Abstract
Studies on spinalized animals indicate that some pharmacological agents may act on receptors in the spinal cord, helping to produce coordinated locomotor movement. Other drugs may help to ameliorate the neuropathological changes resulting from spinal cord injury (SCI), such as spasticity or demyelination, to improve walking. The purpose of this study was to systematically review the effects of pharmacological agents on gait in people with SCI. A keyword literature search of articles that evaluated the effects of drugs on walking after SCI was performed using the databases MEDLINE/PubMed, CINAHL, EMBASE, PsycINFO and hand searching. Two reviewers independently evaluated each study, using the Physiotherapy Evidence Database (PEDro) tool for randomized clinical trials (RCT), and the modified Downs & Black scale for all other studies. Results were tabulated and Levels of Evidence were assigned. Eleven studies met the inclusion criteria. One RCT provided Level 1 evidence that GM-1 ganglioside in combination with physical therapy improved motor scores, walking velocity and distance better than placebo and physical therapy in persons with incomplete SCI. Multiple studies (Levels of Evidence 1–5) showed that Clonidine and Cyproheptadine may improve locomotor function and walking speed in severely impaired individuals with incomplete SCI. Gains in walking speed associated with GM-1, Cyproheptadine, and Clonidine are low compared to those seen with locomotor training. There was also Level 1 evidence that 4-aminopyridine and L-Dopa were no better than placebo in helping to improve gait. Two Level 5 studies showed that Baclofen had little to no effect on improving walking in persons with incomplete SCI. There is limited evidence that pharmacological agents tested so far would facilitate the recovery of walking after spinal cord injury. More studies are needed to better understand the effects of drugs combined with gait training on walking outcomes in people with SCI.
Keywords: Spinal cord injury, locomotor function, medication, rehabilitation
Introduction
The worldwide annual incidence of spinal cord injury (SCI) is estimated to be between 15–39 per million (Cripps et al., 2010). In North America, it is estimated that there are almost 300,000 individuals living with SCI and more than 11,000 new cases arise each year (National Spinal Cord Injury Statistical Center 2006). SCI is a devastating condition that can result in dramatic impairments in motor, sensory, cardiac, and bladder and bowel function. Although it is often cited that walking is not the first priority for individuals with SCI (Anderson 2004), there are several other studies indicating that the recovery of walking and functional mobility, along with bladder and bowel function, are major concerns for people with SCI (Ditunno et al., 2008; Donnelly et al., 2004; Estores 2003). Furthermore, improvements in walking function have the potential to afford secondary benefits, such as improvements in cardiovascular health, muscle composition and metabolism, bone health, and psychological well-being (Hicks and Ginis 2008).
Strategies to improve walking after SCI can be roughly grouped into two categories: those that attempt to harness and potentiate neural pathways underlying locomotor control or those that attempt to minimize or counteract the secondary sequelae of SCI to allow for the expression of locomotion. Pharmacological agents that target one or both of these approaches could theoretically provide a way to facilitate walking function after SCI.
Since the early 20th century, there has been a great deal of progress in our understanding of the neural control of locomotion. Early studies in spinally transected cats showed that the spinal cord, isolated from all supraspinal and peripheral input, was capable of generating alternating flexion and extension activity and a model for a spinal locomotor network capable of producing the basic locomotor rhythm was proposed (Brown 1911). Although there is only indirect evidence for such spinal locomotor networks in humans (Calancie et al., 1994; Dimitrijevic et al., 1998), a great deal of work has been dedicated to understanding and characterizing spinal mechanisms underlying locomotion in the hopes of finding appropriate strategies to facilitate recovery of walking after SCI.
Cat models have been very useful for understanding the contribution of different neurotransmitter systems on spinal locomotor network activity (Rossignol et al., 2001). This has led, in turn, to investigations of the effect of various pharmacological agonists or antagonists of different neurotransmitter systems on locomotor activity (Rossignol et al., 2001). The seminal work of Jankowska et al. (Jankowska et al., 1967a, 1967b) linked the noradrenergic neurotransmitter system to neuronal pathways that could underlie this locomotor-like rhythmicity in acute spinal cats. Noradrenaline (norepinephrine) acts as a hormone and a neurotransmitter within the central and sympathethic nervous system, and it is synthesized by a series of enzymatic reactions that include the noradrenergic precursor L-DOPA (L-3,4-dihydroxyphenylalanine) and dopamine. In fictive (non-behaving) spinal cats, where the spinal cord is isolated from all supraspinal and sensory input, intravenous application of L-DOPA could elicit rhythmic alternating activity (Grillner and Zangger 1979). In behaving spinalized cats, the administration of Clonidine or tizanidine (both are agonists to the α2 adrenergic receptor), has been shown to facilitate expression of locomotion if the animal is supported over a moving treadmill belt (Barbeau and Rossignol 1991; Chau et al., 1998b; Forssberg and Grillner 1973). Clonidine has also been shown to potentiate locomotor training in acute spinal cats (Chau et al., 1998a) and modulate the timing of muscle activity (Barbeau and Rossignol 1991). The dopamine precursor L-DOPA has been shown to induce locomotion in spinalized cats (Grillner and Zangger 1979), but this effect is likely mediated via noradrenergic pathways in the acute preparation since DOPA’s effects on the spinal cord are mediated by inducing the production and release of noradrenaline (Anden et al., 1966). In the chronic state, however, dopaminergic pathways are implicated in the modulation of ongoing locomotor activity in spinalized cats, particularly in increasing flexor burst amplitude (Barbeau and Rossignol 1991).
The serotonergic system has also been implicated in the spinal control of locomotion in both spinal cat and rat models. Unlike the noradrenergic system, serotonergic agonists modulate established locomotor patterns, but are not able to initiate locomotion in spinal cats (Barbeau and Rossignol 1990, 1991). The application of serotonergic agonists have an excitatory effect on the amplitude of ongoing muscle activity, particularly in the extensor muscles (Barbeau and Rossignol 1990, 1991). The excitatory effect of serotonergic agonists sometimes manifest as spasms or clonus, interfering with the locomotor pattern in spinal cats (Barbeau and Rossignol 1990). Indeed, serotonergic agonists also potentiate the response to cutaneous nerve stimulation (Barbeau and Rossignol 1990) and serotonergic pathways have been implicated in hyperactive reflex responses to electrical stimulation (Carlsson et al., 1963; Marley and Vane 1967) or mechanical stretch or vibration (Carp and Rymer 1986). However, there is evidence that quipazine, a serotonergic agonist, can help to improve locomotor function in adult spinal cats. Barbeau and Rossignol (1990) showed that quipazine improved joint angle excursion, foot clearance and step length and in another study, this drug increased step cycle duration, lateral stability, and rhythmic stepping (Brustein and Rossignol 1999). In adult spinalized rats, serotonin or serotonin-agonists help to improve alternating stepping patterns, foot placement (Antri et al., 2002), step shape consistency, the number of steps executed (Fong et al., 2005), interlimb coordination and electromyographic amplitudes during stepping (Feraboli-Lohnherr et al., 1999). It has also been shown in the in vitro neonatal rat spinal cord that serotonin improves left-right and flexor-extensor rhythmicity during fictive locomotion induced by N-methyl-D-aspartate (NMDA) (Pearlstein et al., 2005). More recently, Courtine and colleagues showed that serotonergic agonists used in combination with epidural electrical stimulation and locomotor training in spinalized rats led to kinematics and EMG during full weight bearing stepping that were similar to those pre-injury (Courtine et al., 2009). Together, this evidence suggests that serotonin and serotonergic agonists, likely in combination with other therapies, may potentially facilitate lumbosacral spinal circuits used in walking after spinal cord injury in humans.
