Abstract
Biomedical research in human beings is largely restricted to in vitro studies that lack complexity of a living organism. To overcome this limitation, humanized mouse models are developed based on immunodeficient characteristics of severe combined immunodeficiency (SCID) or recombination activating gene (Rag)null mice, which can accept xenografts. Peripheral constitution of human immunity in SCID or Ragnull mice has been achieved by transplantation of mature human immune cells, foetal human thymus, bone marrow, liver tissues, lymph nodes or a combination of these, although efficiency needs to be improved. These mouse models with constituted human immunity (defined as humanized mice in the present text) have been widely used to investigate the basic principles of human immunobiology as well as complex pathomechanisms and potential therapies of human diseases. Here, elements of an ideal humanized mouse model are highlighted including genetic and non-genetic modification of recipient mice, transplantation strategies and proposals to improve engraftments. The applications of the humanized mice to study the development and response of human immune cells, human autoimmune diseases, virus infections, transplantation biology and tumour biology are reviewed as well.
Keywords: immunodeficient mice, human immune system, biomedicine, transplantation, animal model
Introduction
Recipient mouse selection for engrafting xenogeneic human haematopoietic and immune cells
-
Pre-conditioning regimens for establishing humanized mice
Depletion of innate immune cells in recipient mice
Implication of human growth factors
Making ‘space’ for donor cells by irradiation or chemical agents
-
Transplantation strategies to establish humanized mice
Humanized mice achieved by directly implanting mature human immune cells
Humanized mice established by engrafting human HSCs
Humanized mice achieved by engrafting human thymus and HSCs
A proposed ideal humanized mouse model
-
Applications of humanized mouse models in biomedical research
Ontogeny of human HSCs and immunocell lineages
Autoimmune diseases
Virus infections
Immune response or tolerance to allografts or xenografts
Anti-tumour immune response
Conclusions and perspectives
Introduction
The study of human immunobiology in vivo is limited by technical and ethical constraints. Animal models with humanized immune systems would significantly advance our understanding on human immunobiology and immune-related diseases such as autoimmune diseases, virus infections, as well as tumour and graft rejections.
Immunodeficient mice with constituted human immunity have been developed to overcome these constraints and are now important research tools for the in vivo study of human haematolymphopoiesis and immune responses. Severe combined immunodeficiency (SCID) or recombination activating gene (Rag)null mice, lacking T and B cells, were originally used as recipients to re-build human immunity [1]. Recently, more and more genetically modified SCID or Ragnul mice including SCID/beige [2], non-obese diabetic/severe combined immunodeficiency (NOD/SCID) [3], NOD/SCID/p2Mnull[4] and NOD/SCID/γcnul [5], Ragnull[6–8], NOD/LtSz-Rag1nullPfpnull[9] and Rag2nullγcnul[10] mice have been employed to further enhance the reconstitution efficiency of human immune cells in the periphery, due to their deficiency of innate immunity (Table 1) [11, 12]. On the other hand, to improve the engrafting efficiency of human immune cells and/or tissues, different conditional regimens and transplantation strategies have been intensively pursued, including the depletion of host innate immune cells as well as implanting mature human immune cells, foetal thymus, liver tissues, bone marrow and CD34+ haematopoietic stem cells (HSCs), respectively [13]. In the present review, we will concentrate on the selection and pre-treatment of genetically modified SCID or Ragnull mouse recipients, strategies for implanting human HSCs, mature immune cells and/or tissues, as well as the applications of these models in biomedical research. Generally, mice constituted with human cells, tissues, organs or even human genes [14] may all be considered as humanized mice, including models grafted with foetal human lung, kidney, pancreas, stomach, liver, ovarian, endometrium, nervous and skin tissues [15–24]. To avoid confusion, humanized mice are specifically defined in this review as mice with a human immune system reconstituted by engrafting human haematopoietic or mature immune cells and/or immune tissues.
Table 1.
The characteristics of genetically modified SCID or Ragnull mice
| Mouse strains | Mechanisms | Adaptive immunity | Innate immunity | Refs |
|---|---|---|---|---|
| SCID | DNA-repair and VDJ recombination defect due to deficiency of DNA-PK | T and B cell deficiency | [1] | |
| SCID/beige | Combined effect of SCID and beige mutations | T and B cell deficiency | Reduced NK-cell function | [2] |
| NOD/SCID | VDJ recombination defect added to various NOD anomalies | T and B cell deficiency | Impaired complement, macrophage and NK cell function | [3] |
| NOD/SCID/β2Mnull | VDJ recombination defect, no MHC-I and various NOD anomalies | T and B cell deficiency | Partial NK cell deficiency | [4] |
| NOD/SCIDA/γcnull | Combined effect of SCID and γcnull on NOD background | Severe defect of T and B cell function | Severe defect of NK cell function, impaired DC function | [5] |
| Ragnull | VDJ recombination defect | T and B cell deficiency | [6–8] | |
| NOD/LtSz-Rag1nullPfpnull | VDJ recombination defect and perforin mutation | T and B cell deficiency | Complement, macrophage and NK cell deficiency | [9] |
| Rag2nuγcnull mice | Combined effect of Rag2null and γcnull | T and B cell deficiency | NK cell deficiency, Impaired DC function | [10] |
Recipient mouse selection for engrafting xenogeneic human haematopoietic and immune cells
SCID mice are defective in DNA repair because of a mutation in DNA-dependent protein kinase (DNA-PK) in the CB-17 inbred mouse strain [25, 26], therefore they lack productive rearrangement of T cell receptor and immunoglobulin genes, which subsequently results in the deficiency of T and B cells [27]. The lack of functional T and B cells in SCID mice contributes to the acceptance of allogeneic or xenogeneic grafts without severe rejection [1, 28, 29]. However, SCID mice have a radiation repair deficiency and an uncomplete block of VDJ recombination, so some old SCID mice may become ‘leaky’ mice depending on antigen stimulation (Fig. 1). In addition, residual innate immunity including complement, natural killer (NK) cells, macrophages and granulocytes remains somewhat intact in SCID mice, which may limit the grafting efficiency of xenogeneic cells and tissues (Table 1) [30, 31]. Many efforts have been made to develop modified SCID mice with more deficient innate immunity such as severely reduced NK cell function and phagocytosis by genetic crossings with inbred or other mutant strains of mice [32, 33].
Figure 1.
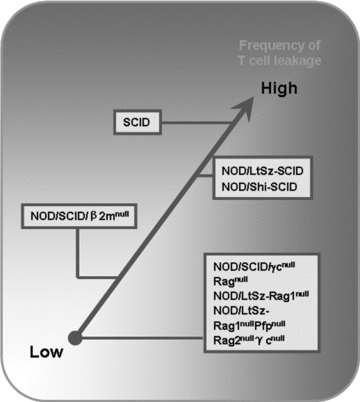
The frequencies of cell leakiness in various immunodeficient mouse strains with aging.
NOD/SCID mice are created by backcrossing the SCID mutation into the NOD/LtSz mice, which have less residual immunity than SCID mice, since NOD mice have defects in the complement pathway, macrophage function and NK cell activity [34]. NOD/Shi-SCID mice also have seriously immune functional impairments similar to NOD/LtSz-SCID mice [35, 36]. The innate immune defects in NOD/LtSz-SCID or NOD/Shi-SCID mice might well account for increased human xenograft survival in vivo[37–40]. CD47 can efficiently protect target cells from phagocytosis by macrophages via reacting with signal regulatory protein α(SIRPα) expressed on effector macrophages [41, 42]. However, in widely disparate xenogeneic combinations, haematopoietic cells can be vigorously engulfed by xenogeneic macrophages due to inefficient interaction between CD47 on the xenogeneic target cells and SIRPaon macrophages [41, 43]. It has been reported that human CD47 cross reacts with SIRPα in NOD mice, but not with SIRPα of other mouse stains [44], which indicates that the improved engraftment of human haematopoietic cells in immunodeficient mice with a NOD genetic background may, at least partially, be due to the functional matching of CD47/SIRPα phagocytosis-inhibiting pathway between human beings and mice in this combination. Furthermore, the NOD/SCIDβ2Mnull mice, which severely lack NK cell activity, are developed and available [4, 45]. NOD/SCIDβ2Mnull mice have higher engraftment of human immune cells compared with other immunodeficient mice such as SCID or NOD/SCID mice after a single i.p. transfer of human peripheral blood lymphocytes (PBLs) (Fig. 2) [46].
Figure 2.
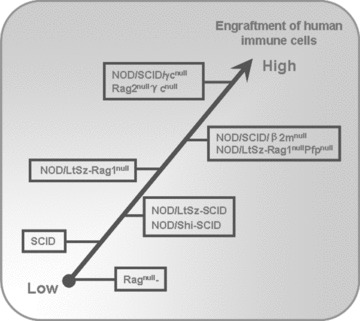
The different efficiency of engrafting human HSCs or immune cells in various immunodeficient mouse recipients.
γc chain is an indispensable component of receptor heterodimers for many cytokines (IL-2, IL-7, IL-9, IL-12, IL-15 and IL-21) [47]. It can dramatically increase the affinity of these receptors to their ligands respectively. The γcnull mice display a significant absence of both mature T and NK cells [47, 48]. NOD/SCID/γcnull mice were created by impairing γc chain in NOD/SCID mice, and these mice are completely defective in T, B and NK activity [5]. Moreover, CD11c+ dendritic cells (DCs) become functionally defective in NOD/SCID/γcnull mice, resulting in the reduction of IFN-γ production. Therefore, NOD/SCID/γcnull mice are currently considered as the optimal recipients of human HSCs [5] because of their complete deficiency in adaptive immunity and more impaired innate immunity (Fig. 2).
The Rag1 or Rag2 are responsible for the initiation of TCR and immunoglobulin genes’ VDJ rearrangement through the generation of a DNA double strand break, therefore homozygous mutants in mice result in the inability to produce mature T and B cells, leading to a SCID-like phenotype [6–8]. These mice do not become leaky with age due to their complete inability to initiate VDJ recombination and display a longer life span than NOD/LtSz-SCID mice. This is due to a delay or prevention of thymic lymphomas associated with the DNA repair defect (Fig. 1) [49]. However, they could not support efficient human engraftment because of the functional innate immunity (Fig. 2) [46]. NOD mice with targeted mutations of both Rag1 and perforin genes were developed, named NOD/LtSz-Rag1nullPfpnull mice, which lacked mature T, B and NK cells (Table 1). These mice supported a 4- to 5-fold increase in percentages and total numbers of human lymphocytes in the periphery compared with NOD/LtSz-Rag1null mice [9]. By crossing γc and Rag2 mutant mice, Rag2nullγcnull mice were developed producing no T, B and NK cells, which provided excellent immunodeficient setting for human engraftment similar to NOD/SCID/γcnull mouse recipients (Fig. 2) [10].
