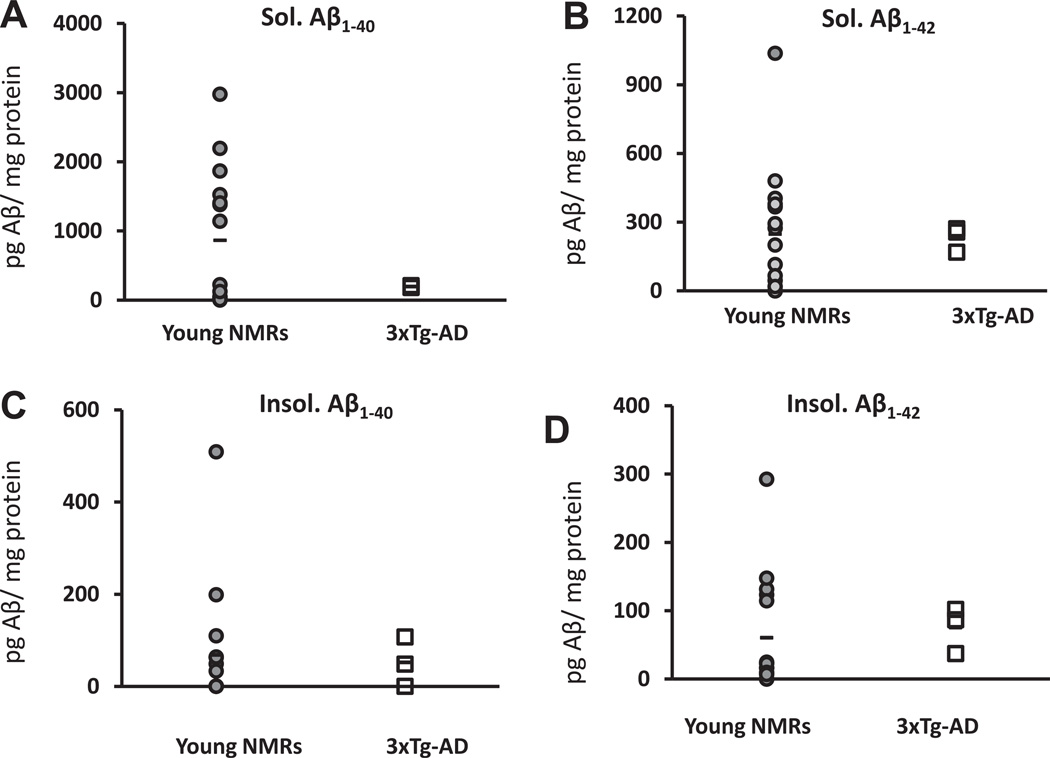
Levels of the soluble Aβ1–40 were highly variable in young NMRs (864.30 ± 253.79 pg Aβ/mg protein, n = 15). Given this high variability, although the average levels were 4-fold greater in the NMR than in the 3xTg-AD mouse model (194.56 ± 8.87; pg Aβ/mg protein, n = 3), data were not statistically different (t test, p = 0.26; Fig. 4A). Average soluble levels of Aβ1–42 (Fig. 4B) were similar (t test, p = 0.92) between NMRs (248.22 ± 70.53 pg Aβ/mg protein, n = 15) and 3xTg-AD mice (232.43 ± 32.13 pg Aβ/mg protein, n = 3). Average levels of insoluble Aβ1–40 (Fig. 4C), although considerably lower than the soluble form, were also similar (t test, p = 0.69) between NMRs (36.89 ± 15.89 pg Aβ/mg protein, n = 14) and 3xTg-AD mice (51.81 ±30.98 pg Aβ/mg protein, n = 3). One NMR sample was omitted from this mean, as it (508.96 pg Aβ/mg protein) was more than 2 standard deviations from the average. Average insoluble Aβ1–42 levels (Fig. 4D) in NMRs (60.25 ± 21.82 pg Aβ/mg protein; n = 15) and 3xTg-AD mice (75.21 ± 19.52 pg Aβ/mg protein; n = 3) were similar (t test, p = 0.77). For NMRs, levels of soluble Aβ1–40 and Aβ1–42 were both significantly higher than their respective insoluble counterpart (t test, p = 0.004 and p = 0.017, respectively).