In addition to the potential contribution of pharmacological agents to the activation of spinal locomotor centers, drugs may also be used to ameliorate neuropathological changes associated with SCI that interfere with functional recovery. Spasticity is one of the most common secondary complications of SCI (Levi et al., 1995; Noreau et al., 2000) and is associated with deleterious effects on ambulation and mobility, among other activities of daily living (Adams and Hicks 2005; Lundqvist et al., 1991). Spasticity is a complex condition that has been defined as a velocity-dependent increase in stretch reflexes but also has encompassed other signs such as clonus and muscle spasms by various authors (Adams and Hicks 2005; Elbasiouny et al., 2010). There are several techniques for reducing spasticity, including pharmacological agents. Baclofen, a derivative of gamma aminobutyric acid (GABA) and a GABAB receptor agonist, is commonly used to treat spasticity after SCI with the intent to enhance motor function. Spasticity has been typically associated with a reduction in presynaptic inhibition (Iles and Roberts 1986), although other changes in motor neuron properties are also implicated (Elbasiouny et al., 2010). The main effect of baclofen is thought to be presynaptic, by reducing neurotransmitter release and thereby reducing the excitability of synaptic inputs onto motor neurons (Li et al., 2004).
Another neuropathological change that negatively impacts locomotor function following SCI is demyelination, which is present following contusion injuries (Guest et al., 2005). In this situation, nerve fibers may remain continuous across the injury site but varying degrees of demyelination will affect axonal conduction (Nashmi and Fehlings 2001; Waxman 1989). The low density of sodium channels in the internodal regions of the axon, along with the presence of ‘fast’ potassium channels in these areas contribute to the reduced electrical excitability of demyelinated axons (Nashmi and Fehlings 2001; Waxman 1989). The blockage of the ‘fast’ potassium channel currents using 4-aminopyridine (4-AP) has been shown to prolong action potentials and facilitate axonal conduction (Blight 1989; Sherratt et al., 1980) and increase synaptic transmission (Smith et al., 2000) in demyelinated neurons. In animal models of SCI, administration of 4-AP has been associated with improved motor and sensory function (Blight and Gruner 1987; Blight et al., 1991). There is also vigorous work being done to address the effects of demyelination using cell-transplant strategies (Keirstead et al., 2005; McDonald and Belegu 2006; Nistor et al., 2005; Reubinoff et al., 2001; Tetzlaff et al., 2010; Wu and Ren 2009).
Finally, there has been a great deal of effort in SCI research towards therapies that could afford neuroregenerative effects, promote neural plasticity, or provide neuroprotective effects (Kwon et al., 2010a; Kwon et al., 2010b; Tetzlaff et al., 2010). There are currently numerous clinical trials testing the effects of different neuroregenerative or neuroprotective compounds and the SCI community is anxiously awaiting the results. Outcomes from two large multi-center clinical trials investigating the neuroprotective effects of methylprednisolone (Bracken et al., 1992; Bracken et al., 1998) and GM-1 ganglioside (Geisler et al., 2001) have already been completed more than a decade ago. Methylprednisolone is a synthetic glucocorticoid steroid with known neuroprotective effects, likely through its anti-inflammatory effects and ability to attenuate lipid peroxidation (Bracken et al., 1992). Gangliosides are naturally occurring glycospingolipids found in the cell membranes of the brain and spinal cord and are thought to have neuroprotective and neurotrophic effects (Mocchetti 2005).
Therapies to improve walking function after SCI have received considerable attention from researchers advancing various rehabilitation therapies and those targeting pharmacological interventions. While there has been much focus on the effect of rehabilitation therapies (Lam et al., 2008; Mehrholz et al., 2008), we also need to understand what has been the effect of pharmacological agents on walking outcomes, especially as clinical trials of new drugs move forward (Baptiste and Fehlings 2008). Hence, we undertook this targeted systematic review to understand the current evidence for the efficacy of pharmacological agents on functional ambulation in people with SCI in order to understand what lessons we may apply towards future clinical research to improve walking after SCI. This work was part of the Spinal Cord Injury Rehabilitation Evidence project (www.scireproject.com).
Materials and Methods
The methods for this systematic review have been described in detail elsewhere (Eng et al., 2007), but will be briefly summarized here. A keyword literature search using multiple databases (MEDLINE/PubMed, CINAHL®, EMBASE, PsycINFO) was used to identify all relevant articles published from 1980–2009. The following keywords were used in the search: spinal cord injury/paraplegia/quadriplegia/tetraplegia, drug therapy/pharmacological agents/4-AP/Clonidine/Cyproheptadine/GM-1 ganglioside/L-Dopa/baclofen, ambulation/gait/walking/locomotion. Studies were only included if they specifically reported outcome measures associated with gait (e.g., walking speed, endurance, spatiotemporal data, gait kinematics). The reference lists of the studies were also hand searched for additional relevant studies. Articles were excluded from the review if they were animal studies, they were not in English, less than half the reported population had a spinal cord injury, or there were no measurable outcomes associated with the intervention. We did not require a minimum sample size because of the resultant limited number of publications.
Quality assessment tools and determination of level of evidence
The rigor and quality of each study were assessed by two of the authors (AD or TL) using either the Physiotherapy Evidence Database (PEDro) scale (Moseley et al., 2002) or a modified version of the Downs & Black tool (Downs and Black 1998). Discrepancies in scoring were resolved by discussion. We extracted data from the reviewed studies and summarized the findings in a table (Tables 1A, 1B, 1C, 1D, 2, 3, and 4). When possible, drug dosage and adverse effects were also extracted.
Table 1A.