Thus, the frequency of leakiness in immunodeficient mice is as follows (in descending order): NOD/SCID/γcnull mice, Rag2nullγc nullmice, NOD/LtSz-Rag1nullPfpnull mice, NOD/LtSz-Rag1null mice< Ragnull mice < NOD/SCID mice < SCID mice (Fig. 1). Until now, the favoured mouse recipients for grafting human haematopoietic or immune cells were generally as follows: NOD/SCID/γcnull mice, Rag2nullγcnull mice or NOD/LtSz-Rag1nullPfpnull mice > NOD/SCID mice or NOD/LtSz-Rag1null mice> SCID mice > Ragnull mice (Fig. 2).
Pre-conditioning regimens for establishing humanized mice
Depletion of innate immune cells in recipient mice
The injected xenogeneic human HSCs or mature immune cells could be rejected in so-called immunodeficient recipient mice by innate immune elements including mouse neutrophils, granulocytes, macrophages and NK cells. So, the residual innate immunity of SCID or Ragnull mice became the main factor limiting the extent of human immune cell reconstitution. The strategies to deplete NK cells and certain subpopulations of monocytes and macrophages in recipient mice could improve human HSCs or immune cell engraftment, especially when limited cell numbers of human HSCs were transplanted [12, 35, 36, 50–52]. Anti-CD122, anti-asialo GM-1 or anti-IL-2Rβchain antibody was used to deplete NK cells, and liposome-encapsulated dichloromethylene-bisphosphonate to deplete macrophages [11]. The neutrophil and granulocyte-depleting mAbs were found to profoundly deplete mouse neutrophils [53] and granulocytes [54], which may control natural immune response of SCID or Ragnull mice against human grafts and be associated with a significant increases in human engraftment (Table 2).
Table 2.
The potential strategies to improve humanized mouse model
| Strategies | Targets/purposes | Refs | |
|---|---|---|---|
| Recipient mouse selection | NOD/SCID/γcnull mice or Rag2nulγcnull mice | Deficient in T, B and NK cells | [5, 10] |
| Anti-CD122, anti-asialo GM-1 or TM-β1 antibody | Depletion of NK cells | [12, 35, 36, 50–52] | |
| Liposome-encapsulated dichloromethylene-bisphosphonate | Depletion of macrophages | [11] | |
| Pre-treatments of recipient mice | Specific antibody | Depletion of neutrophils | [106, 125] |
| Specific antibody | Depletion of granulocytes | [107, 176] | |
| Irradiation | Making space | [66] | |
| TNFα, IL-15, IL-18, rhGH, rhPRL | Growth/Stimulating factors | [56, 58, 62, 64, 65, 177] | |
| Transplantation strategies | Co-transplantation of human foetal thymus/liver tissues and CD34+ HSCs | Enhance the resource for human HSCs and immune cells | [13] |
Implication of human growth factors
Certain growth factors, which play critical roles in haematopoiesis and peripheral immune homeostasis, may be species specific or may have mismatched physiological function in xenogeneic settings. The low engrafting efficiency of human HSCs and immune cells in xenogeneic immunodeficient recipient mice may be somewhat improved by offering human growth factors.
Tumour necrosis factor-α (TNF-α), produced by a variety of cell types including thymic stromal cells, was found to be associated with critical events leading to T lineage commitment and differentiation. In mice, TNF-α-induced expression of CD25 on early immature thymocytes was required for thymocyte maturation to single-positive CD4 and CD8 cells [55]. Administration of human TNF-α to irradiated NOD/SCID mice before transplantation of mobilized human PBLs led to rapid augmentation of human T lymphopoiesis, including the emergence of immature human CD4/CD8 double-positive (DP) T cells and mature CD4+ and CD8+ T cells [56].
Because T cell development, proliferation and survival are critically dependent on epithelially derived IL-7 [57], administration of human Fc-IL7 fusion protein greatly enhances the presence of human T cells in spleens and peripheral blood of NOD/SCIDγcnull mice transplanted with human HSCs [58]. IL-15 is shown to have haematopoiesis-promoting effects, including the proliferation of T cells, maturation of B cells and development of NK cells [59–61]. rhIL-15 could significantly improve the engraftment and reconstitution of human T cells in NOD/SCID mice after transfer of human PBLs [62]. Also, administration of IL-12 or IL-18 enhanced the engraftment of human CD4+ and CD8+ T cells in mice, whereas their co-administration prevented grafting due to IFN-γ-dependent apoptosis and regulation of class-switching IgA [63]. In SCID mice grafted with human PBLs or bone marrow cells (BMCs), injection of recombinant human growth hormone (rhGH) or recombinant human prolactin (rhPRL) strongly promoted human T cell engraftment in the thymus and spleen, as well as enhanced IgG/M levels in sera [64, 65].
Making ‘space’ for donor cells by irradiation or chemical agents
Although the residual immunity of NOD/SCID mice could be depleted by additional pre-treatment with antibodies against NK cells or macrophages, a preparative regimen involving sublethal irradiation or chemical reagents may be required to make ‘space’ for grafting xenogeneic human HSCs or immune cells. These pretreatments could result in depletion of mouse HSCs, increase concentrations of growth factors and chemoattractants and reserve a certain amount of space for the development and repopulation of human HSCs and immune cells in recipient mice [66]. Generally, human HSC transplantation in NOD/SCID mice requires 2–3 Gy pre-irridiation, and human immune cells could repopulate well in irradiated recipients [9, 67, 68]. Some immunosuppressive and alkylating agents have shown similar effects to irradiation, for example, a single dose (35 mg/kg) of Busilvex enabled equivalent engraftment of human cells compared to 3.5 Gy irradiation [66]. It was recently reported that the treatment with anti-c-kit monoclonal antibody (clone ACK2) could transiently remove a majority of recipient HSCs and make niches where HSCs could home to. Subsequent transplantation of donor HSCs could lead to 90% donor chimerism, and this may become an alternative conditioning regimen for engrafting xenogeneic HSCs (Table 2) [69].
Transplantation strategies to establish humanized mice
Certain levels of reconstituted human immune systems have been achieved in immunodeficient mice by different transplantation strategies including implantation of mature human immune cells, HSCs or a combination of human thymus and HSCs.
Humanized mice achieved by directly implanting mature human immune cells
As early as the 1980s, it was found that peritoneal transplantation of human PBLs into SCID mice resulted in the establishment of a partially functional human immune system in mice, in which mature human T and B cells were found to be present in peritoneal cavity, peripheral blood, spleens or lymph nodes. In addition, some human macrophages were found in lymphoid organs [29]. T and B cells were functionally demonstrated by the in vitro T cell proliferative response and the in vivo antigen-specific antibody production when the transplanted mice were immunized with tetanus toxoid in the early stage after transplantation. However anti-mouse reactive clones were subsequently selected and the ‘so-called’ established human immune system became non-functional in the late stage. The intrasplenic or intravenous injection of human PBLs into SCID mice caused mature activated T cells to populate the primary lymphoid tissues [70] and a transient and massive expansion of human B cells in the spleen, which permitted the isolation of human antibodies specific for target antigens [71, 72]. The high rate of reconstitution could be achieved after transfer of human PBLs into SCID, NOD/SCID and NOD/SCIDβ2Mnull mice [46]. Furthermore, the engraftment of human immune cells was significantly improved by the treatment with antibodies toward recipient mouse NK cells, macrophages or granulocytes [33, 54, 73]. Compared with SCID mice, Rag2null mice transplanted intraperitoneally with human PBLs showed more limited human engraftment and lower levels of human immunoglobulins [74]. Although there was less leakiness, the intact macrophage function in these mice were likely too high to overcome for transferred human PBLs to migrate beyond the peritoneal cavity [75]. Additional conditioning with anti-asialo GM1 serum and 600 cGy irradiation could increase the efficiency of human engraftment in Rag2null mice [76].
On the other hand, to provide the appropriate support microenvironment, for the development of human immune responses, human lymph nodes, in which all the necessary cellular components, including T cells, B cells and antigen presenting cells (APCs), were transplanted into SCID mice. In these mice, human T cells supported human immunodeficiency virus (HIV) replication [77, 78] and human B cells differentiated to plasma cells producing human IgM or IgG [1]. But interaction between human T cells and mouse xenoantigens within the mouse environments caused activation phenotypes of human T cells and graft-versus-host disease (GVHD) in this model.
One of limitations of this mouse model was the low levels of human PBL engraftment [29, 70]. Analysis of antigen-specific cellular immune responses was extremely difficult. Various strategies were explored to improve the efficiency of human PBL engraftment in SCID mice, including an increasing number of transferred cells [29, 79], pre-treatment of recipient mice with low-dose irradiation [32], elimination of recipient mouse NK cells by anti-asia-lo-GM1 antibodies [80] or a combination of these [81]. All these protocols showed only marginal effects if both the function and distribution of transferred human PBLs in lymph organs of these models were considered.
Another weakness of this model was the weak primary immune response [82–84]. However, it induced substantial immune responses against recipient mouse xenoantigens, which ‘skew’ the human lymphoid repertoire [85]. This xeno-response mediated by human T and B cells not only caused potentially fatal GVHD [79, 84, 86], but also severely limited the ability of human PBLs to respond to exogenous antigens [70, 87]. Thus, the application of this approach in biomedical research is currently somewhat limited.
Humanized mice established by engrafting human HSCs
Human bone marrow grafts in SCID mice were histologically comparable to normal foetal bone marrow when fragments of foetal human femur and tibia were implanted into SCID mice [88]. In this model, the human cell populations presenting in the circulation, consisted mostly of B cells and a few of cells belonging to the monocytic-granulocytic lineages. Human IgM, IgG and occasionally IgE were detected in the sera, but no mature human T cells could be observed in the bones or the circulation of these mice [88, 89].
The multi-lineage reconstitution of functional T, B and NK cells was achieved in irradiated NOD/SCID/γcnull mice after transplantation of human CD34+ HSCs without co-transplantation of human immune tissues [90–92]. Human T cells developing in the thymus of these mice migrated into peripheral lymphoid organs. These T cells beared polyclonal TCR-αβ and responded not only to mitogenic stimuli, such as PHA and IL-2, but also to allogeneic human cells. These results indicated that functional human T cells could be reconstituted in NOD/SCIDγcnull mice by grafting human CD34+ cells [93].
