Abstract
Background
Leptospirosis is a worldwide zoonosis that is endemic in tropical areas, such as Reunion Island. The species Leptospira interrogans is the primary agent in human infections, but other pathogenic species, such as L. kirschner and L. borgpetersenii, are also associated with human leptospirosis.
Methods and Findings
In this study, a melting curve analysis of the products that were amplified with the primer pairs lfb1 F/R and G1/G2 facilitated an accurate species classification of Leptospira reference strains. Next, we combined an unsupervised high resolution melting (HRM) method with a new statistical approach using primers to amplify a two variable-number tandem-repeat (VNTR) for typing at the subspecies level. The HRM analysis, which was performed with ScreenClust Software, enabled the identification of genotypes at the serovar level with high resolution power (Hunter-Gaston index 0.984). This method was also applied to Leptospira DNA from blood samples that were obtained from Reunion Island after 1998. We were able to identify a unique genotype that is identical to that of the L. interrogans serovars Copenhageni and Icterohaemorrhagiae, suggesting that this genotype is the major cause of leptospirosis on Reunion Island.
Conclusions
Our simple, rapid, and robust genotyping method enables the identification of Leptospira strains at the species and subspecies levels and supports the direct genotyping of Leptospira in biological samples without requiring cultures.
Introduction
Pathogenic Leptospira bacteria are agents of leptospirosis, one of the most widespread zoonoses worldwide[1]. Leptospira spp. can infect a wide range of animal species, which serve as maintenance hosts, but rodents are the most important reservoir of Leptospira spp. Humans typically become infected via contact with urine-contaminated soil or water or, less frequently, by direct contact with the tissues or urine of animals[2]. Leptospira spp. have been subdivided and classified into more than 260 serovars that are grouped into serogroups based on shared antigens [1]. Molecular studies have helped to determine a classification system based on genetic similarities which is currently used in conjunction with antigenic classification [3]. This molecular taxonomy system classifies Leptospira into 21 genomospecies [4,5]: nine pathogenic species, five intermediate species, and seven saprophytic species [3]. In regions of endemicity, the identification of the infecting strain can help to determine the infectious animal source, and thus aid in the implementation of control measures [2]. Various molecular techniques have been described for studying the molecular epidemiology of Leptospira, including pulsed-field gel electrophoresis (PFGE) [6], restriction enzyme analysis (REA) [7], ribotyping [8], randomly amplified polymorphic DNA (RAPD) [9], restriction fragment length polymorphism (RFLP) [10], fluorescent amplified fragment length polymorphism (FAFLP) [11], 16S rRNA sequencing [12] and several PCR-based approaches. Unfortunately, all of these methods suffer from significant drawbacks [13]. In recent years, two genotyping methods, multiple loci variable number of tandem repeats analysis (MLVA) [13] and multilocus sequence typing (MLST) [14], have emerged as noteworthy PCR-based genotyping techniques, but one major limitation of these methods is their reliance on bacterial cultures to generate a significant amount of high-pure DNA [14,15]. Moreover, MLVA requires the determination of the PCR product size, which requires agarose gel electrophoresis, a time-consuming technique with a poor resolution of amplicon size. MLST requires high-throughput sequencing and bioinformatics, but provides a portable, reproducible, and scalable typing system[14,16].
High-resolution melting (HRM) analysis [17], which measures the melting temperatures of amplicons in real time using a fluorescent DNA-binding dye, is a closed-tube, non-sequencing-based system for genotyping and mutation scanning[17,18]. This method has emerged as a valuable tool for the rapid testing of diverse biological specimens and tissues for the presence of micro-organisms and for the differentiation of Brucella spp. genetic variants [19], for example, Chlamydiaceae [20], Mycoplasma pneumoniae [21], Leishmania [22], Bordetella pertussis [23], Staphylococcus aureus [24], Bacillus anthracis [25], Mycoplasma synoviae[26], Pseudomonas aeruginosa [27] adenovirus serotypes [28], and Aspergillus species [29]. HRM has even been used for the identification of members of the Anopheles funestus group [30]. Tulsiani et al.[31] developed a random amplification of polymorphic DNA (RAPD) method associated with HRM analysis (RAPD-HRM) using 13 previously published RAPD primers to genotype 10 Leptospira strains. Traditional HRM curves are difficult to interpret, thus, the interpretation of HRM results can be arbitrary. In this study, we evaluated the potential of an alternative method based on unsupervised high-resolution melting curve (HRM) analysis using ScreenClust HRM (Qiagen, Courtabeuf, France) and examined the ability of this new method to type Leptospira from human Reunion Island specimens rapidly and easily.
Materials and Methods
Leptospira reference strains and human samples
Forty-nine Leptospira reference strains (Table 1) and seven in vitro cultured strains obtained from human patients from Mayotte [32] (L. interrogans sg. Pyrogenes, L. borgpetersenii sg. Mini, L. borgpetersenii sg. Pomona, L. kirchneri sg. Mini/Hebdomadis, L. kirchneri sg. Grippotyphosa, L. mayottensis sg. Pyrogenes/Ballum [33]) were provided by the National Reference Center for Leptospirosis, Institut Pasteur (Paris, France).
Table 1. Determination of species and genotypes by real-time PCR with different sets of primers.