Fig. 4.
Levels of Aβ are detectable in young NMRs and are comparable to those of young 3xTg-AD mice. Young NMRs (2–9 years old; n = 15; gray-shaded circles) show levels of Aβ similar to those of young (8-month-old; n = 3; open squares) 3xTg-AD mice. Six of the samples for soluble Aβ1–40 had undetectable levels (A). Soluble Aβ1–40 and soluble Aβ1–42 (B) were not significantly different from those of 8-month-old 3xTg-AD mice (t test, p = 0.26 and p = 0.92, respectively). Levels of insoluble Aβ1–40 were also similar, with 1 NMR outlier as calculated by having levels more than 2 SDs higher than the average is shown on the graph. It was not included to calculate average levels or statistical analysis (t test, p = 0.69) (C). Levels of insoluble Aβ1–42 were also similar to those of 3xTg-AD mice (t test, p = 0.77) (D). All data points obtained in our experiments are represented, and the average per species appears as a bar.
3.5. Levels of Aβ do not increase with age
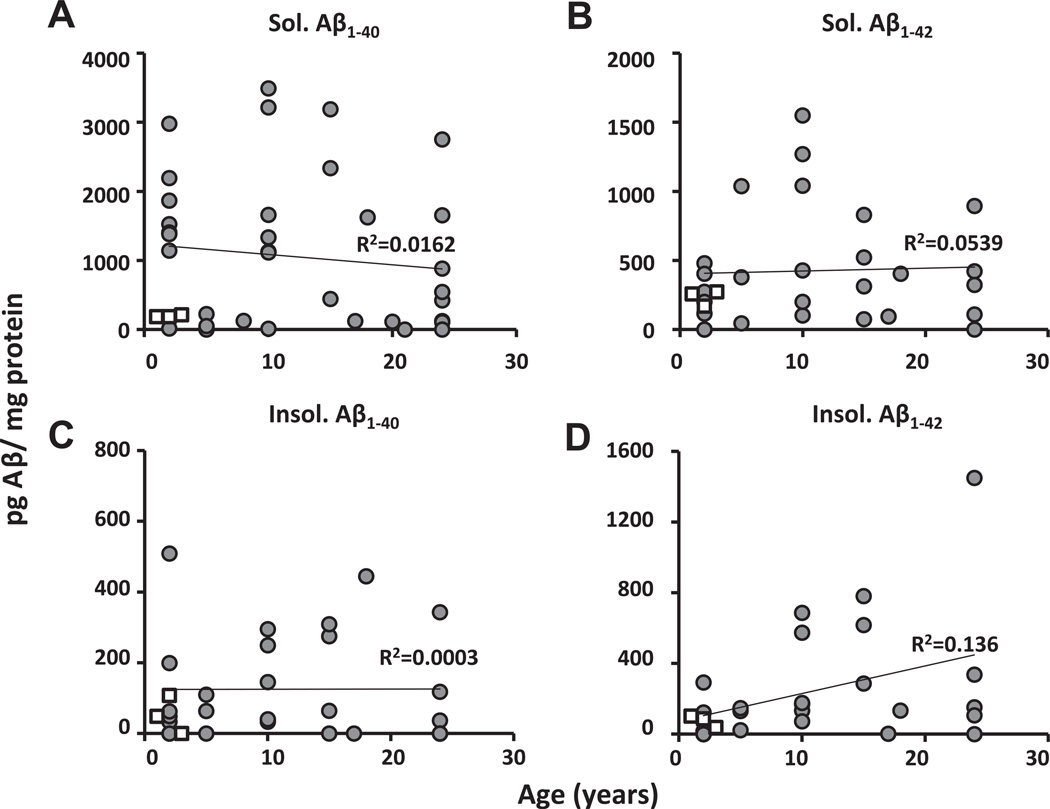
Regardless of the Aβ moiety (soluble and insoluble Aβ1–40 and Aβ1–42), NMR levels (n = 39) were within a range similar to those of young (8-month-old, n = 3) 3xTg-AD mice (Fig. 5A–D). Moreover, there were no significant correlations between age and levels of Aβ. Neither soluble Aβ1–40 (Fig. 5A) nor Aβ1–42 (Fig. 5B) increased with age (R2 = 0.0162, p = 0.441; R2 = 0.0539, p = 0.155, respectively). Similarly, levels of insoluble Aβ1–40 (Fig. 5C) were unchanged with age (R2 = 0.0003, p = 0.914). Levels of the insoluble Aβ1–42 (Fig. 5D) doubled with every doubling of aging (y = 23.536x + 1.314; R2 = 0.136, p = 0.022), with the correlation (r value) accounting for 36.8% of the observed variability. However, this relationship was anchored by 1 very high value in the oldest cohort, and if this outlier is not included in the linear regression analyses, this relationship is no longer observed (y = 12.70x + 108.96; R2 = 0.094, p = 0.112).