When human cord blood CD34+ cells were injected into NOD/SCID foetuses, human immune cells could not efficiently self-renew and differentiate in the foetal mouse environment [94]. However, when foetal human liver mononuclear cells or foetal bone marrow-derived CD34+ cells were injected into NOD/SCID foetuses, multilineages of human haematopoietic cells, including B cells, myelomonocytic cells and haematopoietic progenitor cells, but no human T cells, were detected in the chimaeric mice. Although multilineage engraftment occurred in these foetal recipients, both the frequency and the levels of engraftment were lower than those previously reported when human immune cells were transplanted into adult NOD/SCID recipients [95].
Like NOD/SCID/γcnull mice, Rag2nullγcnull mice engrafted with human cord blood CD34+ cells led to multilineage of human haematopoiesis with the production of T cells, B cells and DCs [96]. Primary and secondary lymphoid organs (thymus, bone marrow, spleen and lymph nodes) were reconstituted in these mice with human immune cells. Human immune responses were demonstrated when these mice were immunized with tetanus tox-oid or infected with Epstein–Barr virus (EBV) [96]. Low levels of antigen-specific human IgG productions upon immunization indicate that an inefficient class switching is present in this model [90, 92]. In addition, the levels of human T cells in these mice are much lower compared to humanized mice that receive human foetal thymus tissue, suggesting that poor efficacy of human thymopoiesis in xenogeneic mouse thymus may exist [90, 92].
Humanized mice achieved by engrafting human thymus and HSCs
Another important approach to establish a humanized mouse model is transplanting foetal human thymus and liver tissue beneath the kidney capsules of SCID mice, which results in the development of a well vascularized human thymus-like organ [97]. The human HSCs from foetal human liver tissue grafts were observed to migrate into the thymic grafts [1, 97–100]. Human T cells in the grafts were similar to those of normal human foetal thymus, with a hierarchal distribution of CD4+CD8+ cortical thymocytes and CD4 or CD8 single positive (SP) medullary thymocytes. The SP T cells expressed a diverse Vβ repertoire that was indistinguishable from those of the foetal thymic donors prior to implantation [101]. Despite the large numbers of human T cells present in the thymus, the levels of peripheral circulating human T cells were relatively low in this model. These human T cells were responsive to mitogenic stimuli and alloantigens, and produced the types and amounts of cytokines similar to compartment of human beings [100, 102]. However, no detectable mature human B cells were generated in these mice [89, 97]. Increasing the quantity of implanted foetal human thymus and liver tissues somehow enhanced the percentages of human CD4+ and CD8+ T cells in the peripheral blood, spleen and lymph nodes [103]. Simultaneous transplantation of foetal human thymus, liver haematopoietic cells and lymph nodes resulted in the improved differentiation of mature human T and B cells, which were tolerant to recipient mouse antigens [1].
Implantation of human foetal thymus together with foetal bone chips under the SCID mouse kidney capsule supported the proliferation and differentiation of human HSCs into mature myeloid and lymphoid cells including human T cells, B cells and macrophages [104]. These structures of bone and thymus were indistinguishable from age-matched human counterparts. These mice produced human IgM and IgG at levels that were consistently higher than those observed in SCID mice grafted with human thymus and liver tissues from the same donor [89]. Furthermore, all four human IgG subclasses, IgA and IgE were detectable [104]. These results showed that human antibody production and isotype switching occured in these mice, indicating that human APCs, T and B cells interacted physiologically in response to environmental antigens.
Importantly, co-transplantation of human foetal thymus/liver tissues and CD34+ HSCs led to the efficient establishment of human haematopoiesis and functional immune systems in NOD/SCID mice, which showed repopulation with multilineage of human haematopoietic cells, including T cells, B cells and DCs in secondary lymphoid tissues [13]. These mice produced high levels of human IgM and IgG antibodies and mediated strong immune responses in vivo as demonstrated by skin xenograft rejection [13]. These humanized mice provided a more powerful model to study human immune function in vivo.
A proposed ideal humanized mouse model
The optimal experimental humanized mouse model, in which all human immune cell lineages can develop and home to specific sites to perform optimal immune response, should be explored.
Firstly, the ideal recipient mice should be deficient of both adaptive and innate immunity. NOD/SCID/γcnunull or Rag2nullγcnull mice are the best choice at present, because they have no T and B cells, no NK activity and impaired DC function [5, 10]. However, the innate immune in these mice were only partially deficient. Therefore, additional treatments including reagents of depleting neutrophils, granulocytes and macrophages need to be pursued [11, 47, 105–107]. In addition, the more genetically immunodeficient mice with more space to accept xenogeneic human cells and optimal microenvironment for the development, survival and homing of human HSCs and immune cells should be identified. Furthermore, in order to observe the ‘pure’ human immune response, the recipient mice should express no mouse major histocompatibility complex (MHC) or express certain human leucocyte antigens (HLA). On the other hand, if the humanized immunity was achieved by grafting foetal human thymus and HSCs, the recipient mice should be athymic to avoid the mixture of human T cells developing in human and mouse thymi.
Secondly, the strategies for implanting human immune cells or their progenitor cells should be considered. Directly transplanting high doses of mature human T cells and other immune cells may not be an ideal choice, due to the high incidence or possibility of GVHD caused by human anti-mouse xenogeneic immune response. Alternatively, implanting foetal human thymus tissue and HSCs in immunodeficient mice to reconstitute human immunity may be an optimal approach. The co-transplantation strategy of foetal human thymus/liver tissues and CD34+ HSCs could sustain development of multilineage of human immune cells, including T cells, B cells and DCs, which provided a more powerful adaptive and innate immunity [13].
Therefore, a combination of modified recipients (immunodeficiency, athymic, available ‘space’, and proper microenvironments including growth factors and homing molecules) and transplantation strategies (human immune tissues and stem cells) would create an optimal humanized mouse model with an approximation of a complete human immune system (Table 2).
Applications of humanized mouse models in biomedical research
Humanized mice were widely employed in research on self-renewal and multilineage differentiation capacity of human HSCs as well as human immunity to virus infection, tumour and transplantation.
Ontogeny of human HSCs and immunocell lineages
Human umbilical cord blood (UCB), bone marrow and mobilized peripheral blood (mPB) were used as sources of human HSCs for transplantation. Transplantation of CD34+ cells from human UCB resulted in higher engraftment levels in NOD/SCID mice as compared to CD34+ cells from bone marrow or mPB [108]. Human CD34+ population could be segregated into two subpopulations with distinct characteristics (CD34+CD38+ and CD34+CD38−). CD34+CD38+ cells repopulated early, whereas in CD34+CD38∼ cells repopulation was more robust at late stages and represented a more primitive population and a higher frequency of T cell precursors [109]. The population of human CD34+Lin∼Thy-1+ progenitor cells could repopulate the thymic grafts. Further, human CD34+ cells or CD34+Lin Thy-1+CD10+ populations gave rise to T, B, NK and DC populations in SCID mice [110]. When human CD34+ cells were transplanted into NOD/SCID/IL2Rγnull mice, mutilineages of immune cells were reconstituted, including human T cells, B cells, monocytes, macrophages, and DCs [58, 91, 111, 112].
The grafts of human thymus in SCID mice, identical to physiological human thymus, efficiently supported human T cell development. Thymocytes of human thymus grafts in mice had a phenotype comparable to those of freshly isolated foetal human thymocytes, and they comprised all the T cell subsets, with normal ratios of double negative, DP and CD4 and CD8 SP thymocytes [1, 97]. The Vβ repertoire in thymocytes from grafts was comparable to those of the foetal thymus before transplantation, demonstrating generation of human T cell diversity in human thymus grafts [101]. Human T cells maturing in human thymus grafts responded to toxic shock syndrome toxin1 superantigens, EBV infection and mitogens, indicating they were functional [111, 113]. Interestingly, human thymic regulatory T cells (Treg cells) might also be generated via thymic development demonstrated by the expression of CD25 and Foxp3 [96]. Our recent studies also showed human CD4+CD25+Foxp3+ T cells could efficiently develop in human thymus grafts and repopulate in peripheral blood and lymphoid tissues in NOD/SCID mice (BJ Zhang and Y Zhao, unpublished data).
Human B cells underwent normal class switching and matured to produce IgM, IgG and IgA in various immunodeficient mice grafted with human CD34+ cells [13, 90, 112]. These human antigen-specific immunoglobulins were produced after immunization with a T cell-independent antigen [90–92, 112]. Human DCs in humanized mice were developmentally, phenotypically, and functionally similar to the DC subsets found in human beings [114]. Human NK cells mainly repopulated in BM and spleen, and showed cytotoxicity against K562 cells in this model [91].
Autoimmune diseases
Human PBLs obtained from patients with organ-specific and multi-system autoimmune diseases survived in SCID mice for several months and produced IgG and autoantibodies with the same specificities found in the donor [115]. Therefore, this provides a possible way to investigate the pathogenesis and effector phases of human autoimmune diseases in in vivo models.
Pemphigus vulgaris (PV) is a severe autoimmmune skin disorder induced by antibodies against desmoglein (Dsg) 3 on epidermal keratinocytes. After transferring PBLs from patients with PV into SCID mice, circulating anti-pemphigus antibodies were found in these mice, and human IgG deposited in their traumatized skin. Allogeneic human skin grafted to SCID mice before reconstitution with patients’ PBLs developed PV-like lesions containing human IgG deposits [116]. These results indicate that humanized mice may serve as models of antibody-mediated human autoimmune skin diseases.
Virus infections
Some viruses can only infect human beings, thus, the humanized models were used to study the interactions between viruses and the human immune system, and to evaluate the efficiacy of vaccines and therapeutics for human viruses such as HIV, severe acute respiratory syndrome (SARS), porcine endogenous retrovirus (PERVs), varicella-zoster virus (VZV), Dengue virus and others.