| Species | Serogroup | Serovar | Strain | Tm LFB1F/R | Tm G1/G2 | Cluster VNTR-4bis | ClusterVNTR-Lb4 | Cluster VNTR Lb5 | Profile | Genotype |
|---|---|---|---|---|---|---|---|---|---|---|
| L. interrogans * | Hebdomadis | Hebdomadis | Hebdomadis | 80.68 | 78.62 | 2 | na | 5 | 2–5 | 1 |
| L. interrogans * | Icterohaemorrhagiae | Copenhageni | Wijinberg | 80.35 | 78.70 | 5 | na | 1 | 5–1 | 2 |
| L. interrogans * | Australis | Australis | Ballico | 80.87 | 78.05 | 1 | na | 7 | 1–7 | 3 |
| L. interrogans * | Autumnalis | Autumnalis | Akiyami A | 80.57 | 77.95 | 10 | na | 3 | 10–3 | 4 |
| L. interrogans * | Canicola | Canicola | Hond Utrech IV | 80.73 | 78.5 | 1 | na | 4 | 1–4 | 5 |
| L. interrogans * | Pyrogenes | Pyrogenes | Salinem | 80.82 | 78.72 | 4 | 1 | 2 | 4–2 | 6 |
| L. interrogans * | Pomona | Pomona | Pomona | 80.90 | 78.45 | 7 | na | 3 | 7–3 | 7 |
| L. interrogans * | Icterohaemorrhagiae | Icterohaemorrhagiae | Verdun | 80.48 | 78.88 | 5 | na | 1 | 5–1 | 2 |
| L. interrogans | Grippotyphosa | Grippotyphosa | Andaman | 80.75 | 78.32 | 15 | na | 3 | 15–3 | 8 |
| L. interrogans | Canicola | Kuwait | 136/2/2 | 80.77 | 78.50 | 16 | na | 4 | 16–4 | 9 |
| L. interrogans | Canicola | Schueffneri | Vleermuis 90 C | 80.83 | 78.53 | 2 | na | 2 | 2–2 | 10 |
| L. interrogans | Pyrogenes | Biggis | Biggs | 81.1 | 78.48 | 11 | na | 2 | 11–2 | 11 |
| L. interrogans | Sejroe | Haemolytica | Marsh | 80.82 | 78.85 | 3 | na | 2 | 3–2 | 12 |
| L. interrogans | Pyrogenes | Guaratuba | An 7705 | 81.00 | 78.78 | 7 | na | 3 | 7–3 | 7 |
| L. interrogans | Canicola | Broomi | Patane | 80.82 | 78.73 | 4 | na | 7 | 4–7 | 13 |
| L. interrogans | Australis | Fugis | Fudge | 80.85 | 78.22 | 7 | na | 6 | 7–6 | 14 |
| L. interrogans | Canicola | Sumneri | Sumner | 80.77 | 78.88 | 12 | na | 1 | 12–1 | 15 |
| L. interrogans | Pomona | Kennewicki | LT 10–26 | 80.98 | 78.85 | 13 | na | 1 | 14–1 | 16 |
| L. interrogans | Icterohaemorrhagiae | Birkini | Birkin | 80.87 | 78.15 | 8 | na | 1 | 8–1 | 17 |
| L. interrogans | Canicola | Jonsis | Jones | 80.90 | 78.77 | 2 | na | 1 | 2–1 | 18 |
| L. interrogans | Sejroe | Ricardi | Richardson | 80.68 | 78.48 | 14 | na | 2 | 14–2 | 19 |
| L. interrogans | Djasiman | Djasiman | Djasiman | 80.42 | 78.15 | 9 | na | 2 | 9–2 | 20 |
| L. interrogans | Djasiman | Gurungi | Gurung | 80.48 | 78.58 | 17 | na | 3 | 17–3 | 21 |
| L. interrogans | Sejroe | Hardjo | Hardjoprajitno | 80.80 | 78.80 | 1 | na | 7 | 1–7 | 3 |
| L. interrogans | Icterohaemorrhagiae | Lai | Lai | 80.85 | 78.82 | 6 | 2 | 2 | 6–2 | 22 |
| L. interrogans | Australis | Bratislava | Jez-Bratislava | 80.78 | 78.33 | 1 | na | 7 | 1–7 | 3 |
| L. interrogans | Icterohaemorrhagiae | Copenhageni | Fiocruz L1-130 | 80.45 | 78.77 | 5 | na | 1 | 5–1 | 2 |
| L. interrogans (Average Tm ± S.D.) | 80.75 (± 0.19) | 78.55 (± 0.27) | ||||||||
| L. borgpetersenii * | Ballum | castellonis | Castellonis 3 | 83.00 | 80.65 | na | 1 | 2 | 1–2 | 1 |
| L. borgpetersenii * | Sejroe | sejroe | M84 | 83.01 | 80.75 | na | 1 | 1 | 1–1 | 2 |
| L. borgpetersenii * | Tarassovi | tarassovi | perepelicin | 82.95 | 80.90 | na | 2 | 3 | 2–3 | 3 |
| L. borgpetersenii * | Sejroe | hardjobovis | sponselee | 82.73 | 80.87 | na | 3 | 3 | 3–3 | 4 |
| L. borgpetersenii | Hebdomadis | Jules | Jules | 83.35 | 81.25 | na | 1 | 2 | 1–2 | 1 |
| L. borgpetersenii | Javanica | Ceylonica | Piyasena | 83.25 | 81.00 | na | 2 | 1 | 2–1 | 5 |
| L. borgpetersenii | Mini | Mini | Sari | 83.30 | 81.03 | na | 3 | 4 | 3–4 | 6 |
| L. borgpetersenii | Hebdomadis | Nona | Nona | 83.40 | 81.20 | na | 2 | 1 | 2–1 | 5 |
| L. borgpetersenii | Hebdomadis | Worsfoldi | Worsfold | 82.50 | 81.25 | na | 4 | 5 | 4–5 | 7 |
| L. borgpetersenii | Pyrogenes | Hamptoni | Hampton | 82.63 | 80.75 | na | 5 | 6 | 5–6 | 8 |
| L. borgpetersenii (Average Tm ± S.D.) | 83.00 (± 0.31) | 81.10 (± 0.31) | ||||||||
| L. kirschneri * | Grippotyphosa | Grippotyphosa | Moskva V | 81.42 | na | 5 | na | 3 | 5–3 | 1 |
| L. kirschneri * | cynopteri | Cynopteri | 3522C | 81.63 | na | 4 | na | 3 | 4–3 | 2 |
| L. kirschneri * | Mini | Hebdomadis | 200801925 | 81.65 | na | 5 | na | 2 | 5–2 | 3 |
| L. kirschneri | Icterohaemorrhagiae | Bogvere | LT 60–69 | 81.90 | na | 1 | na | 1 | 1–1 | 4 |
| L. kirschneri | Icterohaemorrhagiae | Ndambari | Ndambari | 82.08 | na | 1 | na | 2 | 1–2 | 5 |
| L. kirschneri | Bataviae | Djatzi | HS 26 | 81.90 | na | 1 | na | 2 | 1–2 | 4 |
| L. kirschneri | Icterohaemorrhagiae | Mwogolo | Mwogolo | 81.90 | na | 1 | na | 1 | 1–1 | 6 |
| L. kirschneri | Grippotyphosa | Ratnapura | Wumalasena | 82.40 | na | 3 | na | 2 | 3–2 | 4 |
| L. kirschneri | Icterohaemorrhagiae | Ndahambukuje | Ndahambukuje | 81.92 | na | 1 | na | 1 | 1–1 | 6 |
| L. kirschneri | Canicola | Galtoni | LT 1014 | 82.35 | na | 3 | na | 2 | 3–2 | 7 |
| L. kirschneri | Pomona | Tsaratsovo | B 81/7 | 81.90 | na | 2 | na | 1 | 2–1 | |
| L. kirschneri (Average Tm ± S.D.) | 81.90 (± 0.28) | na | ||||||||
| L. noguchii * | Panama | Panama | CZ214K | 81.48 | 79.20 |
*Sixteen reference strains were used to develop the typing method.
Forty-two Leptospira DNA samples were extracted from the sera of leptospirosis-positive patients who were diagnosed between January 2008 and March 2012 at the Groupe Hospitalier Sud Réunion (Saint-Pierre, Reunion Island, France). The PCR-based microbiological diagnoses used the primers G1/G2 [34] before 2010 and lfb1 F/R [35] after 2010. The samples were stored at -80°C.