Monoaminergic drugs - Clonidine
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Stewart et al. 1991 Canada PEDro = 6 RCT N (enrolled) = 12 N (completed) = 9 Not combined with training |
Population: 6 subjects with Frankel level A/B, 3 subjects with Frankel level C/D; age: 19–57 years; C7-T10 lesion level; 1–10 yrs post-injury. Treatment: Double-blind, placebo-controlled, crossover design: Two periods of 4 weeks of medication (Clonidine (up to 0.1–0.5 mg daily) or Placebo, randomly assigned) separated by a 2 week washout period. Outcome measures: Kinematic measures during body weight support treadmill locomotion, spasticity (no statistical analysis). |
|
|
|
Remy-Neris et al. 1999 France Downs & Black = 16 Prospective controlled trial N = 11 N with SCI = 8 Not combined with training |
Population: 11 subjects with incomplete paraplegia; age: 25–66 years; 6 months to 16 yrs post-injury. Treatment: 3 doses of 15–90 μg Clonidine or placebo (0.6 ml saline) by lumbar puncture or intra-thecally. Each injection separated by a minimum of 3 days. Outcome measures: Spatiotemporal gait data, Ashworth scores, soleus H-reflex, and polysynaptic flexion reflexes recorded before and every hour for 4–6 hours after injection. |
|
|
|
Norman et al. 1998 Canada Downs & Black = 13 Pre-post N=12 Not combined with training |
Population: 12 subjects; Frankel C/D; age: 19–35 yrs; C4-T12 lesion level; 1.1–5.3 yrs post-injury. Treatment: 3 different oral tablets in order of convenience: Clonidine (≤0.25 mg/day) or Cyproheptadine (≤24 mg/day) or Baclofen (≤80 mg/day): each drug trial had incremental increase to maximum dose and stable dosing over 3 weeks followed by incremental decrease from maximum dose and washout over 2 weeks. Outcome measures: Surface EMG and kinematic gait analysis during treadmill walking. No statistical analysis. |
|
|
Table 1B.
Monoaminergic drugs – Cyproheptadine
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Wainberg et al. 1990 Canada Downs & Black = 11 Prospective controlled trial N = 8 Not combined with training (Regular therapy sessions, but not defined) |
Population: 1 female, 2 wheelchair-users; age 23–56 years; C4-T11 lesion level; 1–15 yrs post-injury. Treatment: Double-blind, placebo controlled, crossover design: Two periods of 3 weeks of medication (2–8 mg 3x daily Cyproheptadine or Placebo, randomly assigned) separated by a 1 week washout period. Four subjects continued in an open label, long term trial (>6 months). Outcome measures: Temporal measures, EMG, joint angles, spasticity, comfortable walking speed. No statistical analysis. |
|
|
|
Norman et al. 1998 Canada Downs & Black = 13 Pre-post N=12 |
See Table 1A |
Table 1C.
Monoaminergic drugs – Clonidine + Cyproheptadine
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Fung et al. 1990 Canada Downs & Black = 11 Post-test N = 2 Combined with locomotor training |
Population: 2 males; age 26 and 23 yrs; T4-7 and C7-8 lesion level; 11 and 8 months post-injury. Treatment: Cyproheptadine 24 mg/day and Clonidine 0.175 mg/0.20 mg/day. Subjects were also taking baclofen. After medications were stabilized for 2 weeks, subjects participated in locomotor training. Outcome measures: Surface EMG and kinematic gait analysis during treadmill walking. No statistical analysis. |
|
|
|
Norman et al. 1998 Canada Downs & Black = 13 Pre-post N=12 |
See Table 1A |
Table 1D.
Monoaminergic drugs – L-DOPA
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Maric et al. 2008 Switzerland PEDro = 7 RCT with crossover N = 12 Combined with locomotor training |
Population: 12 subjects with incomplete SCI; age 23–75; C4-L3 lesion level; 4–16 weeks post-injury. Treatment: L-Dopa 200 mg (with 50 mg dopa decarboxylase inhibitor) for 6 weeks followed by placebo for 6 weeks (or vice versa; crossover design); physiotherapy 2 times/day for 30–45 min, 5 days/week + locomotor training 45 min, 5 days/week. Outcome Measures: ASIA motor score (AMS); Walking Index for Spinal Cord Injury II (WISCI II); Spinal Cord Independence Measure II (SCIM II). |
|
|
Table 2.
4-aminopyridine
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
DeForge et al. 2004 Canada PEDro = 8 RCT N=15 Not combined with training |
Population: 15 subjects with AIS D; age 22–70 yrs; C3-T12 lesion level; 1–20 years post-injury. Treatment: Double-blind, placebo-controlled, crossover design; 4-AP: up-titration to 10 mg 4x/day stable dosing of 4-AP versus Placebo, 2 weeks each condition. Outcome measures: Isometric muscle force, gait analysis (including temporal-spatial data, EMG, joint kinematics and kinetics). |
|
|
|
van der Bruggen et al. 2001 Netherlands PEDro = 7 RCT N=20 Not combined with training |
Population: 20 subjects, 1 AIS B, 5 AIS C, 14 AIS D; age: 25–70 yrs; C2-L3 lesion level; 3–56 yrs post-injury. Treatment: Double-blind, placebo-controlled, crossover design: up-titration to maximum of 15–45 mg, immediate-release 4-AP capsules or Placebo, 4 weeks each condition. Two-week washout between conditions. Outcome measure: functional status (Darmouth COOP Functional Health Assessment), comfortable and maximum over ground walking speed, vibration perception threshold. |
|
|
|
Segal & Brunnemann 1998 USA Downs & Black = 16 Pre-post N=9 Not combined with training |
Population: 9 subjects with AIS C or D; age 28–60 yrs; C2-L4 lesion level; 4–28 yrs post-injury. Treatment: 4-AP (single 10 mg immediate-release capsule). Comparison of means at baseline and at 9 pre-determined intervals over a 24-hour follow-up. Outcome measures: Over ground gait parameters (e.g. velocity, cadence). |
|
|
Table 3.
GM-1 ganglioside
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Walker & Harris 1993 USA PEDro = 6 RCT N=9 Combined with standard PT |
Population: 9 subjects with incomplete SCI; age 21–44 yrs; C5-L1 lesion level; 18 months to 13 yrs post-injury. Treatment: Double-blind, placebo-controlled crossover study design: Intravenous GM-1 ganglioside or placebo + 2 hr gait training, 6 times/week for 2 months, followed by switch of drug administration (total 4 months). All subjects given 6 months of PT before trial. Outcome measures: Motor scores, walking distance, and velocity. |
|
|
Table 4.
Baclofen
| Authors Year Country Score Research Design Sample Size |
Methods | Outcomes | Side Effects |
|---|---|---|---|
|
Azouvi et al. 1996 France Downs & Black = 11 Prospective pre-post N = 18 Not combined with training |
Population: 12 of 18 subjects with SCI (Frankel A-D); age 21–59; C4-T11 lesion level; 0.5–27 years post-injury. Treatment: Implanted intrathecal baclofen pump. 17 patients had an electronically driven programmable pump filled with 18 cc of 500 or 2000 ug/cc baclofen delivered by continuous infusion or by intermittent bolus. One patient had a manually operated pump delivering a bolus of 50 ug. Follow up assessment was 6–72 months after implantation. Outcome Measures: Ashworth scale, spasm frequency scores, FIM. No statistical analysis on FIM walking score. |
|
|
|
Norman et al. 1998 Canada Downs & Black = 13 Pre-post N=12 |
See Table 1A |
We used the PEDro tool to assess the quality of studies that were randomized controlled trials (RCTs). This scale evaluates the internal and external validity of a study based on an 11-item scale, with a maximum score of 10. Higher scores represent greater methodological quality (9–10, excellent; 6–8, good; 4–5, fair; <4, poor) (Foley et al., 2003).