HIV infections are largely restricted to in vitro or clinical studies because of species tropism, and humanized mice have been extensively used to study HIV pathogenesis and therapy in vivo[117, 118]. In SCID mice grafted with human PBLs, HIV infections were limited to a short period because CD4+ T cells were rapidly depleted and lacked replenishment sources [119]. After intrasplenic transfer of PBLs from untreated HIV-infected patients into NOD/SCID mice, a strong HIV-specific antibody response was observed [120]. This, therefore, is feasible for the study of HIV-specific human humoural immune responses and for the isolation of human mAbs against HIV. In humanized mice grafted with human thymus and liver tissues, HIV infection showed preferential tropism for a population of intrathymic CD3∼CD4+CD8∼ T progenitor cells [121], while other thymocytes were normally generated [122, 123]. After transplantation of human CD34+ CB cells, the long-lasting viremia after infection with both CCR5- and CXCR4-tropic HIV-1 isolates was detected in spleen, bone marrow or thymi of NOD/SCID/IL-2γcnull mice. Also, this high rate of viral infection was accompanied by production of anti-HIV-1 Env gp120 and Gag p24 antibodies [90]. In humanized Rag2nullγcnull mice constructed with cord blood CD34+ cells, CXCR4 and CCR5 expression on human CD4+, T cells in lymphoid organs closely resembled HIV coreceptor expression in human beings [124, 125]. CXCR4-tropic HIV virus infected all lymphoid organs, while CCR5-tropic HIV virus infection was largely restricted to extrathymic tissues. Both viral strains led to long-term lymphoid organ disseminated infection closely resembling HIV infection in human beings.
Humanized mouse models have been developed for pre-clini-cal evaluation of antiviral compounds against HIV including azidothymidine [126], dideoxyinosine, dideoxycytosine, nevirapine, protease inhibitors [127], non-immunosuppressive cyclosporine derivatives, peptide inhibitors of env-CD4 interactions and ‘zinc-finger’ inhibitors [128]. Humanized mice constructed by human thymus, liver tissues and lymph nodes were permissive for HIV replication [78] and antiviral compounds such as azidothymidine would partially suppress replication in a time- and dose-dependent manner [126]. Exposure to cocaine can enhance HIV infection in vivo by activating sigma-1 receptors and modulating the expression of HIV coreceptors. A selective sigma-1 antagonist, BD1047, blocked the effects of cocaine on HIV replication in SCID mice grafted with human PBLs [129]. In addition, humanized mouse models have also been proven useful in evaluating gene therapeutic approaches. Transferring human CD4+ T cells containing the IFN-β retroviral vector drastically reduced the pre-existing HIV infection and enhanced CD4+ T cells survival as well as Th1 cytokine expression [130].
SARS, a kind of corona virus, caused a deadly epidemic in Asia [131]. The humanized mice constructed by human PBLs were used to study novel vaccine candidates against SARS CoV, SARS DNA vaccines induced human cytotoxic T lymphocytes specific for SARS antigens and human neutralizing antibodies against SARS CoV [132]. It was demonstrated that Angiotensin-converting enzyme 2 was a functional receptor for the SARS CoV [133].
VZV appeared to cause viremia by infecting human lymphocytes [134]. Humanized mouse models were used to examine VZV pathogenesis and immunobiology in vivo[135–137]. These experiments provided evidence in support of a critical role for T cell tropism in VZV pathogenesis. Eliminating ORF47 kinase activity impaired virus production and envelopment, which would block VZV infectivity for T cells [134].
Dengue virus is an important mosquito-borne flavivirus and it infects human beings causing a range of illnesses from subclinical infection to acute dengue fever to the severe dengue haemorrhagic fever/dengue shock syndrome [138]. A major limitation to the understanding of dengue virus pathogenesis and immunity has been the lack of an ideal humanized animal model. Irradiated NOD/SCID or Rag2nullγcnull mice grafted with human cord blood haematopoietic progenitor cells or foetal liver-derived CD34+ cells achieved replication of dengue viral infection in a physiological setting. These models were found to be susceptible to dengue virus infection, showed signs typical of fever and thrombocytopenia, and were used to evaluate dengue virus pathogenesis [138, 139]. Also, humanized mice were used to investigate pathogenesis and therapeutics of EBV [140], cytomegalovirus (CMV) [135] and influenza infections [141].
Pigs may be suitable donors for human xenotransplantation. The potential transmission of PERVs to human cells was investigated in humanized mice, and some tissues were positive for PERVs’ DNA [142, 143]. However, the infection was non-productive as PERV transcripts were not detectable in those tissues [144], and might be due to microchimerism or pseudotyping with murine viruses [145]. Importantly, it was shown that human cells and porcine cells could co-exist for many weeks without any evidence for infection of human cells by PERVs in NOD/SCID mice transgenic for certain porcine cytokines and transplanted by porcine BMCs, human foetal thymus and liver tissues [146]. However, it remains to be seen whether immunosuppressive therapy would facilitate retroviral infection of human cells.
Immune response or tolerance to allografts or xenografts
Transplantation of allogeneic HLA-mismatched foetal pancreases in SCID mice grafted with human thymus and liver tissues or PBLs resulted in infiltration of human mononuclear cells in the pancreas and subsequent rejection [89, 147]. Human T cells were responsible for the rejection of skin transplants from allogeneic donors in SCID mice [148, 149]. Like skin grafts performed among human beings, the dermal microvessels were destroyed and the skin became necrotic [150].
SCID mice grafted with human PBLs were established models for delayed-type hypersensitivity (DTH) [151]. Human skin was grafted onto the backs and autologous human immune cells were injected into the peritoneal cavity of SCID mice. Injection of tetanus toxoid into the human skin grafts not adjacent mouse skin caused perivascular human T cell infiltrate, which indicates that human T cells specifically recognize human but not mouse skin as homing sites, and that human T cell responses depends on the human microenvironments. Recently, it was found that adaptive CD4+CD25+Treg cells isolated from kidney transplant recipients who are tolerant to the donor, mediated the suppression of donor-specific DTH in humanized SCID mice [152].
SCID mice transplanted with human foetal liver from donor A and foetal thymus from donor B developed a mixed chimeric human thymus [153]. In this model, human T cells reactive to donor A were clonally deleted by selection in the thymus, while T cells potentially responding to donor B were rendered anergic upon interaction with the allogeneic thymic epithelial cells of donor B [154].
GVHD remains a major cause of morbidity and mortality after allogeneic HSC transplantation. Sublethal irradiated immunodeficient mice reconstituted with human T cells would be a valuable tool for investigating the development and pathophysiology of the disease. NOD/SCID/β2Mnull mice conditioned with 300 cGy total body irradiation developed lethal GVHD after injection of human T cells through the retro-orbital venous plexus, in which high numbers of activated human CD4+ and CD8+ T cells expressing CD25 and CD69 were demonstrated in spleen, liver, lung, kidney and bone marrow of the recipients. These T cells secreted large quantities of human IFN-γ, and specific antibodies (IgM and IgG) to the dengue virus were detected in the majority of the mice that showed viremia [67]. This model replicated similar phases and pathogenesis of human acute GVHD in physiological settings and would contribute to development of therapeutic strategies for GVHD. Furthermore, human CD4+CD25+ Treg cells could efficiently prevent xenogeneic GVHD in Rag2nullγcnull mice injected with autologous human peripheral blood mononuclear cells (PBMCs) [155]. Depletion of CD4+CD25+Treg cells from human PBMCs significantly exacerbated xenogeneic GVHD, whereas co-administration of this subset of Treg cells significantly inhibited the expansion of effector T cells and reduced the lethality of xenogeneic GVHD. Interestingly, the protective role was associated with a significant increase in plasma levels of IL-10 and IFN-γ, indicating the de novo development of Tr1 cells [155].
Humanized mouse models were used to study the immune response to xenogeneic porcine grafts in vivo[156–158]. Circulating human T cells in these mice did not infiltrate pig skin or artery grafts in contrast to the robust immune responses against allogeneic human cells in vitro. However, TNF-treated pig tissue permitted human T cells to infiltrate and injure both pig skin and artery grafts because the actions of this proinflammatory cytokine were not species-restricted and could activate pig endothelial cells to express cell surface molecules that were necessary to recruit a local infiltrate of leucocytes [156, 158]. Thus, by addition of TNF, the humanized mice might be used to study primary human T cell-mediated anti-porcine xenogeneic responses in vivo.
Porcine thymus grafts in SCID mice grafted with foetal porcine thymus and human liver tissues support normal development of polyclonal, functional human T cells from haematopoietic precursors provided by human foetal liver cells [159]. These human T cells were specifically tolerant to donor porcine antigens but normally responded to non-donor porcine xenoantigens and alloantigens. Exogenous IL-2 did not abolish tolerance, suggesting that central clonal deletion rather than anergy was the possible tolerance mechanism [159]. Recently, our results showed that human functional CD4+CD25+Foxp3+ Treg cells could develop in xenogeneic pig thymus grafts in NOD/SCID mice (BJ Zhang and Y Zhao, unpublished data).
Chimerism was constructed by human thymus/liver tissues and porcine HSCs in SCID mice [160]. Human T cells developed in this chimerism showed specific non-responsiveness to the porcine donor because it lacked rejection of porcine haematopoietic cells and anti-donor pig MLR responses, as well as the acceptance of donor swine leucocyte antigen (SLA)-matched skin grafts, whereas human T cells in the mixed chimeric mice rejected the third party porcine skin grafts and responded to the third party pig and allogeneic human antigens in MLR assays [160]. The capacity of haematopoietic chimerism to induce T cell tolerance to donor cells largely resulted from intrathymic clonal deletion of maturing donor-reactive thymocytes [161, 162].
Anti-tumour immune response
Malignant tumours can unlimitedly grow, escaping from human immune surveillance. Athymic nude mice, SCID or Rag−/− mice could be successfully engrafted with xenogeneic human tumours including a wide variety of solid human tumours and haematolog-ical neoplasms [163, 164], in which human tumour biology, growth, angiogenesis and metastasis have been evaluated.
The ability to engraft human tumours and human immunocompetent cells successfully in immunodeficient mice has spawned the development and use of humanized mouse models to evaluate anti-tumour therapies, because the study of tumour biology in human beings was impeded by access to tissues and to the site of tumour growth as well as ethical concerns. Indeed, an ideal model was humanized mice with a complete human immune system, which will permit evaluating the mechanism of tumour immunobiology in the context of an intact human immune microenvironment. Humanized mice can be used for the evaluation of therapeutic approaches for the inhibition of human tumour growth, including the use of angiogenesis inhibitors [165], cell-based therapies [166], humanized antibodies [167], traditional immunosuppressive and immunotherapeutic protocols [168] and tumour-growth inhibitors [169–171].