Fig. 5.
Levels of Aβ do not increase with age in NMRs. Levels of NMR (gray circles) soluble Aβ1–40 (A) and Aβ1–42 (B) did not increase with age (2–26 years; gray circles y=−16.48x + 1274.25; R2 = 0.0162, p = 0.44; y = 14.819x + 264.187; R2 = 0.0539, p = 0.155, respectively). Similarly, levels of insoluble Aβ1–40 (C) did not increase with age (y = −0.806x + 166.723; R2 = 0.0003, p = 0.914). In contrast, although insoluble Aβ1–42 (D) levels in young animals are comparatively very low, because of 1 very high value in the aged group, a significant positive correlation with age, was apparent (y = 23.53x + 1.314; R2 = 0.136, p = 0.022, respectively); however, if this 1 outlier is ignored, there was no significant relationship (y = 12.70x + 108.96; R2 = 0.094, p = 0.112). In all cases, NMR levels are within similar range to those of young (8-month-old) 3xTg-AD mice (open squares). All data points obtained in our experiments are represented; n = 39 for NMRs, n = 3 for 3xTg-AD mice.
3.6. Aβ production does not change with age
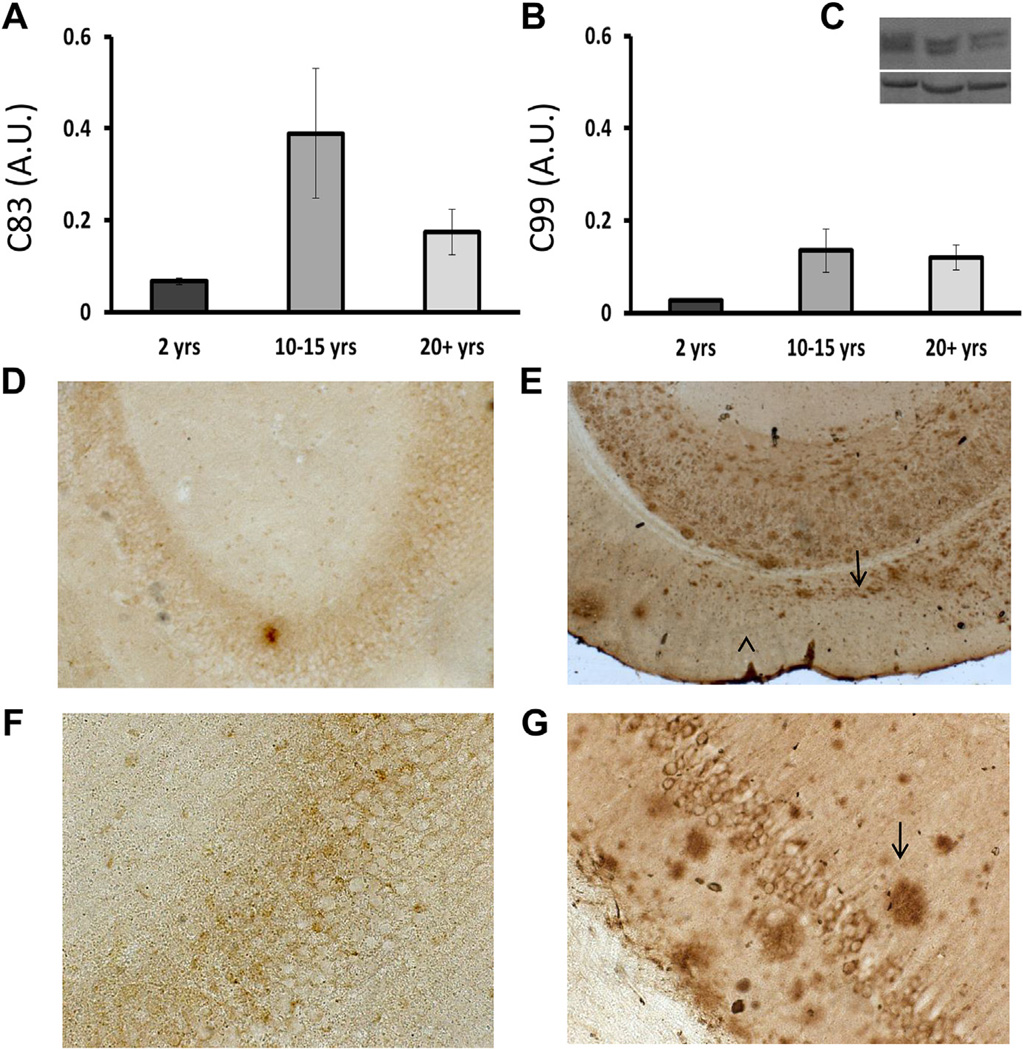
APP processing did not significantly change with age (Fig. 6A and B). C83 and C99 are the C-terminal fragments that remain after APP processing. The former is a footprint of the non-amyloidogenic pathway, whereas the latter implies the occurrence of an amyloidogenic event. Neither C83 (Fig. 6A) nor C99 (Fig. 6B) changed significantly among young (2-year-old), older (10–15 years), and oldest (20+ years) NMRs (ANOVA, F = 2.572, p = 0.171 and F = 2.149, p = 0.212, respectively). In all age groups, the ratio between C83 and C99, however, suggests that there are more non-amyloidogenic events than amyloidogenic ones.
Fig. 6.