A modified version of the Downs &Black tool was used to assess all other studies. This tool evaluates quality based on 27 items assessing reporting, external validity, internal validity (bias and confounding) of the study. The maximum score for this tool is 28, with higher scores indicating better methodological quality.
After each study was individually assessed, we determined the level of evidence of all the studies collectively using a modified scale developed by Sackett et al. (Sackett et al., 2000). We collapsed Sackett’s level of evidence into 5 categories. Level 1 evidence corresponded to studies that were “good” to “excellent” RCTs, or a PEDro score ≥ 6. Level 2 was the rating for evidence that included RCTs with a PEDro score ≤ 5, non-randomized prospective controlled studies, and cohort studies. Level 3 corresponded to evidence from case control studies. Evidence from pre-post/post-test/case series and observational/case report studies were rated Level 4 and 5, respectively. Any study that did not include statistical analysis for the walking outcome measure were automatically rated Level 5.
Effect sizes
We calculated effect sizes and 95% confidence intervals using the Cohen’s d estimate with Hedges adjustment for sample size (Devilly 2004) for studies that measured changes in walking speed, either during pre- and post-treatment or placebo and experimental treatment. To calculate effect size for the pre-post studies, the mean pre-test walking speed was subtracted from the mean post-test walking speed and divided by the weighted average of the standard deviations for both time points. To calculate effect size for the controlled studies, the change in walking speed from baseline in both the experimental and placebo groups was calculated. The difference in the mean changes was then divided by the weighted average of the standard deviations of all the changes. We also calculated effect sizes for 4 different locomotor training studies that had walking speed as an outcome measure (Field-Fote and Roach 2011; Gorassini et al., 2009; Winchester et al., 2009; Wirz et al., 2005) for comparison using the same methods.
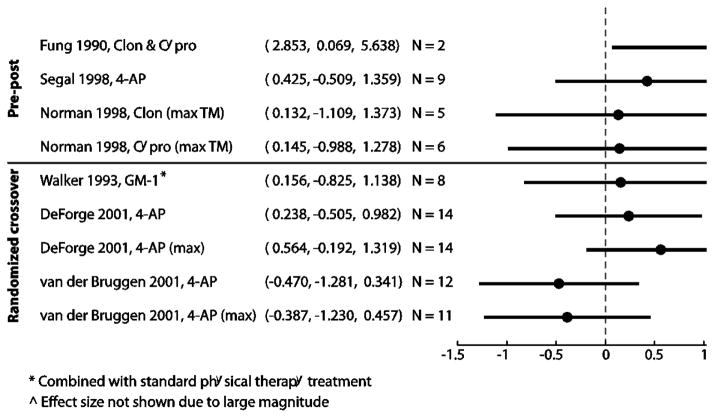
The results were displayed in a forest plot. An effect size of 0.2–0.5 is considered small, 0.5–0.8 medium and ≥ 0.80 large (Cohen 1977). When walking speed data from papers were illustrated in graphical form, we scanned the image to a computer and extracted the data from these graphical plots using a custom-written MATLAB (MathWorks, Inc., Natick, MA, USA) software (GRABIT, J. Doke, MathWorks, Inc.). The changes in walking speed for subjects using body weight support in the Norman study were not included in this analysis because the amount of body weight support may have changed between each evaluation (Norman et al., 1998). In addition, because multiple drugs were tested in each subject, data were only extracted from this study when the drug was immediately preceded by a period where no medication and used for the pre-to-post comparison. This study also reported the effects of Baclofen or the combination of Cyproheptadine and Clonidine on walking, but these results were not included in the forest plot because there was data for only one subject and the effect sizes could not be calculated (Norman et al., 1998).
Results
The literature search resulted in 696 articles related to pharmacological interventions and gait after spinal cord injury. Eleven studies met our criteria and were evaluated using either the PEDro tool, for RCTs, or the modified Downs &Black tool, for all other studies. Six studies examined the effects of monoaminergic drugs (Clonidine, Cyproheptadine, and L-Dopa) on gait after SCI, three studies examined the effects of 4-aminopyridine (4-AP), two studies examined the effects of Baclofen, and one studied the effects of GM-1 ganglioside. Most, but not all subjects had incomplete spinal cord injuries.
Monoaminergic agents
Six studies examined the effects of monoaminergic drugs on walking after complete or incomplete SCI (Table 1A, Table 1B, Table 1C and Table 1D, total N = 51). Reported side effects in each study are also included in each of the tables. In most studies, the effects of each drug were studied individually with the exception of one study by Fung et al. (Fung et al., 1990), where subjects were given a combination of Cyproheptadine and Clonidine in addition to the Baclofen they were already taking before the experiment began.
Clonidine
Our literature search resulted in three studies examining the effects of Clonidine (Norman et al., 1998; Rémy-Néris et al., 1999; Stewart et al., 1991) (Table 1A, total N = 29; 8 months to 10 years post-injury). Two of the studies (Norman et al., 1998; Stewart et al., 1991) used the oral form of Clonidine, while a third study administered the drug intra-thecally via lumbar puncture in order to minimize side effects (Rémy-Néris et al., 1999). In a small randomized controlled trial (n=9, no statistical analysis) improvements were only noted in one of the three subjects with incomplete SCI, where the subject progressed from being unable to walk to walking overground with an assistive device (Stewart et al., 1991). This particular subject was more severely impaired than the other incomplete SCI subjects and was the shortest amount of time post-injury (1 year post-injury). The other studies (1 pre-post (Norman et al., 1998) and 1 non-randomized controlled study (Rémy-Néris et al., 1999)) similarly showed that more severely impaired subjects benefited more from the administration of Clonidine. In addition, the Norman et al. (Norman et al., 1998) pre-post study showed that the effects of Clonidine persisted after washout of the drug.
Cyproheptadine
Cyproheptadine is a serotonergic antagonist and an anti-histamine drug. Two studies examined the effects of this drug on walking in patients with SCI (Norman et al., 1998; Wainberg et al., 1990) (Table 1B, total N = 20; 1–15 years post-injury). Subjects took up to 24 mg of Cyproheptadine daily in the form of oral tablets in both studies.
One Level 5 cross-over study (Wainberg et al., 1990) and one Level 5 post-test study (Norman et al., 1998), both without statistical analysis, described the effects of Cyproheptadine on gait patterns after SCI. The prospective controlled trial showed that maximum comfortable walking speed increased 8–34% in all 6 ambulatory subjects with the medication (Wainberg et al., 1990). In addition, the two patients that required body weight support during treadmill walking while taking the placebo medication were able to make stepping movements with full weight bearing while taking Cyproheptadine. Muscle coordination was improved and clonus was reduced in the patients. Norman et al. (Norman et al., 1998) similarly reported that administration of Cyproheptadine was associated with increased treadmill speed as well as reduced ankle clonus.