The DNA extractions from the in vitro cultured strains and sera were performed using the NucliSens easyMAG system (BioMérieux, Marcy l’Etoile, France). The concentration of the Leptospira DNA was normalized using the quantification analysis module of a RotorGene 6000 cycler (Qiagen, Courtabeuf, France). To normalize the input template concentration for the strains, the Ct (threshold cycle) was set to be between 27 and 30 cycles.
PCR amplification and analysis
We tested seven primer pairs specific to Leptospira on 16 Leptospira reference strains: secY IV F/R [36], lgB F/R [37], A/B [38], lfb1 F/R [35], G1/G2 [34], lpL32 F/R [39], and lpL41 F/R [14], with the aim of selecting specific primers for Leptospira species identification. In all the PCRs, L. biflexa sv. Patoc was used as a negative control.
The sensitivity of each PCR using the selected primer(s) was evaluated by performing PCRs on 10-fold serial dilutions of the DNA extracted from three in vitro cultured strains from Mayotte: L. interrogans sg. Pyrogenes, L. borgpetersenii sg. Mini, and L. kirchneri sg. Mini/Hebdomadis. The quantification of the leptospires was based on genomic DNA mass, taking into consideration that the size of the genome of the L. interrogans strain Fiocruz L1-130 is 4.6 Mb (1 genome is approximately 5 fg). The end-point detection limit was determined by 10 repetitions of the measurement of the last positive point with 100% amplification.
We also cloned a plasmid DNA carrying the G1/G2 gene of L. interrogans sv. Copenhageni strain Wijinberg into the pGEM-T Easy Vector (Promega, USA).
A subspecies determination was performed on the matching reference strains (with the exception of L. noguchii), and eleven primer pairs amplifying polymorphic tandem repeat sequences were tested (VNTR 4, VNTR 7, VNTR 10, VNTR 19, VNTR 23, VNTR 31, VNTR 4bis, VNTR 7bis, VNTR 10bis, VNTR Lb4, and VNTR Lb5) [13,40,]. These VNTR primers were used in a previously published MLVA study and exhibited a noteworthy discriminatory power for the identification of the serovars of L. interrogans, L. borgpetersenii, and L. kirschneri [40].
The reproducibility of our method (which refers to the variation in our results between runs) was evaluated by ten repetitions of PCRs with the selected primer(s) for three strains (L. kirschneri sv. Grippotyphosa str. Moskva V, L. borgpetersenii sv. Hardjobovis str. Sponselee, and L. interrogans sv. Icterohaemorrhagiae str. Verdun). For each strain, if the species identification was identical in the ten repetitions, then the reproducibility was determined to be 100%.
The PCRs were performed using a Type-IT HRM PCR Qiagen Kit (Qiagen, Courtabeuf, France) on a RotorGene 6000 system (Qiagen, Courtaboeuf, France). The 20 μl reactions contained 10 μl of mix 2X master mix HRM, with a 0.7 μM final concentration of each primer (TibMolBiol, Berlin, Germany), and 5 μl of the extracted nucleic acid solution. The following amplification protocol was used: denaturation at 95°C for 5 min, 45 cycles at 95°C for 10 s, 55°C for 30 s, and 72°C for 10 s. These conditions were used for all the primer pairs. For the species determination, a melting curve analysis determined the melting temperature (Tm). To test the reproducibility of the Tm determination, three strains of different species were tested in 10 separate runs with the selected primers. For the subspecies determination, after PCR cycling with the VNTR primers, the samples were heated from 65°C to 95°C with continuous acquisition. For each VNTR locus, a normalization region of the melting curve was selected to improve the analysis per the recommendations of the Rotor-Gene ScreenClust HRM Software User Guide (Qiagen, Courtabeuf, France). Two normalization regions were selected: one before and one after the melting curve transition. The highest fluorescence value was 100 and the lowest fluorescence value was zero. The data were analyzed using ScreenClust HRM (Qiagen, Courtabeuf, France) in unsupervised mode (i.e., there were no known controls for each cluster and the number of clusters was unknown). The unsupervised mode was also employed to analyze Leptospira DNA fluorescence data obtained with human samples, and the results from the control strains were employed as pseudo-unknowns. The data were analyzed by ScreenClust using the principal component analysis statistical method [41], which enabled the maximum separation of genotypes.
A cluster plot was built in three dimensions (principal components) and ellipses representing each cluster were drawn. The probability of each sample fitting into a specific cluster was calculated and required to be at least 0.7. The typicality, which measures how well a sample falls within the cluster to which it has been assigned, was also given for each sample and was required to be at least 0.05.
The genotype [42] of each Leptospira DNA was determined by combining the results of the two clusters that were obtained with the two VNTR primers selected by the species in the subspecies characterization.
To evaluate the ability of the tested primers to correctly identify the Leptospira species, a Mann-Whitney test was used to determine whether the difference in the mean Tm was significant between species.
The typeability of each set of primers for each species of Leptospira (i.e., the proportion of strains that were assigned a type by the typing system) was determined by using the following formula: T = Nt/N, where Nt is the number of strains that were assigned a type and N is the number of strains that were tested [5].
To evaluate the discriminatory power of the VNTR primers, the Simpson diversity index (DI) was used as described by Hunter and Gaston[43].
1). The polymorphism is considered to be high when the Simpson diversity index is greater than 95%[42,44].
Results
Determination of species
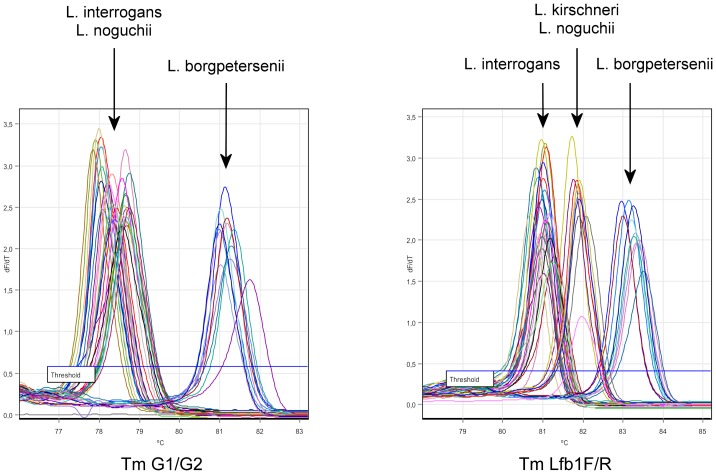
Our results are shown in Table 1 and Figs 1, 2 and 3. In all the runs, the negative control, a DNA extract from an in vitro culture of L. biflexa sv. Patoc, did not show amplification with lfb1F/R nor G1/G2. Seven primers were tested on 16 strains to characterize each species, including L. interrogans, L. borgpetersenii, L kirschneri, and L. noguchii. Only the association of the primer pairs lfb1F/R and G1/G2 resulted in a species-specific Tm (except L kirschneri, which had no amplification with G1/G2) and allowed for the discrimination of the four species (p<0.001); thus, these two primer pairs were selected for further analysis.