No age-related change in APP processing with no evidence for senile plaques. C83 and C99 are the C-terminal fragments that remain after APP processing. The former is a footprint of the non-amyloidogenic pathway, whereas the latter implies an amyloidogenic event. Neither C83 (A) nor C99 (B) changed significantly among young (2 years), older (10–15 years), and the oldest (20+ years) NMRs (ANOVA F = 2.572, p = 0.171; F = 2.149, p = 0.212, respectively). A representative immunoblot of C83 and C99 is shown (C, top) with the corresponding loading control (C, bottom). In all age groups, the ratio between C83 and C99, however, suggests that there are more non-amyloidogenic events than amyloidogenic ones, providing some protection against Aβ production. Immunostaining with 6E10 antibody does not reveal plaques in NMRs. Note that some intraneuronal staining appears; staining with 4G8 antibody revealed the same pattern and is therefore not shown here. No plaques detected in low (D) or high (F) magnification of a 29-year-old NMR. For control purposes, immunohistochemistry was performed in tandem with an 18-month-old 3xTg-AD mouse. Low (E) and high (G) magnification clearly shows Aβ plaques (arrows).
3.7. No evidence of Aβ plaques
Tissues were stained with 2 different antibodies that are traditionally used to detect human (6E10) and mouse (4G8) Aβ. In both cases, based on the primary sequence of NMR Aβ, there appeared to be no recognition artifacts, and neither antibody revealed the existence of extracellular plaques in NMRs (Fig. 6D and F). The study included paraffin-embedded thin sections (5 µm) and free-floating thick sections (50 µm). The latter were also stained with thioflavin-S, which does not rely on antibody recognition and would fluoresce upon binding to mature plaques. This method also did not reveal any indications of plaques in the aged NMR brain (not shown). 3xTg-AD mice were used as positive controls for 6E10 staining (Fig. 6E and G) and thioflavin-S (not shown), and clearly detected Aβ plaques using both techniques. These data reveal that despite high levels of Aβ, histological assessments of the brains of animals as old as 29 years do not reveal Aβ plaques.