Combination of Cyproheptadine + Clonidine
There are two Level 5 post-test studies that examined the effect of the combination of Cyproheptadine and Clonidine (Table 1A and 1C, total N = 4; 8 months-2 years post- injury) (Fung et al., 1990; Norman et al., 1998). In the Norman et al. study (Norman et al., 1998), the combination of Cyproheptadine and Clonidine resulted in increased maximal treadmill speed and decreased assistance with stepping in 2 subjects. In the Fung et al. study (Fung et al., 1990), subjects also underwent locomotor training. The subjects were first stabilized on the combined medication for two weeks (in addition to the Baclofen they were already taking before the study started) before commencing the combined drug therapy in addition to intense locomotor training (body-weight supported treadmill training, 3–5 sessions/week for 3–8 weeks). Treadmill-based gait assessments demonstrated improved lower limb muscle activity and joint kinematic patterns following medication and locomotor training. Muscle activity became more phasic during walking and the subjects experienced less clonus. Subjects were able to walk overground while on medication, and they showed further improvement in functional ambulation with the addition of locomotor training.
Levodopa
One study has examined the effect of L-Dopa in incomplete SCI (Maric et al., 2008) (Table 1D, N = 12, 4–16 weeks post-injury). Subjects underwent a randomized crossover trial where 6 weeks of placebo was followed by 6 weeks of L-Dopa (or vice versa) combined with locomotor training (45 minutes, 5 days/week). This study provides Level 1 evidence that there is no difference between L-Dopa and placebo combined with locomotor training on changes in voluntary muscle strength, walking function, and activities of daily living in sub-acute SCI.
4-aminopyridine
The effects of 4-AP on gait after SCI has been studied in two Level 1 RCTs (DeForge et al., 2004; van der Bruggen et al., 2001) and one Level 4 pre-post study (Segal and Brunnemann 1998) (Table 2, total N = 44, 1–56 years post-injury). Although an earlier pre-post trial showed promising results (36% improvement in gait speed) (Segal and Brunnemann 1998), later controlled trials did not support this result (DeForge et al., 2004; van der Bruggen et al., 2001). Level 1 evidence showed that there was no benefit from 4-AP over placebo on gait parameters in chronic incomplete SCI (DeForge et al., 2004; van der Bruggen et al., 2001). The varied dosages between all three of the reviewed studies (10 mg, 4 times daily in DeForge et al. (DeForge et al., 2004); between 15–45 mg daily in van der Bruggen et al. (van der Bruggen et al., 2001); and 10 mg single dose in Segal & Brunneman (Segal and Brunnemann 1998)) complicates interpretation of the outcomes and makes it difficult to draw clear conclusions from the available evidence.
GM-1 ganglioside
One study reported the effects of GM-1 on walking after SCI (Walker and Harris 1993) (Table 3, N = 9, 1–13 years post-injury). Subjects were given GM-1 intravenously or intramuscularly in conjunction with physical therapy, compared to placebo via crossover design. This study provides Level 1 evidence shows that GM-1 ganglioside is beneficial for the recovery of walking in chronic spinal cord injury (Walker and Harris 1993). The levels of injury (i.e., ASIA Impairment Scale (AIS) classification or functional level) of the subjects were not clearly described by the authors, but all subjects were chronic wheelchair users at the start of the study. Subjects had significantly higher motor scores with GM-1 ganglioside, with no placebo effect. All but one subject increased walking speed and walking distance with GM-1 ganglioside. The subject who did not improve in gait speed or distance did, however, switch from long-leg bracing to below-knee bracing.
Baclofen
There is limited evidence that Baclofen may improve walking after SCI. Two Level 5 pre-post studies that examined the effects of Baclofen on gait (Table 4, total N = 21, 0.5–27 years post-injury (Azouvi et al., 1996; Norman et al., 1998). The Avouzi et al. study (Azouvi et al., 1996) showed there was increase in the Functional Independence Measure (FIM) walking scores in 5 of 18 patients, and 2 people acquired the ability to climb stairs with using Baclofen. Subjects in the Norman et al. study (Norman et al., 1998) only showed minor changes in walking when using this drug.
Effect sizes
Figure 1 is a forest plot of the effect sizes for all studies from which we were able to extract data on changes in walking speed. Effect sizes were small for the majority of the studies (d < 0.20) for which we were able to extract walking speed data (Fig. 1). One randomized crossover study looking at the effects of 4-aminopyridine actually resulted in slower walking speeds (van der Bruggen et al., 2001). The crossover study that investigated the effects of 4-aminopyridine on walking (DeForge et al., 2004) had a medium effect size (d = 0.564, maximum walking speed).
Figure 1.
Health and drug screening
Few of the studies presented in this review reported that health screenings of other organ systems (e.g., liver function) were performed to ensure that the subjects were healthy enough to properly metabolize the drugs. Only 1 of the studies (DeForge et al., 2004) stated that blood count and liver function tests were performed. Two of the studies involving Clonidine (Norman et al., 1998; Stewart et al., 1991) measured blood pressure when increasing dosage because of its hypotensive effects. Possible drug-drug interactions were also not addressed. Six studies stated that subjects were taking other drugs at entry to the study (DeForge et al., 2004; Fung et al., 1990; Norman et al., 1998; Stewart et al., 1991; van der Bruggen et al., 2001; Wainberg et al., 1990), but only 3 specifically listed what these drugs were (DeForge et al., 2004; Fung et al., 1990; Norman et al., 1998).
In the Norman et al. study, one subject continued to take anticonvulsant medication (divalporex sodium) and an anti-depressant (imipramine) throughout the study and another subject took an anxiolytic medication (bromazepam) occasionally during the study to aid with sleeping (Norman et al., 1998). In the DeForge et al. 4-AP study, 6 of 15 subjects had been stabilized on medication for spasticity. Three of these subjects were taking Clonidine, 2 were taking Baclofen, and 1 was taking Cyproheptadine (DeForge et al., 2004). Subjects in the Fung et al. study were also taking Baclofen in addition to the combination of Clonidine and Cyproheptadine throughout the study (Fung et al., 1990). In the van der Bruggen et al. 4-AP study, subjects were asked to maintain stable dose of prescribed medications throughout study, but the types of drugs that were taken or the number of subjects taking other drugs were not reported (van der Bruggen et al., 2001). Anti-spasticity medications were also taken by 3 subjects in the Stewart et al. Clonidine study (Stewart et al., 1991). Subjects in the Wainberg et al. study were stabilized on their medications for spasticity for at least 3 months before they started the study with Cyproheptadine (Wainberg et al., 1990).
Discussion
This systematic review found 11 articles that examined the effects of pharmacological agents on walking outcomes after spinal cord injury. The median PEDro score for the RCTs was 7 out of 10 [1st quartile = 6, 2nd quartile = 7] (two studied the effects of 4-AP, one studied L-Dopa, one studied Clonidine, and one studied GM-1 ganglioside), showing that these studies were of good quality. The non-RCTs that were reviewed were of lower quality, with the median Downs & Black score for these studies being 12 out of 28 [11, 15.25].