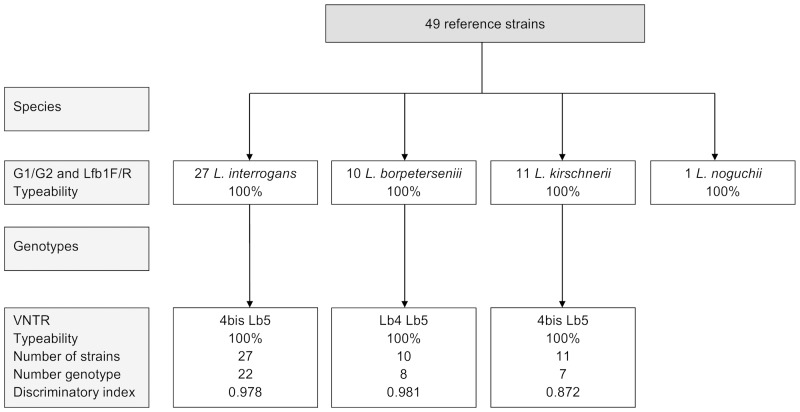
Fig 1. Determination of the Leptospira species and genotypes with reference strains.
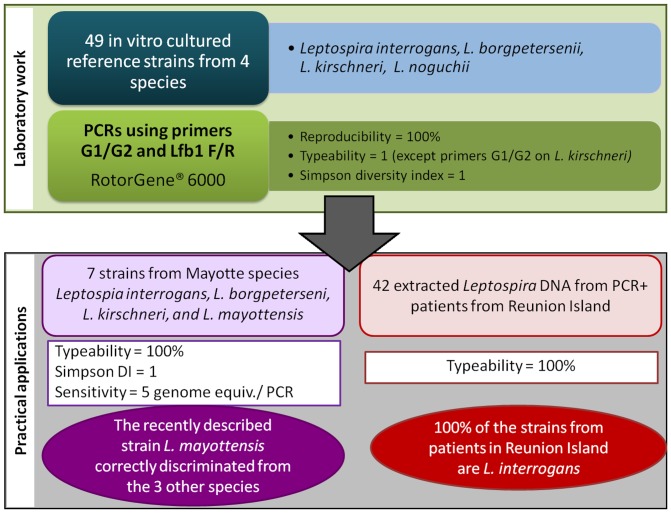
Fig 2. The materials and methods implemented in this study for the genotyping of Leptospira at the species level, and practical applications.
Fig 3. Melting curve analysis of pathogenic Leptospira strains after real-time PCR amplification using the G1/G2 and LFb1F/R primer sets.
The reproducibility (evaluated on ten runs) was 100% for the three tested strains using the two primer pairs lfb1F/R and G1/G2.
The coefficient of variation of the Tm was 0.19% for strain L. kirschneri sv. Grippotyphosa str. Moskva V, 0.4% for L. borgpetersenii sv. Hardjobovis str. Sponselee and 0.22% for L. interrogans sv. Icterohaemorrhagiae str. Verdun with lfb1 F/R and 0.5% for L. borgpetersenii sv. Hardjobovis str. Sponselee and 0.45% for L. Interrogans sv. Icterohaemorrhagiae str. Verdun with G1/G2.
Among the seven strains from Mayotte, those from the species L. interrogans, L. borgpetersenii, and L. kirschneri were well discriminated by the primer pairs lfb1F/R and G1/G2. L. mayottensis was not amplified by G1/G2, similar to L. kirschneri, but the Tm resulting from the lfb1 F/R amplification was significantly different for L. mayottensis (Tm 83.76±0.3) and L. kirschneri (81.90 ± 0.28).
The sensitivity was evaluated for the two selected pairs of primers, lfb1F/R and G1/G2, using three strains. For each PCR assay (with the exception of G1/G2, which did not amplify the L. kirschneri strain) the two sets of primers showed that 100% positive amplification was obtained for an average of 5 genome equivalents per PCR (Ct ≈ 37 cycles).
Because one leptospire contains an average of five genome equivalents [49], our analytical sensitivity was calculated to be 1 bacterium/μl. This result was confirmed by using the plasmid DNA of the G1/G2 gene of L. interrogans sv. Copenhageni strain Wijinberg because we had obtained a limit of detection of 25 copies per PCR for L. interrogans sg. Pyrogenes.
The limits of detection on plasma were assessed using DNA that was extracted, diluted from identical strains and spiked. The limit of detection on the spiked plasma was estimated to be 5 genome equivalents per PCR, which was similar to that of the two sets of primers on the different strains and the extracted unspiked DNA.
Genotype determination of Leptospira reference strains using ScreenClust HRM Software
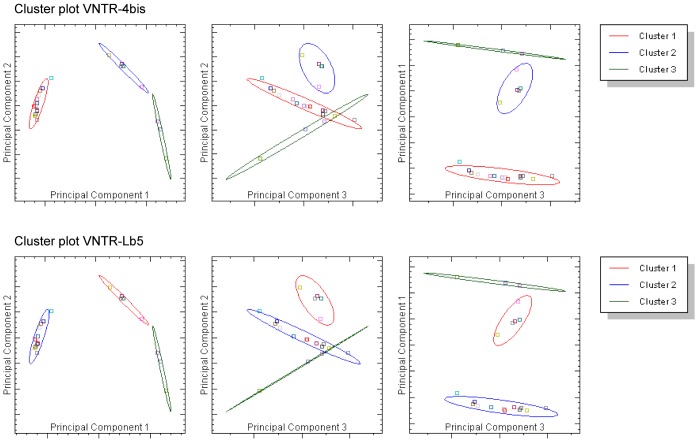
Of the 11 primer sets tested, three VNTR primer sets were considered to be noteworthy for discriminating the strains and were considered further: VNTR-Lb4, VNTR-4 bis and VNTR-Lb5 (Table 1, Figs 1, 4 and 5). Two VNTR primer pairs per species were used to genotype Leptospira at the subspecies level. Using these three VNTR primers, the probability of a strain belonging to a specific cluster was ≥ 0.95, and the typicality was ≥ 0.05 for all the strains.