4. Discussion
In this study we assessed whether extraordinarily long-lived NMRs showed signs of Aβ accrual and damage as is commonly the case in humans with AD. Both humans and NMRs live 4-to 5-fold longer than expected by body size and are considered long-lived species. We and others have shown that the NMRs’ extreme longevity goes hand in hand with sustained good health, and that NMRs show both a delayed and attenuated aging profile with compression of morbidity restricted to only the last few years of life (Edrey et al., 2011A; Edrey et al., 2012; Lambert et al., 2007; Pérez et al., 2009; Ungvari et al., 2008). We questioned whether these long-lived rodents live long enough to accrue high levels of Aβ and senile plaques, and whether NMR Aβ properties differ substantially from those of humans. We therefore characterized the sequence, aggregation propensity, and neurotoxicity properties of NMR Aβ and assessed whether NMRs have detectable levels of Aβ, and evaluated whether these accrue with age.
The sequence of NMR Aβ (GenBank ID: ADL59579.1) is, for the most part, identical to both mouse and human Aβ, and differs from that of mouse/rat Aβ at only 2 amino acid sites and from human Aβ by only 1 amino acid (Fig. 1A). This small species difference between humans and NMRs nevertheless appeared to be sufficient to alter in vitro Aβ aggregation but not toxicity properties. The single amino acid substitution in the NMR (compared to human Aβ) reduced the tendency of Aβ to aggregate, confirming earlier chemical studies that changes to certain amino acids had profound effects on aggregation properties (Boyd-Kimball et al., 2004; Cecchi et al., 2007; Hilbich et al., 1991; Liu et al., 1999). That a single amino acid sequence change can have an impact on the functional properties of Aβ is fascinating, and must reflect 3-dimensional structural differences. Human Aβ has 2 adjacent histidines (at positions 13 and 14), and these are proposed as important metal binding domains that would promote aggregation, neurotoxicity (Atwood et al., 1998; Liu et al., 1999; Tickler et al., 2005), or, conversely, would serve as sites for Aβ to bind and internalize itself into neurons (Diaz et al., 2006; Poduslo et al., 2010). The change in 1 amino acid at position 13, a key site for Cu(II) binding (Atwood et al., 1998) is missing in the NMR Aβ1–42 compared to human Aβ1–42. This suggests that in the NMR, Cu(II) binding is likely deficient (either in amount or affinity). Given the high levels of oxidative stress and similar neurotoxicity properties in NMRs, Cu(II) may not play as prominent a role in Aβ metabolism. Interestingly, the Octodon degu shares the same Aβ sequence as the NMR, and it does, in fact, show plaques and AD-like symptoms Inestrosa et al., 2005, Van Groen et al., 2011, so it is difficult to conclude the role of this change from the data that we have gathered here.
Cecchi et al. (2007) reported that the propensity for Aβ aggregation differs also by depending upon the total peptide size. For example, Aβ1–40 and Aβ1–42 show different aggregation properties; Aβ1–42 assemblies reach high aggregation states within minutes, contrary to Aβ1–40. Based on the size of the aggregates as viewed with AFM, NMR Aβ1–42 formed both significantly smaller and fewer aggregates than the more abundant, larger fibril human aggregates (Figs. 2,3). Once human Aβ monomeric peptides (~4 kDa) are produced, they readily form dimers, oligomers, and fibrils. The oligomers, not the large fibrils, are currently regarded as the key culprits for damaging and killing cells in the central nervous system (Fodero-Tavoletti et al., 2011).
NMR and human Aβ1–42 in this study induced similar degrees of neurotoxicity (Fig. 3G). Given that human Aβ1–42 takes less than 72 hours to form fibrils, it is possible that species’ differences in toxicity would be evident if human Aβ1–42 were allowed to aggregate before toxicity experiments. In this case, we would predict that because NMR Aβ does not aggregate into large fibrils, NMR Aβ may be more toxic than the human form. If this were the case, our findings would support the premise that insoluble fibrils/plaques may be protective, removing the toxin from the cell before it can essentially do much harm.