Studies show small effects
Where over ground walking speed data was available, we calculated and compared the effect sizes of each reviewed study. Most studies showed small to negative effects on walking speed (d = −0.470 – 0.564) with only one study showing a medium effect size (DeForge et al., 2004; Fung et al., 1990; Segal and Brunnemann 1998; van der Bruggen et al., 2001; Walker and Harris 1993). To determine how these effect sizes compared to changes in walking speed following physical rehabilitation alone, we looked at the results from 4 studies where subjects with chronic incomplete spinal cord injury underwent different forms of gait training and where walking speed was an outcome measure (Field-Fote and Roach 2011; Gorassini et al., 2009; Winchester et al., 2009; Wirz et al., 2005). Our calculated effect sizes from these studies were 0.1–0.62 (N = 10–30; mean ± SD: d = 0.32±0.16), indicating that locomotor training will result in as good, or more likely better gait outcomes than using any of the drugs presented in this review.
It is striking that few of the studies we found combined intensive locomotor training with pharmacological agents. Only two studies (Fung et al., 1990; Maric et al., 2008) employed intensive task-specific gait training techniques in conjunction with the drug therapy. Two other studies reported that subjects underwent regular therapy sessions that appeared to consist largely of non-specific range of motion and strengthening exercises, with some gait training (Wainberg et al., 1990; Walker and Harris 1993). Given the importance of task-specific training on functional recovery (Edgerton and Roy 2009; Harkema 2001; Ichiyama et al., 2008), it will be important to understand the combinatorial effects of rehabilitation along with pharmacological interventions.
Only a fraction of patients were able to improve their walking speed with Clonidine or Cyproheptadine. The subjects that typically benefited from the medications were more severely impaired and with higher levels of spasticity, but also had a minimum level of function (could stand or walk with assistance). This suggests that these drugs may be more useful in helping individuals with more severe impairments as a way to initiate or facilitate locomotor training, particularly through their effect in reducing severe spasticity and clonus (Norman et al., 1998; Stewart et al., 1991). In addition, there is evidence that Clonidine has deleterious effects on walking in cats with partial spinal lesions (Rossignol et al., 2001), so it may not be surprising that this drug did not greatly improve walking in humans with incomplete SCI.
Another possible reason that minimal gains in walking were seen with the monoaminergic drugs may have to do with the drugs’ side effects. Subjects with SCI may already have low tolerance to changes in blood pressure or heart rate due to abnormal cardiovascular control (Krassioukov and Claydon 2006), and therefore may be more sensitive to the medications that have hypotensive effects, such as Clonidine, than the general population. Also, because Clonidine is an α2-receptor agonist that inhibits the release of norepinephrine and decreases sympathetic tone (AHFS Drug Information 2011), it could cause a decrease in the general excitability of the nervous system. Cyproheptadine is an anti-serotonergic drug (a CNS depressant) and is also an anti-histamine drug. Side effects of anti-histamine medications are drowsiness and fatigue, which may have an obvious detrimental impact on walking outcomes. Thus, the side effects of these drugs may have out-weighed the desired effects of the medication.
There may be several explanations as to why L-Dopa did not improve locomotor function in persons with SCI. Maric and colleagues hypothesized that the locomotor spinal circuitry would be facilitated during walking training in the presence of a noradrenergic drug (Maric et al., 2008). However, animal studies have shown that noradrenergic terminals degenerate within a week after spinal cord injury (Anden et al., 1964) and in chronic spinalized cats, L-Dopa modulates muscle activity in ongoing locomotion through dopaminergic receptors, not noradrenergic receptors (Barbeau and Rossignol 1991). There is also a reduction in the capacity of the spinal cord to synthesized dopamine from L-Dopa in acutely injured rats (10 days post-injury), although this capacity recovers to normative values by the time animals are 100 days post-injury (Commissiong 1985). The participants in the study of Maric et al. (2008) had SCI of between 4 and 16 weeks duration and the administration of L-Dopa occurred over a 12-week period. Thus, changes in the capacity for dopamine synthesis during this sub-acute injury phase could have confounded the results. The authors also justified the use of this drug in persons with SCI because it improved motor function in stroke in combination with exercise training (Scheidtmann et al., 2001). However, the mechanisms underlying the recovery of walking function in stroke could be quite different than what occurs in SCI. These factors may have contributed to the lack of effect of L-Dopa on walking outcomes in persons with SCI (Maric et al., 2008).
Conflicting evidence exists regarding the benefits of GM-1 on the recovery of locomotion after SCI. In the reviewed study, GM-1 combined with physical therapy improved motor scores, walking distance and walking speed in a small RCT with chronic SCI (Walker and Harris 1993). Later, a large multicenter RCT in patients with acute SCI (N = 760) showed that although GM-1 treatment accelerated initial recovery during the first 8 weeks of treatment, it was no different than placebo by the end of the trial (26 weeks) (Geisler et al., 2001). However, specific walking-related outcomes were not reported in this larger trial. This supports the idea that treatment effects tend to be exaggerated in smaller, non-controlled trials and that larger trials are needed to evaluate the effectiveness of therapeutic interventions.
Intrathecal baclofen showed slightly better results (an increase in the walking component of the FIM score in 5 of 18 patients (Azouvi et al., 1996)) than oral baclofen (only minor changes in walking (Norman et al., 1998)). This is consistent with the evidence on the effects of Baclofen on spasticity. A systematic review showed that intrathecal Baclofen is effective in reducing spasticity compared to controls, while oral Baclofen had no effects (Taricco et al., 2000). Currently, there are investigations into using other drugs to target spasticity through different neural mechanisms (serotonergic or noradrenergic pathways) (Rank et al., 2007).
Methodological limitations of reviewed studies
There are several possibilities as to why the studies evaluated in the present review had small or even negative effects on walking, many of which may be related to the variability of subject characteristics and in the testing protocols between studies. In addition to the small sample sizes in many of the studies, the subject groups may have been too heterogeneous in injury chronicity, level and severity, participant age, or too small in number to show a strong effect on the outcome measures. The dose and frequency of the different medications varied within and between studies, creating even more variability in study design. It is also possible that the subjects tested had impairments that would have only minimum benefits from these drugs. In addition, it was reported that some of the subjects in the reviewed studies were taking other medications. The presence of other drugs may have led to drug-drug interactions that may have ultimately affected locomotor outcomes.
Varied outcome measures were used in the reviewed studies, making the results difficult to compare. There was a lack of measurable functional ambulation outcomes in most of the studies. Quantitative and accurate functional outcome measures are essential to SCI clinical trials (Steeves et al., 2007). In addition, the relatively weak level of evidence for the effects of these drugs on walking is due to the fact that more than half the studies reviewed were not randomized or blinded, which may exaggerate the actual treatment effects (Carlson and Schmidt 1999; Lipsey and Wilson 1993).
In most studies, it was unknown whether subjects had organ systems healthy enough to metabolize the drugs they were given. For example, impairments in liver or kidney function could limit drug metabolism, distribution, and excretion. Liver function and blood count tests were reported in only one study (DeForge et al., 2004). Clonidine, 4-AP, and Baclofen dosages should be adjusted according to the degree of renal function (AHFS Drug Information 2011), yet none of the studies involving these drugs reported they tested renal function of the subjects.