Humanized mice provide an opportunity to evaluate human cytokines and chemokines that augment both innate and adaptive anti-tumour immune responses of human leucocytes, thus providing a model that is more clinically relevant. This model has been utilized to test the anti-tumour efficacy of a wide spectrum of human cytokines [172–174] and cytokine-delivery strategies including the direct injection of soluble cytokines [175], cytokine gene transfer [173, 174] and the use of slow-release polymer particles [175].
Conclusions and perspectives
Humanized mouse models have made tremendous progress in modification of recipient mice and transplantation strategies in recent years. These models are becoming powerful and versatile tools to investigate human haematolymphoid differentiation, autoimmunity, virus infections, transplantation rejection and tolerance induction. However, much effort should be directed in minimizing innate immunity mediated by NK cells and macrophages in SCID or Ragnull mouse recipients. Some incompatibility of cytokines and adhesive molecules between mice and human beings could descend the human immune cell levels in immunodeficient recipients. Immunodeficient mice with some important human growth factors will be ideal recipients to establish humanized mice. Optimized humanized mouse models will offer a powerful approach for us to study human immunity in vivo, which will significantly advance biomedical research.
Acknowledgments
The authors wish to thank Drs David E. Corbin, Aqeel Javeed and Iram Murtaza for reviewing the manuscript. This work was supported by grants from the National Natural Science Foundation for Distinguished Young Scholars (C03020504, YZ), Knowledge Innovation Program of Chinese Academy of Sciences (KSCX2-SW-333, YZ) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars of State Education Ministry (2005-546, YZ).
References
- 1.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 2.Mosier DE, Stell KL, Gulizia RJ, Torbett BE, Gilmore GL. HomozygousSCID/SCID; beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J Exp Med. 1993;177:191–4. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prochazka M, Gaskins HR, Shultz LD, Leiter EH. The nonobese diabetic SCID mouse: model for spontaneous thymomagenesis associated with immunodeficiency. Proc Natl Acad Sci USA. 1992;89:3290–4. doi: 10.1073/pnas.89.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson SW, Greiner DL, Hesselton RA, Leif JH, Wagar EJ, Schweitzer IB, Rajan TV, Gott B, Roopenian DC, Shultz LD. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-SCID mice. J Immunol. 1997;158:3578–86. [PubMed] [Google Scholar]
- 5.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Shinkai Y, Young F, Alt FW. Probing immune functions in RAG-deficient mice. Curr Opin Immunol. 1994;6:313–9. doi: 10.1016/0952-7915(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 7.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 8.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 9.Shultz LD, Banuelos S, Lyons B, Samuels R, Burzenski L, Gott B, Lang P, Leif J, Appel M, Rossini A, Greiner DL. NOD/LtSz-Rag1nullPfpnull mice: a new model system with increased levels of human peripheral leukocyte and hematopoietic stem-cell engraftment. Transplantation. 2003;76:1036–42. doi: 10.1097/01.TP.0000083041.44829.2C. [DOI] [PubMed] [Google Scholar]
- 10.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz VL, Di Santo JP. Dissecting NK cell development using a novel alymphoid mouse model: investigating the role of the c-abl proto-oncogene in murine NK cell differentiation. J Immunol. 1999;162:2761–5. [PubMed] [Google Scholar]
- 11.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176:2053–8. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 12.McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood. 2005;106:1259–61. doi: 10.1182/blood-2005-03-1081. [DOI] [PubMed] [Google Scholar]
- 13.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–92. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 14.Taneja V, David CS. HLA transgenic mice as humanized mouse models of disease and immunity. J Clin Invest. 1998;101:921–6. doi: 10.1172/JCI2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesbron JY, Agut H, Gosselin B, Candotti D, Raphael M, Puech F, Grandadam M, Debre P, Capron A, Autran B. SCID-Hu mouse as a model for human lung HIV-1 infection. C R Acad Sci III. 1994;317:669–74. [PubMed] [Google Scholar]
- 16.Dekel B, Amariglio N, Kaminski N, Schwartz A, Goshen E, Arditti FD, Tsarfaty I, Passwell JH, Reisner Y, Rechavi G. Engraftment and differentiation of human metanephroi into functional mature nephrons after transplantation into mice is accompanied by a profile of gene expression similar to normal human kidney development. J Am Soc Nephrol. 2002;13:977–90. doi: 10.1681/ASN.V134977. [DOI] [PubMed] [Google Scholar]
- 17.Aubard Y. Ovarian tissue xenografting. Eur J Obstet Gynecol Reprod Biol. 2003;108:14–8. doi: 10.1016/s0301-2115(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 18.Lozniewski A, Muhale F, Hatier R, Marais A, Conroy MC, Edert D, Le Faou A, Weber M, Duprez A. Human embryonic gastric xenografts in nude mice: a new model of Helicobacter pylori infection. Infect Immun. 1999;67:1798–805. doi: 10.1128/iai.67.4.1798-1805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maltaris T, Koelbl H, Fischl F, Seufert R, Schmidt M, Kohl J, Beckmann MW, Binder H, Hoffmann I, Mueller A, Dittrich R. Xenotransplantation of human ovarian tissue pieces in gonadotropin-stimulated SCID mice: the effect of ovariectomy. Anticancer Res. 2006;26:4171–6. [PubMed] [Google Scholar]
- 20.Matsuura-Sawada R, Murakami T, Ozawa Y, Nabeshima H, Akahira J, Sato Y, Koyanagi Y, Ito M, Terada Y, Okamura K. Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice. Hum Reprod. 2005;20:1477–84. doi: 10.1093/humrep/deh783. [DOI] [PubMed] [Google Scholar]
- 21.Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M, Tamaoki N, Nomura T, Okano H, Matsuzaki Y, Yoshimura Y. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci USA. 2007;104:1925–30. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein LG, Cvetkovich TA, Lazar ES, DiLoreto D, Saito Y, James H, Del Cerro C, Kaneshima H, McCune JM, Britt WJ, Cerro MD. Human neural xenografts: progress in developing an in-vivo model to study human immunodeficiency virus (HIV) and human cytomegalovirus (HCMV) infection. Adv Neuroimmunol. 1994;4:257–60. doi: 10.1016/s0960-5428(06)80264-0. [DOI] [PubMed] [Google Scholar]
- 23.Raychaudhuri SP, Dutt S, Raychaudhuri SK, Sanyal M, Farber EM. Severe combined immunodeficiency mouse-human skin chimeras: a unique animal model for the study of psoriasis and cutaneous inflammation. Br J Dermatol. 2001;144:931–9. doi: 10.1046/j.1365-2133.2001.04178.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim YH, Woodley DT, Wynn KC, Giomi W, Bauer EA. Recessive dystrophic epidermolysis bullosa phenotype is preserved in xenografts using SCID mice: development of an experimental in vivo model. J Invest Dermatol. 1992;98:191–7. doi: 10.1111/1523-1747.ep12555849. [DOI] [PubMed] [Google Scholar]
- 25.Bosma GC, Davisson MT, Ruetsch NR, Sweet HO, Shultz LD, Bosma MJ. The mouse mutation severe combined immune deficiency (SCID) is on chromosome 16. Immunogenetics. 1989;29:54–7. doi: 10.1007/BF02341614. [DOI] [PubMed] [Google Scholar]
- 26.Fulop GM, Phillips RA. The SCID mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–82. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 27.Kirchgessner CU, Patil CK, Evans JW, Cuomo CA, Fried LM, Carter T, Oettinger MA, Brown JM. DNA-dependent kinase (p350) as a candidate gene for the murine SCID defect. Science. 1995;267:1178–83. doi: 10.1126/science.7855601. [DOI] [PubMed] [Google Scholar]
- 28.Kamel-Reid S, Dick JE. Engraftment of immune-deficient mice with human hematopoietic stem cells. Science. 1988;242:1706–9. doi: 10.1126/science.2904703. [DOI] [PubMed] [Google Scholar]
- 29.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 30.Dorshkind K, Keller GM, Phillips RA, Miller RG, Bosma GC, O’Toole M, Bosma MJ. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984;132:1804–8. [PubMed] [Google Scholar]
- 31.Dorshkind K, Pollack SB, Bosma MJ, Phillips RA. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (SCID) J Immunol. 1985;134:3798–801. [PubMed] [Google Scholar]
- 32.Nonoyama S, Smith FO, Bernstein ID, Ochs HD. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150:3817–24. [PubMed] [Google Scholar]
- 33.Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, Schweitzer PA, Hesselton RM, Shultz LD. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186–99. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 34.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, Leiter EH. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 35.Ueda T, Tsuji K, Yoshino H, Ebihara Y, Yagasaki H, Hisakawa H, Mitsui T, Manabe A, Tanaka R, Kobayashi K, Ito M, Yasukawa K, Nakahata T. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105:1013–21. doi: 10.1172/JCI8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koyanagi Y, Tanaka Y, Tanaka R, Misawa N, Kawano Y, Tanaka T, Miyasaka M, Ito M, Ueyama Y, Yamamoto N. High levels of viremia in hu-PBL-NOD-scid mice with HIV-1 infection. Leukemia. 1997;11(Suppl 3):109–12. [PubMed] [Google Scholar]
- 37.Serreze DV, Gaedeke JW, Leiter EH. Hematopoietic stem-cell defects underlying abnormal macrophage development and maturation in NOD/Lt mice: defective regulation of cytokine receptors and protein kinase C. Proc Natl Acad Sci USA. 1993;90:9625–9. doi: 10.1073/pnas.90.20.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kataoka S, Satoh J, Fujiya H, Toyota T, Suzuki R, Itoh K, Kumagai K. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983;32:247–53. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- 39.Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993;42:1574–8. doi: 10.2337/diab.42.11.1574. [DOI] [PubMed] [Google Scholar]