Unlike both human and mouse Aβ, the NMR Aβ1–42 showed divergent aggregation and neurotoxicity properties, revealing that these are not necessarily codependent but, rather, are uncoupled. Interestingly, the NMR Aβ sequence is identical to that of a small South American rodent, the Octodon degus (degu) (Inestrosa et al., 2005) that belongs to the same taxonomic suborder of rodents, the hystricognathae. Aβ deposits have been reported in the brains of old degus. Moreover, by 6 years of age, degus had symptoms and behavior reminiscent of AD-like neurodegeneration (Ardiles et al., 2012, Inestrosa et al., 2005, van Groen et al., 2008). Clearly, therefore, the NMR and degu Aβ sequence has the potential to be both neurotoxic and aggregation prone in vivo.
Surprisingly, on average, young adult NMR brains had high amounts of both soluble and insoluble Aβ1–40 and Aβ1–42. These were within range of those measured in 8-month-old 3xTg-AD mice, which are specifically designed to express high levels of human APP to model AD-like pathology. The large variance evident in the NMR data set may be reflective of the fact that these are second-generation captive, non-manipulated individuals, originating from different wild colonies. It is unlikely that this is reflective of housing or dietary conditions, as all individuals in our colony are maintained under identical conditions and fed the same diet. It may also reflect the high variance in a natural population toward developing AD-like pathology, similar to what occurs in humans. In contrast, the low variation observed within the 3xTg-AD data set reflects the fact that these mouse mutants are deliberately bred to maintain a specific genetic identity and are thereby considered robust models for the study of familial AD. To date, there is no information regarding age-related brain pathology (e.g., strokes or compromises to the blood–brain barrier) in NMRs. Housing conditions and diets were identical in all individuals, and although all of the animals chosen for this study were healthy, it is possible that our data set may include individual variation due to life history. For example, our data set included both breeding and non-breeding individuals, which could introduce variation resulting from differences in sex and stress hormones between these groups (Clarke and Faulkes, 1997; Faulkes and Abbott, 1993).
Because oxidative stress is implicated as a key risk factor for Aβ accumulation, the high levels of Aβ observed in relatively young NMRs may be linked to earlier findings of high levels of oxidative stress in our captive colony (Andziak et al., 2006) and divergent individual cytoprotective responses to this artificial “hyperoxic” milieu (Edrey et al., 2011B). NMRs have led a strictly subterranean existence since the early Miocene and have evolved to tolerate the hypoxic (~8%–15% oxygen) conditions encountered by living in large groups in poorly ventilated underground burrows. Life in captivity thus presents a pronounced oxidative stress to these animals living in a gaseous atmosphere of 20.9% oxygen normally found above ground. Clearly, NMRs are extremely resilient and capable of tolerating these stressors. Living permanently in dark sealed burrows, this species is chronically vitamin D deficient, with undetectable levels of circulating 24-hydroxyvitamin D3 and very low levels of calcitriol (Buffenstein and Yahav, 1991). Vitamin D is thought to play a role in the removal of Aβ from the brain (Masoumi et al., 2009; Smolders et al., 2011), and deficiency has been implicated as a risk factor for AD. Both oxidative damage and vitamin D status may therefore contribute to the observed high variability and high levels of Aβ in the brains of captive young NMRs.
Despite the observed high levels of Aβ in brain tissues, there were no signs of plaques even in a 29-year-old animal (Fig. 6D and F). This finding was most surprising, as the levels of Aβ were comparable to those observed in an AD mouse model. Furthermore, degus with the same Aβ sequence show signs of plaques and cognitive deficits even in middle age (Ardiles et al., 2012; Inestrosa et al., 2005; van Groen et al., 2011). The NMR is therefore a rare species in that, unlike other long-lived mammals (e.g., canines, primates), it does not show age-related pathology reminiscent of AD. In this regard, NMRs seem to be a natural model for studying the mechanisms that protect against an AD-like phenotype with age.
Given that NMRs endogenously produce high levels of Aβ at a young age, yet live more than 2 decades with these high levels, it appears that this species is exceedingly tolerant of high levels of Aβ1–42 and has evolved mechanisms to counter its neurotoxic effects. We have previously reported that NMRs have high levels of the neurotrophic factor Neuregulin 1 (NRG-1) known to maintain neuronal integrity, which remain elevated throughout life (Edrey et al., 2012). NRG-1 may serve NMRs from age-related and other neuronal impairments (e.g., Aβ-induced damage) and contribute to the observed attenuated aging profile in the NMR brain. As such, this species may prove to be an exciting new animal model with which to investigate the mechanisms that may protect against AD-like pathology.