Future directions
Although the studies to-date of the effects of pharmacological agents on walking outcomes in SCI have been disappointing, there could be potential in investigating combinatorial strategies. Although it had a small sample size, the Fung et al. study suggested that Cyproheptadine and Clonidine in combination with locomotor training improved gait patterns and over ground walking speed (Fung et al., 1990). There are other promising combinations of multiple therapeutic interventions currently under study. One new drug combination (Spinalon), which consists of L-Dopa and the 5-HT agonist Buspirone, has been shown to facilitate reflex stepping during treadmill training by activating spinal locomotor networks (Guertin et al., 2010). A recent case study testing safety has shown that this combination has no significant side effects in a subject with incomplete spinal cord injury (Guertin and Brochu 2009). Combining pharmacological agents with active rehabilitation therapy may also be promising. In spinalized rats, combining pharmacological agents with locomotor training and electrical stimulation led to reorganization of central pattern generator circuits, enabling full weight-bearing stepping (Courtine et al., 2009). Future studies should also benefit from a combination of expertise from clinical pharmacologists along with neuroscientists and rehabilitation specialists.
Acknowledgments
This work was supported by the King Saud University-International Twinning Program and CIHR (114559). We thank the Rick Hansen Man-in-Motion Foundation and Ontario Neurotrauma Fund for their support of the Spinal Cord Injury Rehabilitation Evidence (SCIRE) project. TL was supported by a New Investigator Award from the Canadian Institutes of Health Research. JJE is a Michael Smith Foundation for Health Research Senior Scholar and was with a salary support by Canadian Institutes of Health Research (CIHR MSH-63617).
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Adams M, Hicks A. Spasticity after spinal cord injury. Spinal Cord. 2005;43:577–586. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- AHFS Drug Information. American Society of Health-System Pharmacists, Inc; 2011. [Google Scholar]
- Anden NE, Haeggendal J, Magnusson T, Rosengren E. The time course of the disappearance of noradrenaline and 5-Hydroxytryptamine in the spinal cord after transection. Acta Physiol Scand. 1964;62:115–118. doi: 10.1111/j.1748-1716.1964.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Anden NE, Jukes MG, Lundberg A. The effect of DOPA on the spinal cord 2. A pharmacological analysis. Acta Physiol Scand. 1966;67:387–397. doi: 10.1111/j.1748-1716.1966.tb03325.x. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996;77:35–39. doi: 10.1016/s0003-9993(96)90217-8. [DOI] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG. Emerging drugs for spinal cord injury. Expert Opin Emerg Drugs. 2008;13:63–80. doi: 10.1517/14728214.13.1.63. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Res. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Blight AR. Effect of 4-aminopyridine on axonal conduction-block in chronic spinal cord injury. Brain Res Bull. 1989;22:47–52. doi: 10.1016/0361-9230(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Blight AR, Gruner JA. Augmentation by 4-aminopyridine of vestibulospinal free fall responses in chronic spinal-injured cats. J Neurol Sci. 1987;82:145–159. doi: 10.1016/0022-510x(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Blight AR, Toombs JP, Bauer MS, Widmer WR. The effects of 4-aminopyridine on neurological deficits in chronic cases of traumatic spinal cord injury in dogs: a phase I clinical trial. J Neurotrauma. 1991;8:103–119. doi: 10.1089/neu.1991.8.103. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, Jr, Holford TR, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon JC, Marshall LF, Perot PL, Piepmeier J, Sonntag VK, Wagner FC, Wilberger JL, Winn HR, Young W. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci. 1911;84:308–319. [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. J Neurophysiol. 1999;81:1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117(Pt 5):1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- Carlson KD, Schmidt FL. Impact of Experimental Design on Effect Size: Findings From the Research Literature on Training. Journal of Applied Psychology. 1999;84:851–862. [Google Scholar]
- Carlsson A, Magnusson T, Rosengren E. 5-Hydroxytryptamine of the Spinal Cord Normally and after Transection. Experientia. 1963;19:359. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- Carp JS, Rymer WZ. Enhancement by serotonin of tonic vibration and stretch reflexes in the decerebrate cat. Exp Brain Res. 1986;62:111–122. doi: 10.1007/BF00237407. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Early locomotor training with clonidine in spinal cats. J Neurophysiol. 1998a;79:392–409. doi: 10.1152/jn.1998.79.1.392. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal alpha1- and alpha2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol. 1998b;79:2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Academic Press; New York, NY: 1977. [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps RA, Lee BB, Wing P, Weerts E, Mackay J, Brown D. A global map for traumatic spinal cord injury epidemiology: towards a living data repository for injury prevention. Spinal Cord. 2010;49:493–501. doi: 10.1038/sc.2010.146. [DOI] [PubMed] [Google Scholar]
- DeForge D, Nymark J, Lemaire E, Gardner S, Hunt M, Martel L, Curran D, Barbeau H. Effect of 4-aminopyridine on gait in ambulatory spinal cord injuries: a double-blind, placebo-controlled, crossover trial. Spinal Cord. 2004;42:674–685. doi: 10.1038/sj.sc.3101653. [DOI] [PubMed] [Google Scholar]
- Devilly GJ. The Effect Size Generator for Windows. Centre for Neuropsychology, Swinburne University; Australia: 2004. [Google Scholar]
- Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- Ditunno P, Patrick M, Stineman M, Ditunno JF. Who wants to walk? Preferences for recovery after SCI: a longitudinal and cross-sectional study. Spinal Cord. 2008;46:500–506. doi: 10.1038/sj.sc.3102172. [DOI] [PubMed] [Google Scholar]
- Donnelly C, Eng JJ, Hall J, Alford L, Giachino R, Norton K, Kerr DS. Client-centred assessment and the identification of meaningful treatment goals for individuals with a spinal cord injury. Spinal Cord. 2004;42:302–307. doi: 10.1038/sj.sc.3101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain Res Bull. 2009;78:4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair. 2010;24:23–33. doi: 10.1177/1545968309343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng JJ, Teasell R, Miller WC, Wolfe DL, Townson AF, Aubut J-A, Abramson C, Hsieh J, Connolly S. SCIRE: Spinal Cord Injury Rehabilitation Evidence, Chapter 2: Methods of Systematic Reviews. ICORD; Vancouver: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estores IM. The consumer’s perspective and the professional literature: what do persons with spinal cord injury want? Journal of rehabilitation research and development. 2003;40:93–98. doi: 10.1682/jrrd.2003.08.0093. [DOI] [PubMed] [Google Scholar]