- 40.Greiner DL, Shultz LD, Yates J, Appel MC, Perdrizet G, Hesselton RM, Schweitzer I, Beamer WG, Shultz KL, Pelsue SC, Leif JH, Rajan TV. Improved engraftment of human spleen cells in NOD/LtSz-SCID/SCID mice as compared with C.B-17-SCID/SCID mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci USA. 2007;104:13744–9. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, Oldenborg PA, Sykes M, Yang YG. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109:836–42. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–23. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 45.Kollet O, Peled A, Byk T, Ben-Hur H, Greiner D, Shultz L, Lapidot T. Beta2 microglobulin-deficient (B2m(null)) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95:3102–5. [PubMed] [Google Scholar]
- 46.Berney T, Molano RD, Pileggi A, Cattan P, Li H, Ricordi C, Inverardi L. Patterns of engraftment in different strains of immunodeficient mice reconstituted with human peripheral blood lymphocytes. Transplantation. 2001;72:133–40. doi: 10.1097/00007890-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 47.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–8. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 48.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 49.Shultz LD, Lang PA, Christianson SW, Gott B, Lyons B, Umeda S, Leiter E, Hesselton R, Wagar EJ, Leif JH, Kollet O, Lapidot T, Greiner DL. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol. 2000;164:2496–507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 50.Tournoy KG, Depraetere S, Meuleman P, Leroux-Roels G, Pauwels RA. Murine IL-2 receptor beta chain blockade improves human leukocyte engraftment in SCID mice. Eur J Immunol. 1998;28:3221–30. doi: 10.1002/(SICI)1521-4141(199810)28:10<3221::AID-IMMU3221>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 51.Kerre TC, De Smet G, De Smedt M, Zippelius A, Pittet MJ, Langerak AW, De Bosscher J, Offner F, Vandekerckhove B, Plum J. Adapted NOD/SCID model supports development of phenotypically and functionally mature T cells from human umbilical cord blood CD34(+) cells. Blood. 2002;99:1620–6. doi: 10.1182/blood.v99.5.1620. [DOI] [PubMed] [Google Scholar]
- 52.Yoshino H, Ueda T, Kawahata M, Kobayashi K, Ebihara Y, Manabe A, Tanaka R, Ito M, Asano S, Nakahata T, Tsuji K. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 2000;26:1211–6. doi: 10.1038/sj.bmt.1702702. [DOI] [PubMed] [Google Scholar]
- 53.Santini SM, Rizza P, Logozzi MA, Sestili P, Gherardi G, Lande R, Lapenta C, Belardelli F, Fais S. The SCID mouse reaction to human peripheral blood mononuclear leukocyte engraftment. Neutrophil recruitment induced expression of a wide spectrum of murine cytokines and mouse leukopoiesis, including thymic differentiation. Transplantation. 1995;60:1306–14. [PubMed] [Google Scholar]
- 54.Santini SM, Spada M, Parlato S, Logozzi M, Lapenta C, Proietti E, Belardelli F, Fais S. Treatment of severe combined immunodeficiency mice with anti-murine granulocyte monoclonal antibody improves human leukocyte xenotransplantation. Transplantation. 1998;65:416–20. doi: 10.1097/00007890-199802150-00022. [DOI] [PubMed] [Google Scholar]
- 55.Zuniga-Pflucker JC, Di J, Lenardo MJ. Requirement for TNF-alpha and IL-1 alpha in fetal thymocyte commitment and differentiation. Science. 1995;268:1906–9. doi: 10.1126/science.7541554. [DOI] [PubMed] [Google Scholar]
- 56.Samira S, Ferrand C, Peled A, Nagler A, Tovbin Y, Ben-Hur H, Taylor N, Globerson A, Lapidot T. Tumor Necrosis factor promotes human T-cell development in nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2004;22:1085–100. doi: 10.1634/stemcells.22-6-1085. [DOI] [PubMed] [Google Scholar]
- 57.El Kassar N, Lucas PJ, Klug DB, Zamisch M, Merchant M, Bare CV, Choudhury B, Sharrow SO, Richie E, Mackall CL, Gress RE. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–27. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 58.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-SCID IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 59.Bykovskaia SN, Buffo M, Zhang H, Bunker M, Levitt ML, Agha M, Marks S, Evans C, Ellis P, Shurin MR, Shogan J. The generation of human dendritic and NK cells from hemopoietic progenitors induced by interleukin-15. J Leukoc Biol. 1999;66:659–66. doi: 10.1002/jlb.66.4.659. [DOI] [PubMed] [Google Scholar]
- 60.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–6. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 61.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 62.Sun A, Wei H, Sun R, Xiao W, Yang Y, Tian Z. Human interleukin-15 improves engraftment of human T cells in NOD-SCID mice. Clin Vaccine Immunol. 2006;13:227–34. doi: 10.1128/CVI.13.2.227-234.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Senpuku H, Asano T, Matin K, Salam MA, Tsuha Y, Horibata S, Shimazu Y, Soeno Y, Aoba T, Sata T, Hanada N, Honda M. Effects of human interleukin-18 and interleukin-12 treatment on human lymphocyte engraftment in NOD-scid mouse. Immunology. 2002;107:232–42. doi: 10.1046/j.1365-2567.2002.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy WJ, Durum SK, Anver M, Frazier M, Longo DL. Recombinant human growth hormone promotes human lymphocyte engraftment in immunodeficient mice and results in an increased incidence of human Epstein Barr virus-induced B-cell lymphoma. Brain Behav Immun. 1992;6:355–64. doi: 10.1016/0889-1591(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 65.Sun R, Zhang J, Zhang C, Zhang J, Liang S, Sun A, Wang J, Tian Z. Human prolactin improves engraftment and reconsti-tution of human peripheral blood lymphocytes in SCID mice. Cell Mol Immunol. 2004;1:129–36. [PubMed] [Google Scholar]
- 66.Robert-Richard E, Ged C, Ortet J, Santarelli X, Lamrissi-Garcia I, De Verneuil H, Mazurier F. Human cell engraftment after busulfan or irradiation conditioning of NOD/SCID mice. Haematologica. 2006;91:1384. [PubMed] [Google Scholar]
- 67.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, Bonyhadi ML, Berenson RJ, Prior JL, Piwnica-Worms D, Nolta JA, Dipersio JF. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2m(null) mice. Exp Hematol. 2007;35:1823–38. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice AM, Wood JA, Milross CG, Collins CJ, McCarthy NF, Vowels MR. Conditions that enable human hematopoietic stem cell engraftment in all NOD-SCID mice. Transplantation. 2000;69:927–35. doi: 10.1097/00007890-200003150-00044. [DOI] [PubMed] [Google Scholar]
- 69.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tary-Lehmann M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J Exp Med. 1992;175:503–16. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Depraetere S, Verhoye L, Leclercq G, Leroux-Roels G. Human B cell growth and differentiation in the spleen of immunodeficient mice. J Immunol. 2001;166:2929–36. doi: 10.4049/jimmunol.166.5.2929. [DOI] [PubMed] [Google Scholar]
- 72.Martino G, Anastasi J, Feng J, Mc Shan C, DeGroot L, Quintans J, Grimaldi LM. The fate of human peripheral blood lymphocytes after transplantation into SCID mice. Eur J Immunol. 1993;23:1023–8. doi: 10.1002/eji.1830230506. [DOI] [PubMed] [Google Scholar]
- 73.Tournoy KG, Depraetere S, Pauwels RA, Leroux-Roels GG. Mouse strain and conditioning regimen determine survival and function of human leucocytes in immunodeficient mice. Clin Exp Immunol. 2000;119:231–9. doi: 10.1046/j.1365-2249.2000.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinsvik TE, Gaarder PI, Aaberge IS, Lovik M. Engraftment and humoral immunity in SCID and RAG-2-deficient mice transplanted with human peripheral blood lymphocytes. Scand J Immunol. 1995;42:607–16. doi: 10.1111/j.1365-3083.1995.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 75.Shibata S, Asano T, Noguchi A, Naito M, Ogura A, Doi K. Peritoneal macrophages play an important role in eliminating human cells from severe combined immunodeficient mice transplanted with human peripheral blood lymphocytes. Immunology. 1998;93:524–32. doi: 10.1046/j.1365-2567.1998.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Friedman T, Shimizu A, Smith RN, Colvin RB, Seebach JD, Sachs DH, Iacomini J. Human CD4+ T cells mediate rejection of porcine xenografts. J Immunol. 1999;162:5256–62. [PubMed] [Google Scholar]
- 77.Kaneshima H, Shih CC, Namikawa R, Rabin L, Outzen H, Machado SG, McCune JM. Human immunodeficiency virus infection of human lymph nodes in the SCID-hu mouse. Proc Natl Acad Sci USA. 1991;88:4523–7. doi: 10.1073/pnas.88.10.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–6. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 79.Hoffmann-Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood. 1993;81:3440–8. [PubMed] [Google Scholar]
- 80.Shpitz B, Chambers CA, Singhal AB, Hozumi N, Fernandes BJ, Roifman CM, Weiner LM, Roder JC, Gallinger S. High level functional engraftment of severe combined immunodeficient mice with human peripheral blood lymphocytes following pretreatment with radiation and anti-asialo GM1. J Immunol Methods. 1994;169:1–15. doi: 10.1016/0022-1759(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 81.Barry TS, Jones DM, Richter CB, Haynes BF. Successful engraftment of human postnatal thymus in severe combined immune deficient (SCID) mice: differential engraftment of thymic components with irradiation versus anti-asialo GM-1 immunosuppressive regimens. J Exp Med. 1991;173:167–80. doi: 10.1084/jem.173.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delhem N, Hadida F, Gorochov G, Carpentier F, De Cavel JP, Andreani JF, Autran B, Cesbron JY. Primary Th1 cell immunization against HIVgp160 in SCID-hu mice coengrafted with peripheral blood lymphocytes and skin. J Immunol. 1998;161:2060–9. [PubMed] [Google Scholar]
- 83.Mazingue C, Cottrez F, Auriault C, Cesbron JY, Capron A. Obtention of a human primary humoral response against schistosome protective antigens in severe combined immunodeficiency mice after the transfer of human peripheral blood mononuclear cells. Eur J Immunol. 1991;21:1763–6. doi: 10.1002/eji.1830210728. [DOI] [PubMed] [Google Scholar]
- 84.Sandhu J, Shpitz B, Gallinger S, Hozumi N. Human primary immune response in SCID mice engrafted with human peripheral blood lymphocytes. J Immunol. 1994;152:3806–13. [PubMed] [Google Scholar]
- 85.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994;180:1817–27. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy WJ, Bennett M, Anver MR, Baseler M, Longo DL. Human-mouse lymphoid chimeras: host-vs.-graft and graft-vs.-host reactions. Eur J Immunol. 1992;22:1421–7. doi: 10.1002/eji.1830220614. [DOI] [PubMed] [Google Scholar]