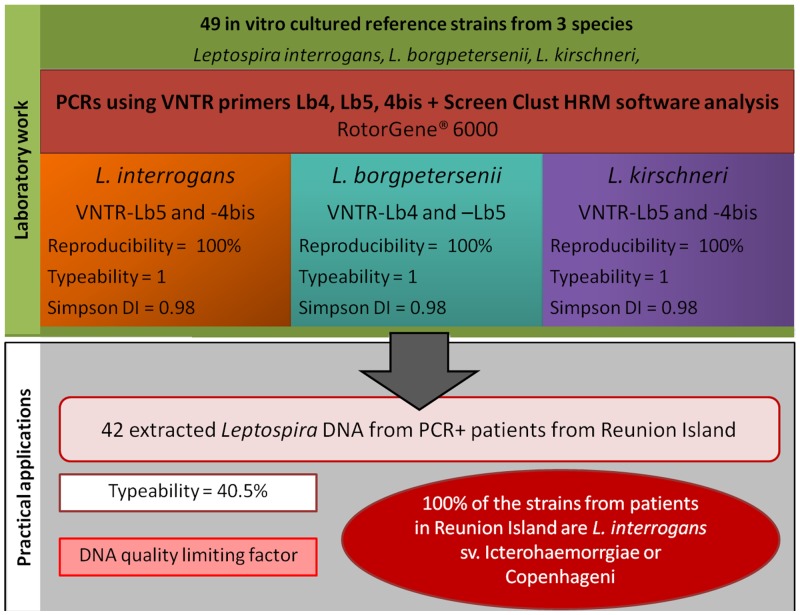
Fig 4. The materials and methods implemented in this study for the genotyping of Leptospira at the subspecies level, and practical applications.
Fig 5. Cluster plot obtained after the HRM analysis using the VNTR-4bis and VNTR-Lb5.
L. interrogans
Using VNTR-Lb4, the typeability value obtained for the 27 L. interrogans strains tested was low (0.074); consequently, VNTR-Lb4 was not employed further. The typeability values for VNTR-4bis and -Lb5 were both 1; thus, these two VNTRs were selected for further study, and their normalization temperatures were 83.7–89.3°C and 84.5–88.4°C, respectively. The reproducibility of the PCR runs with the two primers VNTR-4bis and -Lb5 was 100%, which was tested on L. interrogans sv. Icterohaemorrhagiae str. Verdun.
The HRM analysis of the amplicons obtained with primers VNTR-4bis and -Lb5 was highly discriminating, with 17 and seven clusters generated, respectively, for the 27 different L. interrogans strains tested. By combining the results using VNTR-4bis and -Lb5, we found 22 genotypes in the 27 strains tested, with 19 strains having a unique genotype (DI = 0.98) (Table 1). However, some strains could not be discriminated: cluster 2 included three strains belonging to the serogroup Icterohaemorrhagiae (sv. Copenhageni str. Wijinberg, sv. Icterohaemorrhagiae str. Verdun, and sv. Copenhageni str. Fiocruz L1-130) and cluster 3 included two serovars from the serogroup Australis (sv. Australis str. Ballico and sv. Bratislava str. Jez-Bratislava) and sv. Sejroe. Moreover, cluster 7 included sv. Pomona str. Pomona and sv. Guaratuba str. An 7705.
L. borgpetersenii
UsingVNTR-4bis, the typeability value obtained for the 10 L. borgpetersenii strains tested was low (0.1); consequently, VNTR-4bis was not employed further. The typeability for the 10 L. borgpetersenii strains with VNTR-Lb4 and -Lb5 was 1; thus these two VNTRs were selected for further study, and their normalization temperatures were 79.8–82.9°C and 84.3–86°C, respectively.
The PCR reproducibility with the two primers VNTR-Lb4 and -Lb5 was 100%, which was tested on L. borgpetersenii sv. Hardjobovis str. Sponselee.
The HRM analysis of the amplicons obtained with primerVNTR-Lb4 generated five clusters for the ten L. borgpetersenii strains, while VNTR-Lb5 discriminated six different clusters. Thus, in the analysis of the L. borgpetersenii strains, the combination of Lb4 and -Lb5 generated eight out of 10 genotypes, with six strains having a unique genotype (DI = 0.98) (Table 1). The method could not discriminate sv. Ceylonica str. Piyasena from sg. Javanica, and sv. Nona str. Nona from sg. Hebdomadis, which were grouped into cluster 5, nor sv. Castellonis str. Castellonis 3 sg. Ballum from sv. Jules str. Jules sg. Hebdomadis, which were grouped into cluster 1.
L. kirschneri
Using VNTR-Lb4, the typeability value obtained for the 11 L. kirschneri strains tested was 0; consequently, VNTR-Lb4 was not employed further. The typeability using VNTR-4bis and -Lb5 was 1; thus, these two VNTRs were selected for further study, and their normalization temperatures were 81.3–83.3°C and 83.7–86.7°C, respectively.
The PCR reproducibility using the primers VNTR-4bis and -Lb5 was 100%, which was tested on L. kirschneri sv. Grippotyphosa str. Moskva V.
The HRM analysis of the amplicons obtained with the primer VNTR-4bis enabled the discrimination of five clusters for the 11 L. kirschneri strains, whereas VNTR-Lb5 discriminated three clusters within this species. A combination of these two VNTRs generated seven genotypes in the 11 L. kirschneri strains, and five strains had a unique genotype (DI = 0.87) (Table 1). Cluster 4 included three serovars of the serogroup Icterohaemorrhagiae (sv. Bogvere str. LT 60–69, sv. Mwogolo str. Mwogolo and sv. Ndahambukuje str. Ndahambukuje). Moreover, sv. Ndambari str. Ndambari from sg. Icterohaemorrhagiae and sv. Djatzi str. HS 26 from sg. Bataviae were not discriminated using our method (cluster 5), and similarly, sv. Ratnapura str. Wumalasena from sg. Grippotyphosa and sv. Galtoni str. LT 1014 from sg. Canicola, were included together in cluster 6.
Our method generated 37 genotypes in 48 reference strains. Thirty strains had a unique genotype while seven clusters contained two to three strains. Thus, the global DI of our method was 0.98.
Application of ScreenClust software to biological samples (Table 2, Figs 2 and 3)
Table 2. Clusters of species and DNA extracted from sera with the VNTR 4bis and Lb5 primers shown in Fig 5.
| Species | Serogroup | Serovar | Strains or DNA extracted from sera | Cluster VNTR-4bis | ClusterVNTR-Lb5 | Genotype |
|---|---|---|---|---|---|---|
| L. interrogans | Icterohaemorrhagiae | Icterohaemorrhagiae | Verdun | 1 | 2 | 1 |
| L. interrogans | Icterohaemorrhagiae | Birkini | Birkin | 3 | 3 | 2 |
| L. interrogans | Australis | Fugis | Fudge | 3 | 3 | 2 |
| L. interrogans | Autumnalis | Autumnalis | Akiyami A | 3 | 3 | 2 |
| L. interrogans | Pyrogenes | Biggis | Biggis | 2 | 1 | 3 |
| L. interrogans | Canicola | Kuwait | 136/2/2 | 2 | 1 | 3 |
| L. interrogans | Canicola | Canicola | Hond Utrech | 2 | 1 | 3 |
| L. interrogans | Grippotyphosa | Grippotyphosa | Andaman | 2 | 1 | 3 |
| L. interrogans | Sejroe | Ricardi | Richardson | 2 | 1 | 3 |
| NA | NA | NA | DNA of Leptospiraextracted from sera 1–13 | 1 | 2 | 1 |
The 42 Leptospira DNAs that were extracted from patient serum samples showed a Tm corresponding to that of the species L. interrogans using the primer pairs lfb1F/R and G1/G2.