It is widely accepted that AD pathology takes considerable time to develop, and that short-lived species may die of other causes before AD-like pathology may present. Given that NMRs live more than 3 decades, it is more likely that these rodents have sufficient time to develop some or all of pathology associated with AD. The absence of age-associated signs of plaques concurs with our earlier findings of “negligible senescence” in NMR behavior, physiology, and biochemistry of the visceral tissues (Buffenstein, 2008), as well as unchanged gene expression in the NMR brain with age (Kim et al., 2011). Indeed, there were no significant age-related changes in amyloidogenic Aβ production in the brain and the non-amyloidogenic pathway (C83, non-amyloidogenic remnant) continues to predominate over the amyloidogenic pathway (C99, amyloidogenic remnant), even in old age. Transcriptome analysis of 4 –year-old and 20-year-old brains concurs with this finding, for no age-related changes in any of the enzymes involved in the cleavage of APP to form the C-fragments (Kim et al., 2011) were evident.
Aβ is correlated with the severity of AD; however, levels of Aβ, at least in NMRs, may not be causally linked to AD progression. This may be due to protective mechanisms that prevent the manifestation of harmful effects of Aβ (e.g., antioxidants) (Baruch-Suchodolsky and Fischer, 2009) or Aβ may be a symptom of the disease and not a key causative player. Understanding the mechanisms that protect the NMR brain from the harmful effects of Aβ may reveal novel insights into putative translational strategies to protect against the age-associated progression into AD-like pathology.
Acknowledgements
This work was supported by grants to R.B. from the National Institute on Aging/National Institutes of Health (NIA/NIH AG022891) and the Glenn foundation.
Footnotes
Disclosure statement
The authors state that they have nothing to disclose, and that there are no potential conflicts of interest.
References
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Ardiles AO, Tapia-Rojas CC, Mandal M, Alexandre F, Kirkwood A, Inestorsa NC, Palacios AG. Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2012;109:13835–13840. doi: 10.1073/pnas.1201209109. http://dx.doi.org/10.1073/pnas.1201209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada P, Growdon J, Hedley-Whyte E, Hyman B. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry. 2009;48:4354–4370. doi: 10.1021/bi802361k. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Yahav S. Cholecalciferol has no effect on calcium and inorganic phosphorus balance in a naturally cholecalciferol-deplete subterranean mammal, the naked mole rat (Heterocephalus glaber) J. Endocrinol. 1991;129:21–26. doi: 10.1677/joe.0.1290021. [DOI] [PubMed] [Google Scholar]
- Cecchi C, Fiorillo C, Baglioni S, Pensalfini A, Bagnoli S, Nacmias B, Sorbi S, Nosi D, Relini A, Liguri G. Increased susceptibility to amyloid toxicity in familial Alzheimer’s fibroblasts. Neurobiol. Aging. 2007;28:863–876. doi: 10.1016/j.neurobiolaging.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA. Rodent Abeta(1–42) exhibits oxidative stress properties similar to those of human Abeta(1–42): implications for proposed mechanisms of toxicity. J. Alzheimers Dis. 2004;6:515–525. doi: 10.3233/jad-2004-6509. [DOI] [PubMed] [Google Scholar]
- Clarke F, Faulkes C. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc. Biol. Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JC, Linnehan J, Pollard H, Arispe N. Histidines 13 and 14 in the Abeta sequence are targets for inhibition of Alzheimer’s disease Abeta ion channel and cytotoxicity. Biol. Res. 2006;39:447–460. doi: 10.4067/s0716-97602006000300007. [DOI] [PubMed] [Google Scholar]