- 87.Bankert RB, Umemoto T, Sugiyama Y, Chen FA, Repasky E, Yokota S. Human lung tumors, patients’ peripheral blood lymphocytes and tumor infiltrating lymphocytes propagated in SCID mice. Curr Top Microbiol Immunol. 1989;152:201–10. doi: 10.1007/978-3-642-74974-2_24. [DOI] [PubMed] [Google Scholar]
- 88.Kyoizumi S, Baum CM, Kaneshima H, McCune JM, Yee EJ, Namikawa R. Implantation and maintenance of functional human bone marrow in SCID-hu mice. Blood. 1992;79:1704–11. [PubMed] [Google Scholar]
- 89.Roncarolo MG, Carballido JM, Rouleau M, Namikawa R, De Vries JE. Human T-and B-cell functions in SCID-hu mice. Semin Immunol. 1996;8:207–13. doi: 10.1006/smim.1996.0026. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, Shiozawa Y, Dewan MZ, Yu Z, Ito M, Morio T, Shimizu N, Honda M, Yamamoto N. Hematopoietic stem cell-engrafted NOD/SCID/IL2R gamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–8. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- 91.Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, Nakahata T. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–80. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 92.Matsumura T, Kametani Y, Ando K, Hirano Y, Katano I, Ito R, Shiina M, Tsukamoto H, Saito Y, Tokuda Y, Kato S, Ito M, Motoyoshi K, Habu S. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31:789–97. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 93.Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, Kato S, Hotta T. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol. 2002;169:204–9. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 94.Schoeberlein A, Schatt S, Troeger C, Surbek D, Holzgreve W, Hahn S. Engraftment kinetics of human cord blood and murine fetal liver stem cells following in utero transplantation into immunodeficient mice. Stem Cells Dev. 2004;13:677–84. doi: 10.1089/scd.2004.13.677. [DOI] [PubMed] [Google Scholar]
- 95.Turner CW, Archer DR, Wong J, Yeager AM, Fleming WH. In utero transplantation of human fetal haemopoietic cells in NOD/SCID mice. Br J Haematol. 1998;103:326–34. doi: 10.1046/j.1365-2141.1998.01003.x. [DOI] [PubMed] [Google Scholar]
- 96.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 97.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–63. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 99.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–8. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krowka JF, Sarin S, Namikawa R, McCune JM, Kaneshima H. Human T cells in the SCID-hu mouse are phenotypically normal and functionally competent. J Immunol. 1991;146:3751–6. [PubMed] [Google Scholar]
- 101.Vandekerckhove BA, Baccala R, Jones D, Kono DH, Theofilopoulos AN, Roncarolo MG. Thymic selection of the human T cell receptor V beta repertoire in SCID-hu mice. J Exp Med. 1992;176:1619–24. doi: 10.1084/jem.176.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vandekerckhove BA, Krowka JF, McCune JM, De Vries JE, Spits H, Roncarolo MG. Clonal analysis of the peripheral T cell compartment of the SCID-hu mouse. J Immunol. 1991;146:4173–9. [PubMed] [Google Scholar]
- 103.Kollmann TR, Pettoello-Mantovani M, Zhuang X, Kim A, Hachamovitch M, Smarnworawong P, Rubinstein A, Goldstein H. Disseminated human immunodeficiency virus 1 (HIV-1) infection in SCID-hu mice after peripheral inoculation with HIV-1. J Exp Med. 1994;179:513–22. doi: 10.1084/jem.179.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carballido JM, Schols D, Namikawa R, Zurawski S, Zurawski G, Roncarolo MG, De Vries JE. IL-4 induces human B cell maturation and IgE synthesis in SCID-hu mice. Inhibition of ongoing IgE production by in vivo treatment with an IL-4/IL-13 receptor antagonist. J Immunol. 1995;155:4162–70. [PubMed] [Google Scholar]
- 105.Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL., Jr Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–7. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marshall AJ, Denkers EY. Toxoplasma gondii triggers granulocyte-dependent cytokine-mediated lethal shock in D-galactosamine-sensitized mice. Infect Immun. 1998;66:1325–33. doi: 10.1128/iai.66.4.1325-1333.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Noort WA, Wilpshaar J, Hertogh CD, Rad M, Lurvink EG, Van Luxemburg-Heijs SA, Zwinderman K, Verwey RA, Willemze R, Falkenburg JH. Similar myeloid recovery despite superior overall engraftment in NOD/SCID mice after transplantation of human CD34(+) cells from umbilical cord blood as compared to adult sources. Bone Marrow Transplant. 2001;28:163–71. doi: 10.1038/sj.bmt.1703120. [DOI] [PubMed] [Google Scholar]
- 109.Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci USA. 2002;99:413–8. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–73. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 111.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 112.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vandekerckhove BA, Barcena A, Schols D, Mohan-Peterson S, Spits H, Roncarolo MG. In vivo cytokine expression in the thymus. CD3high human thymocytes are activated and already functionally differentiated in helper and cytotoxic cells. J Immunol. 1994;152:1738–43. [PubMed] [Google Scholar]
- 114.Cravens PD, Melkus MW, Padgett-Thomas A, Islas-Ohlmayer M, Del PMM, Garcia JV. Development and activation of human dendritic cells in vivo in a xenograft model of human hematopoiesis. Stem Cells. 2005;23:264–78. doi: 10.1634/stemcells.2004-0116. [DOI] [PubMed] [Google Scholar]
- 115.Elkon KB, Ashany D. Autoimmunity versus allo- and xeno-reactivity in SCID mice. Int Rev Immunol. 1994;11:283–93. doi: 10.3109/08830189409051175. [DOI] [PubMed] [Google Scholar]
- 116.Juhasz I, Lazarus GS, Murphy GF, Shih IM, Herlyn M. Development of pemphigus vulgaris-like lesions in severe combined immunodeficiency disease mice reconstituted with lymphocytes from patients. J Clin Invest. 1993;92:2401–7. doi: 10.1172/JCI116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kollmann TR, Pettoello-Mantovani M, Katopodis NF, Hachamovitch M, Rubinstein A, Kim A, Goldstein H. Inhibition of acute in vivo human immunodeficiency virus infection by human interleukin 10 treatment of SCID mice implanted with human fetal thymus and liver. Proc Natl Acad Sci USA. 1996;93:3126–31. doi: 10.1073/pnas.93.7.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Withers-Ward ES, Amado RG, Koka PS, Jamieson BD, Kaplan AH, Chen IS, Zack JA. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–9. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 119.Mosier DE, Gulizia RJ, MacIsaac PD, Torbett BE, Levy JA. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–92. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 120.Steyaert S, Verhoye L, Beirnaert E, Donners H, Fransen K, Heyndrickx L, Vanham G, Leroux-Roels G, Vanlandschoot P. The intraspleen huPBL NOD/SCID model to study the human HIV-specific antibody response selected in the course of natural infection. J Immunol Methods. 2007;320:49–57. doi: 10.1016/j.jim.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune JM. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 122.Kraft DL, Weissman IL, Waller EK. Differentiation of CD3-4-8- human fetal thymocytes in vivo: characterization of a CD3–4 + 8- intermediate. J Exp Med. 1993;178:265–77. doi: 10.1084/jem.178.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galy A, Verma S, Barcena A, Spits H. Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymic development. J Exp Med. 1993;178:391–401. doi: 10.1084/jem.178.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck RF. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci USA. 2006;103:15951–6. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109:2978–81. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCune JM, Namikawa R, Shih CC, Rabin L, Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247:564–6. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- 127.Rabin L, Hincenbergs M, Moreno MB, Warren S, Linquist V, Datema R, Charpiot B, Seifert J, Kaneshima H, McCune JM. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob Agents Chemother. 1996;40:755–62. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Datema R, Rabin L, Hincenbergs M, Moreno MB, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune JM. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–4. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol. 2005;78:1198–203. doi: 10.1189/jlb.0405219. [DOI] [PubMed] [Google Scholar]
- 130.Vieillard V, Jouveshomme S, Leflour N, Jean-Pierre E, Debre P, De Maeyer E, Autran B. Transfer of human CD4(+) T lymphocytes producing beta interferon in Hu-PBL-SCID mice controls human immunodeficiency virus infection. J Virol. 1999;73:10281–8. doi: 10.1128/jvi.73.12.10281-10288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drosten C, Gunther S, Preiser W, Van Der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 132.Okada M, Okuno Y, Hashimoto S, Kita Y, Kanamaru N, Nishida Y, Tsunai Y, Inoue R, Nakatani H, Fukamizu R, Namie Y, Yamada J, Takao K, Asai R, Asaki R, Kase T, Takemoto Y, Yoshida S, Peiris JS, Chen PJ, Yamamoto N, Nomura T, Ishida I, Morikawa S, Tashiro M, Sakatani M. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine. 2007;25:3038–40. doi: 10.1016/j.vaccine.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ku CC, Besser J, Abendroth A, Grose C, Arvin AM. Varicella-Zoster virus pathogenesis and immunobiology: new concepts emerging from investigations with the SCIDhu mouse model. J Virol. 2005;79:2651–8. doi: 10.1128/JVI.79.5.2651-2658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moffat JF, Stein MD, Kaneshima H, Arvin AM. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–42. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baiker A, Bagowski C, Ito H, Sommer M, Zerboni L, Fabel K, Hay J, Ruyechan W, Arvin AM. The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J Virol. 2004;78:1181–94. doi: 10.1128/JVI.78.3.1181-1194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sato B, Ito H, Hinchliffe S, Sommer MH, Zerboni L, Arvin AM. Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse in vivo. J Virol. 2003;77:5607–20. doi: 10.1128/JVI.77.10.5607-5620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(-/-) gamma(c)(-/-) (RAG-hu) mice. Virology. 2007;369:143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–9. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lim WH, Kireta S, Russ GR, Coates PT. Human plasmacytoid dendritic cells regulate immune responses to Epstein-Barr virus (EBV) infection and delay EBV-related mortality in humanized NOD-SCID mice. Blood. 2007;109:1043–50. doi: 10.1182/blood-2005-12-024802. [DOI] [PubMed] [Google Scholar]