- Feraboli-Lohnherr D, Barthe JY, Orsal D. Serotonin-induced activation of the network for locomotion in adult spinal rats. J Neurosci Res. 1999;55:87–98. doi: 10.1002/(SICI)1097-4547(19990101)55:1<87::AID-JNR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91:48–60. doi: 10.2522/ptj.20090359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NC, Teasell RW, Bhogal SK, Speechley MR. Stroke rehabilitation evidence-based review: methodology. Top Stroke Rehabil. 2003;10:1–7. [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal Cord-Transected Mice Learn to Step in Response to Quipazine Treatment and Robotic Training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J Neurol Sci. 1990;100:85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine. 2001;26:S87–98. doi: 10.1097/00007632-200112151-00015. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang J. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol. 2009;101:969–979. doi: 10.1152/jn.91131.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Brochu C. Preliminary evidence of safety following administration of L-DOPA and buspirone in an incomplete monoplegic patient. Spinal Cord. 2009;47:91–92. doi: 10.1038/sc.2008.70. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Ung RV, Rouleau P. Oral administration of a tri-therapy for central pattern generator activation in paraplegic mice: proof-of-concept of efficacy. Biotechnol J. 2010;5:421–426. doi: 10.1002/biot.200900278. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- Hicks A, Ginis KA. Treadmill training after spinal cord injury: it’s not just about the walking. Journal of rehabilitation research and development. 2008;45:241–248. doi: 10.1682/jrrd.2007.02.0022. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Roberts RC. Presynaptic inhibition of monosynaptic reflexes in the lower limbs of subjects with upper motoneuron disease. J Neurol Neurosurg Psychiatry. 1986;49:937–944. doi: 10.1136/jnnp.49.8.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res. 2006;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W. A Systematic Review of Non-Invasive Pharmacologic Neuroprotective Treatments for Acute Spinal Cord Injury. J Neurotrauma. 2010a doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon EB, Plunet W, Baptiste D, Fouad K, Hillyer J, Weaver LC, Fehlings MG, Tetzlaff W. A Systematic Review of Directly Applied Biologic Therapies for Acute Spinal Cord Injury. J Neurotrauma. 2010b doi: 10.1089/neu.2009.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Noonan V, Eng J, Team SR. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246–254. doi: 10.1038/sj.sc.3102134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi R, Hultling C, Seiger A. The Stockholm Spinal Cord Injury Study: 2. Associations between clinical patient characteristics and post-acute medical problems. Paraplegia. 1995;33:585–594. doi: 10.1038/sc.1995.125. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ. Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol. 2004;92:2694–2703. doi: 10.1152/jn.00164.2004. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am Psychol. 1993;48:1181–1209. doi: 10.1037//0003-066x.48.12.1181. [DOI] [PubMed] [Google Scholar]
- Lundqvist C, Siosteen A, Blomstrand C, Lind B, Sullivan M. Spinal cord injuries. Clinical, functional, and emotional status. Spine (Phila Pa 1976) 1991;16:78–83. [PubMed] [Google Scholar]
- Maric O, Zorner B, Dietz V. Levodopa therapy in incomplete spinal cord injury. J Neurotrauma. 2008;25:1303–1307. doi: 10.1089/neu.2008.0583. [DOI] [PubMed] [Google Scholar]
- Marley E, Vane JR. Tryptamines and spinal cord reflexes in cats. Br J Pharmacol Chemother. 1967;31:447–465. doi: 10.1111/j.1476-5381.1967.tb00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane database of systematic reviews (Online) 2008:CD006676. doi: 10.1002/14651858.CD006676.pub2. [DOI] [PubMed] [Google Scholar]
- Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci. 2005;62:2283–2294. doi: 10.1007/s00018-005-5188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley AM, Herbert RD, Sherrington C, Maher CG. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48:43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Injury Statistical Center. Facts and figures at a glance. Birmingham, AB: 2006. [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil. 2000;79:526–535. doi: 10.1097/00002060-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Norman KE, Pépin A, Barbeau H. Effects of drugs on walking after spinal cord injury. Spinal Cord. 1998;36:699–715. doi: 10.1038/sj.sc.3100674. [DOI] [PubMed] [Google Scholar]
- Pearlstein E, Ben Mabrouk F, Pflieger JF, Vinay L. Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord. Eur J Neurosci. 2005;21:1338–1346. doi: 10.1111/j.1460-9568.2005.03971.x. [DOI] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97:3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rémy-Néris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Experimental brain research Experimentelle Hirnforschung Expérimentation cérébrale. 1999;129:433–440. doi: 10.1007/s002210050910. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharmacological aids to locomotor training after spinal injury in the cat. J Physiol. 2001;533:65–74. doi: 10.1111/j.1469-7793.2001.0065b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. Churchill Livingstone; Toronto, ON: 2000. [Google Scholar]
- Scheidtmann K, Fries W, Muller F, Koenig E. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: a prospective, randomised, double-blind study. Lancet. 2001;358:787–790. doi: 10.1016/S0140-6736(01)05966-9. [DOI] [PubMed] [Google Scholar]
- Segal JL, Brunnemann SR. 4-Aminopyridine alters gait characteristics and enhances locomotion in spinal cord injured humans. J Spinal Cord Med. 1998;21:200–204. doi: 10.1080/10790268.1998.11719527. [DOI] [PubMed] [Google Scholar]
- Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283:570–572. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Felts PA, John GR. Effects of 4-aminopyridine on demyelinated axons, synapses and muscle tension. Brain. 2000;123(Pt 1):171–184. doi: 10.1093/brain/123.1.171. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Barbeau H, Gauthier S. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci. 1991;18:321–332. doi: 10.1017/s0317167100031887. [DOI] [PubMed] [Google Scholar]
- Taricco M, Adone R, Pagliacci C, Telaro E. Pharmacological interventions for spasticity following spinal cord injury. Cochrane database of systematic reviews. 2000:CD001131. doi: 10.1002/14651858.CD001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J Neurotrauma. 2010 doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen MA, Huisman HB, Beckerman H, Bertelsmann FW, Polman CH, Lankhorst GJ. Randomized trial of 4-aminopyridine in patients with chronic incomplete spinal cord injury. J Neurol. 2001;248:665–671. doi: 10.1007/s004150170111. [DOI] [PubMed] [Google Scholar]
- Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiatr. 1990;53:754–763. doi: 10.1136/jnnp.53.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JB, Harris M. GM-1 ganglioside administration combined with physical therapy restores ambulation in humans with chronic spinal cord injury. Neurosci Lett. 1993;161:174–178. doi: 10.1016/0304-3940(93)90287-u. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Demyelination in spinal cord injury. J Neurol Sci. 1989;91:1–14. doi: 10.1016/0022-510x(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Winchester P, Smith P, Foreman N, Mosby JM, Pacheco F, Querry R, Tansey K. A prediction model for determining over ground walking speed after locomotor training in persons with motor incomplete spinal cord injury. The journal of spinal cord medicine. 2009;32:63–71. doi: 10.1080/10790268.2009.11760754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Archives of Physical Medicine and Rehabilitation. 2005;86:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Wu B, Ren X. Promoting axonal myelination for improving neurological recovery in spinal cord injury. J Neurotrauma. 2009;26:1847–1856. doi: 10.1089/neu.2008.0551. [DOI] [PubMed] [Google Scholar]