Using VNTR-4 bis and VNTR-Lb5, the genotypes of 17 out of 42 (40.5%) DNA samples were identified. These 17 Leptospira DNA samples exhibited the same genotype, which was consistent with the strains L. interrogans sv. Copenhageni or Icterohaemorrhagiae.
Using VNTR-4 bis and VNTR-Lb5, the genotype determination was 100% when the Ct was ≤ 30 cycles (8250 copies/ml, data not shown) and 28.5% (10/35) when the Ct was >30. We then evaluated the integrity of three genotypable DNAs and three non-genotypable DNAs by performing a standard PCR targeting the secY gene. Agarose gel electrophoresis revealed multiple bands of different sizes for the non-genotypable DNAs resulting from nonspecific amplification, and a 202-bp band for the three genotypable DNAs. To understand how the DNA lost its integrity, we subjected three good quality DNAs to several freezing-defrosting processes. After each freezing-defrosting process, the migration of the amplified fragments showed progressive DNA degradation.
Discussion
The main aim of this study was not to diagnose pathogenic leptospirosis infection but to describe a new genotyping method to identify Leptospira species and subspecies. For a better understanding of the epidemiology of leptospirosis in a region, information about the prevalent circulating serovars or genotypes is essential.
The first objective of the present study was to provide a rapid technique to discriminate between species of pathogenic Leptospira (only the four most frequent pathogenic leptospires were considered [45]), which is a necessary step for further levels of genotyping, i.e., at the serovar level. Using two sets of primers (G1/G2 and lfb1 R/F), the melting temperature profiles of the strains are able to accurately discriminate L. interrogans, L. kirschneri, L: borgpetersenii, L. noguchii, and the newly described L. mayottensis [33] with a specificity and reproducibility of 100% with less than 0.5% Tm coefficients of variation. Our method enabled us to accurately determine the infectious Leptospira species from DNA extracted from the blood of sick patients at Reunion Island. Previous methods for the identification of Leptospira species, such as PCR amplification followed by electrophoresis on a non-denaturing polyacrylamide gel [46] or the sequencing of partial 16SrDNAs [47], are time-consuming. In contrast, this method allows for species discrimination in a short time period. Recently described PCR methods for the diagnosis of acute infection [48] may have a lower end-point detection than our PCRs, consequently, a PCR-positive sample that has been identified with these highly sensitive methods might be unsuitable for characterization by melting temperature analysis. In a previous study, Merien et al. [35] used a similar melting curve analysis with the primer pairs lfb1 F/R, which facilitated the differentiation of the pathogenic species L. interrogans, L. borgpetersenii, and L. kirschneri, but not L. noguchii and L. kirschneri. In our study, the use of a second set of primers provided improved specificity. In a recent study, Feirrera et al [49] developed sets of species-specific probes with primers designed from ompL1 and secY with 100% analytical specificity for the species L. interrogans, L. kirschneri, L. borgpetersenii, and L. noguchii. These probes were specifically designed for these four species and do not allow for the detection and identification of the new species L. mayottensis.
Tulsiani et al. [31] applied a high-resolution melting curve analysis of random-amplified-polymorphic DNA to reference collection strains. MLVA has been previously proposed for typing the L. interrogans, L. borgpetersenii, and L. kirschneri strains [40,13]. In our study, the HRM analysis of the VNTR sequences was performed using ScreenClust HRM software. To the best of our knowledge, our study signifies the first time that Leptospira strains have been genotyped using VNTR primers. Using our method, we obtained a high Hunter-Gaston's discriminatory index (0.98) compared to a serological classification of all the reference strains tested. This high index (> 0.95) shows the genetic polymorphism within each tested species and validates the high sensitivity and specificity of auto-calling genotypes from HRM data using ScreenClust software.
The discriminatory index was relatively lower for L. kirschneri (0.87), which is potentially due to the decreased polymorphism of this species. Using MLVA, Salaün et al.[40] showed that the genetic diversity of L. kirschneri serovars was lower than L. interrogans serovars.
Using the ‘unsupervised analysis’ mode, HRM analysis using ScreenClust HRM software permitted the determination of clusters without genotypic knowledge. HRM analysis using ScreenClust HRM software also enabled the automated genotyping of unknown samples.
Although the performance measurements of our typing method [41] were excellent (reproducibility 100%, typeability 100%, discriminatory index 0.98), not all the strains (37/48, 77.1%) could be discriminated at the serovar level. The identification of Leptospira serovars by serological methods relies on surface-exposed lipopolysaccharides (LPS) and there is a poor correlation between serological and molecular typing methods that are not LPS-based. Like MLST, PFGE, and MLVA, our HRM analysis, which is based on DNA sequence analysis, did not always correlate with the serological characterization. The MLST, PFGE, and MLVA, and HRM methods have the ability to discriminate Leptospira strains at the subspecies level. However, depending on the technique used, distinct levels of discrimination can be obtained. In general, PFGE discrimination is superior to that of MLST, MLVA, and HRM analysis. For example, serovars Pomona and Guaratuba, which do not belong to the same serogroup, share the same ST [40,50] and HRM profiles but are distinguishable by PFGE [51]. For L. kirschneri, the genotype determined by HRM for serovars Bogvere and Djatzi was identical, but they had different profiles using MLVA and PFGE. MLST, PFGE, MLVA, and HRM cannot distinguish between strains belonging to the serovars Copenhageni and Icterohaemorrhagiae. Currently, no single molecular typing method is ideal and fulfils all the characteristics required to unambiguously determine if the isolates from numerous patients and/or animals are identical. However, HRM curve analysis is a simple and effective technique that does not require culture isolation and can be performed in a single test tube in less than two hours.
Our molecular investigation of the Leptospira DNA extracted from blood samples revealed that the Leptospira species responsible for 100% of the clinical cases in humans on Reunion Island was L. interrogans. In contrast, on the sister island of Mayotte, only 8.5% of the isolated strains belong to L. interrogans, and no cases were attributed to the serogroup Icterohaemorrhagiae [52]. In our study, the Reunion Island patient infecting strains were all clustered by ScreenClust HRM software using the VNTR-4bis and -Lb5 primers into the L. interrogans strain serogroups Icterohaemorrhagiae serovar Copenhageni or Icterohaemorrhagiae. We can therefore conclude that only one genotype has been involved in human symptomatic leptospirosis on Reunion Island since 2008. This result reflects the persistence of a serovar over time in a geographical area. These results are also confirmed by our previous studies on human patients. Using a serological method, the strains we isolated in culture in 1998 and 2012 were identified as belonging to the L. interrogans serogroup Icterohaemorrhagiae. Between 1998 and 2009, the serogroup Icterohaemorrhagiae was reported to represent 59.3% of the human cases diagnosed on Reunion Island using MAT [53].