- Edrey YH, Casper D, Huchon D, Gelfond J, Mele J, Kristan DM, Nevo E, Buffenstein R. Sustained high levels of neuregulin-1 in the longest-lived rodents: a key determinant of rodent longevity. Aging Cell. 2012;11:213–222. doi: 10.1111/j.1474-9726.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. 2011A;52:41–53. doi: 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- Edrey YH, Park TJ, Kang H, Biney A, Buffenstein R. Endocrine function and neurobiology of the longest-living rodent, the naked mole-rat. Exp. Gerontol. 2011B;46:116–123. doi: 10.1016/j.exger.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Faulkes CG, Abbott DH. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats (Heterocephalus glaber) J. Reprod. Fertil. 1993;99:225–230. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- Fodero-Tavoletti MT, Villemagne VL, Rowe CC, Masters CL, Barnham KJ, Cappai R. Amyloid-beta: the seeds of darkness. Int. J. Biochem. Cell. Biol. 2011;43:1247–1251. doi: 10.1016/j.biocel.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann F, Bussière T, Bouras C, Kövari E, Perl D, Morrison J, Gold G, Hof P. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K. Human and rodent sequence analogs of Alzheimer’s amyloid beta A4 share similar properties and can be solubilized in buffers of pH 7.4. Eur. J. Biochem. 1991;201:61–69. doi: 10.1111/j.1432-1033.1991.tb16256.x. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Reyes AE, Chacón MA, Cerpa W, Villalón A, Montiel J, Merabachvili G, Aldunate R, Bozinovic F, Aboitiz F. Human-like rodent amyloid-b-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol Aging. 2005;26:1023–1028. doi: 10.1016/j.neurobiolaging.2004.09.016. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Boysen HM, Buckingham JA, Yang T, Podlutsky A, Austad SN, Kunz TH, Buffenstein R, Brand MD. Low rates of hydrogen peroxide production by isolated heart mitochondria associate with long maximum life-span in vertebrate homeotherms. Aging Cell. 2007;6:607–618. doi: 10.1111/j.1474-9726.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Liu ST, Howlett G, Barrow CJ. Histidine-13 is a crucial residue in the zinc ion-induced aggregation of the A beta peptide of Alzheimer’s disease. Biochemistry. 1999;38:9373–9378. doi: 10.1021/bi990205o. [DOI] [PubMed] [Google Scholar]
- Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwickie M, Cashman J, Fiala M. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Yim SH, Zhao X, Kasaikina MV, Stoletzki N, Peng C, Polak P, Xiong Z, Kiezun A, Zhu Y, Chen Y, Kryukov GV, Zhang Q, Peshkin L, Yang L, Bronson RT, Buffenstein R, Wang B, Han C, Li Q, Chen L, Zhao W, Sunyaev SR, Park TJ, Zhang G, Wang J, Gladyshev VN. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature. 2011;479:223–227. doi: 10.1038/nature10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y, Leslie FM, LaFerla FM. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2005;102:3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J. Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A, Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int. J. Biochem. Cell. Biol. 2009;41:1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo JF, Gilles EJ, Ramakrishnan M, Howell KG, Wengenack TM, Curran GL, Kandimalla KK. HH domain of Alzheimer’s disease Abeta provides structural basis for neuronal binding in PC12 and mouse cortical/hippocampal neurons. PLoS One. 2010;5:e8813. doi: 10.1371/journal.pone.0008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J, Moen SM, Damoiseaux J, Huitinga I, Holmoy T. Vitamin D in the healthy and inflamed central nervous system: access and function. J. Neurol. Sci. 2011;311:37–43. doi: 10.1016/j.jns.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Tickler AK, Smith DG, Ciccotosto GD, Tew DJ, Curtain CC, Carrington D, Masters CL, Bush AI, Cherny RA, Cappai R, Wade JD, Barnham KJ. Methylation of the imidazole side chains of the Alzheimer disease amyloid-beta peptide results in abolition of superoxide dismutase-like structures and inhibition of neurotoxicity. J. Biol. Chem. 2005;280:13355–13363. doi: 10.1074/jbc.M414178200. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front. Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Popovic N, Popovic M, Caballero-Bleda M, Bano-Otalora B, Vivanco P, Rol MA, Madrid JA. Age-related brain pathology in Octodon degu: blood vessel, white matter and Alzheimer-like pathology. Neurobiol. Aging. 2011;32:1651–1661. doi: 10.1016/j.neurobiolaging.2009.10.008. [DOI] [PubMed] [Google Scholar]