- 141.Palucka AK, Gatlin J, Blanck JP, Melkus MW, Clayton S, Ueno H, Kraus ET, Cravens P, Bennett L, Padgett-Thomas A, Marches F, Islas-Ohlmayer M, Garcia JV, Banchereau J. Human dendritic cell subsets in NOD/SCID mice engrafted with CD34+ hematopoietic progenitors. Blood. 2003;102:3302–10. doi: 10.1182/blood-2003-02-0384. [DOI] [PubMed] [Google Scholar]
- 142.McKane BW, Ramachandran S, Yang J, Xu XC, Mohanakumar T. Xenoreactive anti-Galalpha(1, 3)Gal antibodies prevent porcine endogenous retrovirus infection of human in vivo. Hum Immunol. 2003;64:708–17. doi: 10.1016/s0198-8859(03)00081-8. [DOI] [PubMed] [Google Scholar]
- 143.Van Der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, Salomon DR. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature. 2000;407:90–4. doi: 10.1038/35024089. [DOI] [PubMed] [Google Scholar]
- 144.Kuddus RH, Metes DM, Nalesnik MA, Logar AJ, Rao AS, Fung JJ. Porcine cell microchimerism but lack of productive porcine endogenous retrovirus (PERV) infection in naive and humanized SCID-beige mice treated with porcine peripheral blood mononuclear cells. Transpl Immunol. 2004;13:15–24. doi: 10.1016/j.trim.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 145.Irgang M, Karlas A, Laue C, Specke V, Tacke SJ, Kurth R, Schrezenmeir J, Denner J. Porcine endogenous retroviruses PERV-A and PERV-B infect neither mouse cells in vitro nor SCID mice in vivo. Intervirology. 2005;48:167–73. doi: 10.1159/000081745. [DOI] [PubMed] [Google Scholar]
- 146.Yang YG, Wood JC, Lan P, Wilkinson RA, Sykes M, Fishman JA, Patience C. Mouse retrovirus mediates porcine endogenous retrovirus transmission into human cells in long-term human-porcine chimeric mice. J Clin Invest. 2004;114:695–700. doi: 10.1172/JCI21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Banuelos SJ, Shultz LD, Greiner DL, Burzenski LM, Gott B, Lyons BL, Rossini AA, Appel MC. Rejection of human islets and human HLA-A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1(null)Prf1(null) mice. Clin Immunol. 2004;112:273–83. doi: 10.1016/j.clim.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 148.Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003;25:167–80. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 149.Rayner D, Nelson R, Murray AG. Noncytolytic human lymphocytes injure dermal microvessels in the huPBL-SCID skin graft model. Hum Immunol. 2001;62:598–606. doi: 10.1016/s0198-8859(01)00252-x. [DOI] [PubMed] [Google Scholar]
- 150.Murray AG, Petzelbauer P, Hughes CC, Costa J, Askenase P, Pober JS. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc Natl Acad Sci USA. 1994;91:9146–50. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Petzelbauer P, Groger M, Kunstfeld R, Petzelbauer E, Wolff K. Human delayedtype hypersensitivity reaction in a SCID mouse engrafted with human T cells and autologous skin. J Invest Dermatol. 1996;107:576–81. doi: 10.1111/1523-1747.ep12582823. [DOI] [PubMed] [Google Scholar]
- 152.Xu Q, Lee J, Jankowska-Gan E, Schultz J, Roenneburg DA, Haynes LD, Kusaka S, Sollinger HW, Knechtle SJ, VanBuskirk AM, Torrealba JR, Burlingham WJ. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178:3983–95. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 153.Vandekerckhove BA, Namikawa R, Bacchetta R, Roncarolo MG. Human hematopoietic cells and thymic epithelial cells induce tolerance viadifferent mechanisms in the SCID-hu mouse thymus. J Exp Med. 1992;175:1033–43. doi: 10.1084/jem.175.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schols D, Vandekerckhove B, Jones D, Roncarolo MG. IL-2 reverses human T cell unresponsiveness induced by thymic epithelium in SCID-hu mice. J Immunol. 1994;152:2198–206. [PubMed] [Google Scholar]
- 155.Mutis T, Van Rijn RS, Simonetti ER, Aarts-Riemens T, Emmelot ME, Van Bloois L, Martens A, Verdonck LF, Ebeling SB. Human regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2-/-gammac-/-immunodeficient mice. Clin Cancer Res. 2006;12:5520–5. doi: 10.1158/1078-0432.CCR-06-0035. [DOI] [PubMed] [Google Scholar]
- 156.Kirkiles-Smith NC, Tereb DA, Kim RW, McNiff JM, Schechner JS, Lorber MI, Pober JS, Tellides G. Human TNF can induce nonspecific inflammatory and human immune-mediated microvascular injury of pig skin xenografts in immunodeficient mouse hosts. J Immunol. 2000;164:6601–9. doi: 10.4049/jimmunol.164.12.6601. [DOI] [PubMed] [Google Scholar]
- 157.Sultan P, Murray AG, McNiff JM, Lorber MI, Askenase PW, Bothwell AL, Pober JS. Pig but not human interferon-gamma initiates human cell-mediated rejection of pig tissue in vivo. Proc Natl Acad Sci USA. 1997;94:8767–72. doi: 10.1073/pnas.94.16.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tereb DA, Kirkiles-Smith NC, Kim RW, Wang Y, Rudic RD, Schechner JS, Lorber MI, Bothwell AL, Pober JS, Tellides G. Human T cells infiltrate and injure pig coronary artery grafts with activated but not quiescent endothelium in immunodeficient mouse hosts. Transplantation. 2001;71:1622–30. doi: 10.1097/00007890-200106150-00023. [DOI] [PubMed] [Google Scholar]
- 159.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162:3402–7. [PubMed] [Google Scholar]
- 160.Lan P, Wang L, Diouf B, Eguchi H, Su H, Bronson R, Sachs DH, Sykes M, Yang YG. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964–9. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 161.Lechler RI, Garden OA, Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003;3:147–58. doi: 10.1038/nri1002. [DOI] [PubMed] [Google Scholar]
- 162.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–24. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 163.Reddy S, Piccione D, Takita H, Bankert RB. Human lung tumor growth established in the lung and subcutaneous tissue of mice with severe combined immunodeficiency. Cancer Res. 1987;47:2456–60. [PubMed] [Google Scholar]
- 164.Hudson WA, Li Q, Le C, Kersey JH. Xenotransplantation of human lymphoid malignancies is optimized in mice with multiple immunologic defects. Leukemia. 1998;12:2029–33. doi: 10.1038/sj.leu.2401236. [DOI] [PubMed] [Google Scholar]
- 165.O’Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–92. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 166.Siegler U, Kalberer CP, Nowbakht P, Sendelov S, Meyer-Monard S, WodnarFilipowicz A. Activated natural killer cells from patients with acute myeloid leukemia are cytotoxic against autologous leukemic blasts in NOD/SCID mice. Leukemia. 2005;19:2215–22. doi: 10.1038/sj.leu.2403985. [DOI] [PubMed] [Google Scholar]
- 167.Flavell DJ, Warnes SL, Bryson CJ, Field SA, Noss AL, Packham G, Flavell SU. The anti-CD20 antibody rituximab augments the immunospecific therapeutic effectiveness of an anti-CD19 immunotoxin directed against human B-cell lymphoma. Br J Haematol. 2006;134:157–70. doi: 10.1111/j.1365-2141.2006.06155.x. [DOI] [PubMed] [Google Scholar]
- 168.Trieu Y, Wen XY, Skinnider BF, Bray MR, Li Z, Claudio JO, Masih-Khan E, Zhu YX, Trudel S, McCart JA, Mak TW, Stewart AK. Soluble interleukin-13Ralpha2 decoy receptor inhibits Hodgkin’s lymphoma growth in vitro and in vivo. Cancer Res. 2004;64:3271–5. doi: 10.1158/0008-5472.can-03-3764. [DOI] [PubMed] [Google Scholar]
- 169.Watanabe M, Dewan MZ, Okamura T, Sasaki M, Itoh K, Higashihara M, Mizoguchi H, Honda M, Sata T, Watanabe T, Yamamoto N, Umezawa K, Horie R. A novel NF-kappaB inhibitor DHMEQ selectively targets constitutive NF-kappaB activity and induces apoptosis of multiple myeloma cells in vitro and in vivo. Int J Cancer. 2005;114:32–8. doi: 10.1002/ijc.20688. [DOI] [PubMed] [Google Scholar]
- 170.Dewan MZ, Terashima K, Taruishi M, Hasegawa H, Ito M, Tanaka Y, Mori N, Sata T, Koyanagi Y, Maeda M, Kubuki Y, Okayama A, Fujii M, Yamamoto N. Rapid tumor formation of human T-cell leukemia virus type 1-infected cell lines in novel NOD-SCID/gammac(null) mice: suppression by an inhibitor against NF-kappaB. J Virol. 2003;77:5286–94. doi: 10.1128/JVI.77.9.5286-5294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bentzien F, Struman I, Martini JF, Martial J, Weiner R. Expression of the antiangiogenic factor 16K hPRL in human HCT116 colon cancer cells inhibits tumor growth in Rag1(-/-) mice. Cancer Res. 2001;61:7356–62. [PubMed] [Google Scholar]
- 172.Iwanuma Y, Chen FA, Egilmez NK, Takita H, Bankert RB. Antitumor immune response of human peripheral blood lymphocytes coengrafted with tumor into severe combined immunodeficient mice. Cancer Res. 1997;57:2937–42. [PubMed] [Google Scholar]
- 173.Tanaka F, Abe M, Akiyoshi T, Nomura T, Sugimachi K, Kishimoto T, Suzuki T, Okada M. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res. 1997;57:1335–43. [PubMed] [Google Scholar]
- 174.Parney IF, Petruk KC, Zhang C, Farr-Jones M, Sykes DB, Chang LJ. Granulocyte-macrophage colony-stimulating factor and B7-2 combination immunogene therapy in an allogeneic Hu-PBL-SCID/beige mousehuman glioblastoma multiforme model. Hum Gene Ther. 1997;8:1073–85. doi: 10.1089/hum.1997.8.9-1073. [DOI] [PubMed] [Google Scholar]
- 175.Egilmez NK, Jong YS, Hess SD, Jacob JS, Mathiowitz E, Bankert RB. Cytokines delivered by biodegradable microspheres promote effective suppression of human tumors by human peripheral blood lymphocytes in the SCID-Winn model. J Immunother. 2000;23:190–5. doi: 10.1097/00002371-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 176.Lozupone F, Luciani F, Venditti M, Rivoltini L, Pupa S, Parmiani G, Belardelli F, Fais S. Murine granulocytes control human tumor growth in SCID mice. Int J Cancer. 2000;87:569–73. doi: 10.1002/1097-0215(20000815)87:4<569::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 177.Bombil F, Latinne D, Kints JP, Bazin H. Human recombinant interleukin-4 (HurIL-4) improves SCID mouse reconstitution with human peripheral blood lymphocytes. Immunobiology. 1996;196:437–48. doi: 10.1016/S0171-2985(96)80065-9. [DOI] [PubMed] [Google Scholar]