Conclusions
Our genotyping method was able to identify a change in genotype that was responsible for the clinical cases on Reunion Island. The unsupervised mode of ScreenClust HRM should also enable one to discover new genotypes that are present in clinical samples. Our genotyping method is limited by the number of leptospires in blood samples, wherein approximately 8250 copies/ml are necessary to obtain 100% typeability.
In this study, the use of HRM confirmed the predominance of a clonal subpopulation of L. interrogans as the cause of human leptospirosis on Reunion Island, suggesting that this method would be useful for epidemiological studies.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Adler B, de la Peña Moctezuma A (2010) Leptospira and leptospirosis. VetMicrobiol 140: 287–296. 10.1016/j.vetmic.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 2. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA et al. (2003) Leptospirosis: a zoonotic disease of global importance. Lancet Infectious Dis 3: 757–771. [DOI] [PubMed] [Google Scholar]
- 3. Ko AI, Goarant C, Picardeau M (2009) Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7: 736–747. 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Picardeau M (2013) Diagnosis and epidemiology of leptospirosis. Med Mal Infect 43: 1–9. 10.1016/j.medmal.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 5. Brenner DJ, Kaufmann AF, Sulzer KR, Steigerwalt AG, Rogers FC, Weyant RSl (1999) Further determination of DNA relatedness between serogroups and serovars in the family Leptospiraceae with a proposal for Leptospira alexanderi sp. nov. and four new Leptospira genomospecies. Int J Syst Microbiol 49: 839–858. [DOI] [PubMed] [Google Scholar]
- 6. Taylor KA, Barbour AG, Thomas DD (1991) Pulsed-field gel elctrophoretic analysis of leptospiral DNA. Infect Immun 59: 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shukla J, Tuteja U, Batra HV (2002) Use of restriction endonucleases for differentiation of pathogenic & saprophytic leptospires. Indian J Med Res 116: 192–200. [PubMed] [Google Scholar]
- 8. Kositanont U, Chotinantakul K, Phulsuksombati D, Tribuddharat C (2007) Assessment of Southern blot ribotyping for differentiation of Leptospira strains isolated from field rats. J Microbiol Methods 69: 288–297. [DOI] [PubMed] [Google Scholar]
- 9. Corney BG, Colley J, Djordjevic SP, Whittington R, Graham GC (1993) Rapid identification of some Leptospira isolates from cattle by random amplified polymorphic DNA fingerprinting. J Clin Microbiol 31: 2927–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zuerner RL, Ellis WA, Bolin CA, Montgomery JM (1993) Restriction fragment length polymorphisms distinguish Leptospira borgpetersenii serovar hardjo type hardjo-bovis isolates from different geographical locations. J Clin Microbiol 31: 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vijayachari P, Ahmed A, Sugunan AP, Ghousunnissa S, Rao KR, Hasnain SE et al. (2004) Use of fluorescent amplified fragment length polymorphism for molecular epidemiology of leptospirosis in India. J Clin Microbiol 42: 3575–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morey RE, Galloway RL, Bragg SL, Steigerwalt AG, Mayer LW, et al. (2006) Species-specific identification of Leptospiraceae by 16S rRNA gene sequencing. J ClinMicrobiol 44: 3510–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Majed Z, Bellenger E, Postic D, Pourcel C, Baranton G, Picardeau M (2005) Identification of variable-number tandem-repeat loci in Leptospira interrogans sensu stricto. J Clin Microbiol 43: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmed N, Devi SM, de los A Valverde M, Vijayachari P, Machang'u R, Ellis WA et al. (2006) Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann Clin Microbiol Antimicrob 5: 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caimi K, Varni V, Melendez Y, Koval A, Brihuega B, Ruybal P (2012) A combined approach of VNTR and MLST analysis: improving molecular typing of Argentinean isolates of Leptospira interrogans. Mem Inst Oswaldo Cruz 107: 644–651. [DOI] [PubMed] [Google Scholar]
- 16. Urwin R, Maiden MCJ (2003) Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol 11: 479–487. [DOI] [PubMed] [Google Scholar]
- 17. Gundry CN, Vandersteen JG, Reed GH, Pryor RJ, Chen J, Wittver CTl (2003) Amplicon melting analysis with labeled primers: a closed-tube method for differentiating homozygotes and heterozygotes. Clinl Chem 49: 396–406. [DOI] [PubMed] [Google Scholar]
- 18. Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ (2003) High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem 49: 853–860. [DOI] [PubMed] [Google Scholar]
- 19. Winchell JM, Wolff BJ, Tiller R, Bowen M, Hoffmaster A (2010) Rapid identification and discrimination of Brucella isolates using real-time PCR and high resolution melt analysis. J Clin Microbiol: JCM.02021–02009. 10.1128/JCM.02021-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robertson T, Bibby S, O'Rourke D, Belfiore T, Lambie H, Noormohammadi AH (2009) Characterization of Chlamydiaceae species using PCR and high resolution melt curve analysis of the 16S rRNA gene. J Appl Microbiol 107: 2017–2028. 10.1111/j.1365-2672.2009.04388.x [DOI] [PubMed] [Google Scholar]
- 21. Schwartz SB, Mitchell SL, Thurman KA, Wolff BJ, Winchell JM (2009) Identification of P1 variants of Mycoplasma pneumoniae by use of high-resolution melt analysis. JClin Microbiol 47: 4117–4120. 10.1128/JCM.01696-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talmi-Frank D, Nasereddin A, Schnur LF, Schönian G, Toz SO, Jaffe CL, et al. (2010) Detection and identification of old world Leishmania by high resolution melt analysis. Plos Negl Trop Dis 4: e581 10.1371/journal.pntd.0000581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan WF, Maharjan RP, Reeves PR, Sintchenko V, Gilbert GL, Lau R (2009) Rapid and accurate typing of Bordetella pertussis targeting genes encoding acellular vaccine antigens using real time PCR and High Resolution Melt analysis. J Microbiol Methods 77: 326–329. 10.1016/j.mimet.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 24. Stephens AJ, Inman-Bamber J, Giffard PM, Huygens F (2008) High-Resolution Melting analysis of the spa repeat region of Staphylococcus aureus . Clin Chem 54: 432–436. 10.1373/clinchem.2007.093658 [DOI] [PubMed] [Google Scholar]
- 25. Fortini D, Ciammaruconi A, De Santis R, Fasanella A, Battisti A, D’Amelio R et al. (2007) Optimization of high-resolution melting analysis for low-cost and rapid screening of allelic variants of Bacillus anthracis by multiple-locus variable-number tandem repeat analysis. Clin Chem 53: 1377–1380. [DOI] [PubMed] [Google Scholar]
- 26. Jeffery N, Gasser RB, Steer PA, Noormohammadi AH (2007) Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region. Microbiology 153: 2679–2688. [DOI] [PubMed] [Google Scholar]
- 27. Naze F, Jouen E, Randriamahazo RT, Simac C, Laurent P, Bleriot A, et al. (2010) Pseudomonas aeruginosa outbreak linked to mineral water bottles in a neonatal intensive care unit: fast typing by use of high-resolution melting analysis of a variable-number tandem-repeat locus.J Clin Microbiol 48: 3146–3152. 10.1128/JCM.00402-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steer PA, Kirkpatrick NC, O'Rourke D, Noormohammadi AH (2009) Classification of fowl adenovirus serotypes by use of high-resolution melting-curve analysis of the hexon gene region. J Clin Microbiol 47: 311–321. 10.1128/JCM.01567-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erali M, Pounder JI, Woods GL, Petti CA, Wittwer CT (2006) Multiplex single-color PCR with amplicon melting analysis for identification of Aspergillus species. Clin Chem 52: 1443–1445. [DOI] [PubMed] [Google Scholar]
- 30. Vezenegho SB, Bass C, Puinean M, Williamson MS, Field LM, Coetzee M, et al. (2009) Development of multiplex real-time PCR assays for identification of members of the Anopheles funestus species group. Malar J 8: 282 10.1186/1475-2875-8-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tulsiani SM, Craig SB, Graham GC, Cobbold RC, Dohnt MF, Burns MA, et al. (2010) High-resolution melt-curve analysis of random-amplified-polymorphic-DNA markers, for the characterisation of pathogenic Leptospira. Ann Trop Med Parasitol 104: 151–161. 10.1179/136485910X12607012374037 [DOI] [PubMed] [Google Scholar]
- 32. Desvars A, Michault A, Bourhy P (2013) Leptospirosis in the western Indian Ocean islands: what is known so far? Vet Res 44: 80 10.1186/1297-9716-44-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bourhy P, Collet L, Brisse S, Picardeau M (2014) Leptospira mayottensis sp. nov., a pathogenic species of the genus Leptospira isolated from humans. Int J Syst Evol Microbiol 64: 4061–4067. 10.1099/ijs.0.066597-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gravekamp C, Van de Kemp H, Franzen M, Carrington D, Schoone GJ, Van Eys G, et al. (1993) Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J Gen Microbiol 139: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 35. Merien F, Portnoi D, Bourhy P, Charavay F, Berlioz-Arthaud A, Baranton G (2005) A rapid and quantitative method for the detection of Leptospira species in human leptospirosis. FEMS Microbiol Lett 249: 139–147. [DOI] [PubMed] [Google Scholar]
- 36. Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA (2009) Development and validation of a real-time PCR for detection of pathogenic leptospira species in clinical materials. PLoS ONE 4: e7093 10.1371/journal.pone.0007093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerqueira GM, McBride AJ, Picardeau M, Ribeiro AA, Moreira AN, Morel V et al. (2009) Distribution of the leptospiral immunoglobulin-like (Lig) genes in pathogenic Leptospira spp. and application of ligB to typing leptospiral isolates. J Med Microbiol 58: 1173–1181. 10.1099/jmm.0.009175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mérien F, Amouriaux P, Pérolat P, Baranton G, Saint-Girons I (1992) Polymerase chain reaction for detection of Leptospira spp. in clinical sample. J Clin Microbiol 30: 2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Levett PN, Morey RE, Galloway RL, Turner DE, Steigerwalt AG, Mayer LW (2005) Detection of pathogenic leptospires by real-time quantitative PCR. J Med Microbiol 54: 45–49. [DOI] [PubMed] [Google Scholar]
- 40. Salaun L, Merien F, Gurianova S, Baranton G, Picardeau M (2006) Application of multilocus variable-number tandem-repeat analysis for molecular typing of the agent of leptospirosis. J Clin Microbiol 44: 3954–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reja V, Kwok A, Stone G, Yang L, Missel A, Menzel C, et al. (2010) ScreenClust: Advanced statistical software for supervised and unsupervised high resolution melting (HRM) analysis. Methods 50: S10–14. 10.1016/j.ymeth.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 42. van Belkum A, Tassios PT, Dijkshoorn L, Haeggman S, Cookson B, Fry NK, et al. (2007) Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin Microbiol Inf 13: 1–46. [DOI] [PubMed] [Google Scholar]
- 43. Hunter PR, Gaston MA (1988) Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol 26: 2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Struelens MJ (1996) Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect 2: 2–11. [DOI] [PubMed] [Google Scholar]
- 45. Bourhy P, Bremont S, Zinini F, Giry C, Picardeau M (2011) Comparison of real-time PCR assays for detection of pathogenic Leptospira spp. in blood and identification of variations in target sequences. J Clin Microbiol 49: 2154–2160. 10.1128/JCM.02452-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira MA, Caballero OL, Dias Neto E, Koury MC, Romanha AJ, Carvalho J, et al. (1995) Use of nondenaturing silver-stained polyacrylamide gel analysis of polymerase chain reaction amplification products for the differential diagnosis of Leptospira interrogans infection. Diagn Microbiol Infect Dis 22: 343–348. [DOI] [PubMed] [Google Scholar]
- 47. Postic D, Riquelme-Sertour N, Merien F, Perolat P, Baranton G (2000) Interest of partial 16S rDNA gene sequences to resolve heterogeneities between Leptospira collections: application to L. meyeri. Res Microbiol 151: 333–341. [DOI] [PubMed] [Google Scholar]
- 48. Waggoner JJ, Balassiano I, Abeynayake J, Sahoo MK, Mohamed-Hadley A, Liu Y et al. (2014) Sensitive real-time PCR detection of pathogenic Leptospira spp. and a comparison of nucleic acid amplification methods for the diagnosis of leptospirosis. PLoS One 9: e112356 10.1371/journal.pone.0112356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferreira AS, Costa P, Rocha T, Amaro A, Vieira ML, Ahmed A, et al. (2014) Direct detection and differentiation of pathogenic Leptospira species using a multi-gene targeted real time PCR approach. PLoS One 9: e112312 10.1371/journal.pone.0112312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boonsilp S, Thaipadungpanit J, Amornchai P, Wuthiekanun V, Bailey MS, Holden M, et al. (2013) A single multilocus sequence typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl Trop Dis 7: e1954 10.1371/journal.pntd.0001954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galloway RL, Levett PN (2008) Evaluation of a modified pulsed-field gel electrophoresis approach for the identification of Leptospira serovars. Am J Trop Med Hyg 78: 628–632. [PubMed] [Google Scholar]
- 52. Bourhy P, Collet L, Lernout T, Zinini F, Hartskeerl RA, van der Linden H, et al. (2012) Human leptospira isolates circulating in Mayotte (Indian Ocean) have unique serological and molecular features. J Clin Microbiol 50: 307–311. 10.1128/JCM.05931-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Desvars A, Jego J, Chiroleu F, Bourhy P, Cardinale E, Michault A (2011) Seasonality of human leptospirosis in Reunion Island (Indian Ocean) ans its association with meteorological data. PLoS One 6(5): e20377 10.1371/journal.pone.0020377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.