Abstract
The aortic ring model has become one of the most widely used methods to study angiogenesis and its mechanisms. Many factors have contributed to its popularity including reproducibility, cost effectiveness, ease of use and good correlation with in vivo studies. In this system aortic rings embedded in biomatrix gels and cultured under chemically defined conditions generate arborizing vascular outgrowths which can be stimulated or inhibited with angiogenic regulators. Originally based on the rat aorta, the aortic ring model was later adapted to the mouse for the evaluation of specific molecular alterations in genetically modified animals. Viral transduction of the aortic rings has enabled investigators to overexpress genes of interest in the aortic cultures. Experiments on angiogenic mechanisms have demonstrated that formation of neovessels in aortic cultures is regulated by macrophages, pericytes and fibroblasts through a complex molecular cascade involving growth factors, inflammatory cytokines, axonal guidance cues, extracellular matrix (ECM) molecules and matrix-degrading proteolytic enzymes. These studies have shown that endothelial sprouting can be effectively blocked by depleting the aortic explants of macrophages or by interfering with the angiogenic cascade at multiple levels including growth factor signalling, cell adhesion and proteolytic degradation of the ECM. In this paper, we review the literature in this field and retrace the journey from our first morphological descriptions of the aortic outgrowths to the latest breakthroughs in the cellular and molecular regulation of aortic vessel growth and regression.
Keywords: neovascularization, endothelial cells, mural cells, angiogenic factors, integrins, proteolytic enzymes, fibronectin, laminin, collagen, fibrin
-
Introduction
Early history of the aortic ring model
Morphology of the angiogenic response
Source of microvessels in aortic cultures
Stimulatory effect of angiogenesis on cancer spread
Regulation of angiogenesis by the extracellular matrix
Role of integrin receptors in aortic angiogenesis
Paracrine regulation of angiogenesis: role of endogenous growth factors
Paracrine regulation of angiogenesis: role of resident macrophages and inflammatory cytokines and chemokines
Expression and function of neural related genes
Signal transduction pathways
Pericyte origin and recruitment mechanisms
Role of proteolytic enzymes in angiogenesis and vascular regression
Influence of genetic background and aging on angiogenic response
Adaptation of the aortic ring model to different species and vessel types
Limitations of the aortic ring assay
Summary and conclusion
Introduction
Angiogenesis, the growth of new blood vessels, plays a critical role in the progression of many diseases [32, 63]. Studies conducted during the past four decades have identified key mechanisms of the angiogenic process, leading to the development of potent anti-angiogenic drugs. This field has come of age with the recognition of anti-angiogenic therapy as a new modality to treat cancer and wet age related macular degeneration of the retina [61]. Instrumental in the attainment of this milestone have been the many experimental models developed to study the mechanisms of the angiogenic process and test the efficacy of anti-angiogenic drugs [14]. In vivo models such as the corneal micropocket and the chorioallantoic membrane of the chick embryo have provided invaluable information on the growth of blood vessels in the complex setting of the live animal [179, 194]. In vitro models with isolated endothelial cells have enabled investigators to further analyse mechanisms of angiogenesis in the simplified environment of the culture dish [77].
One of the most commonly used assays of angiogenesis is the aortic ring model. This model is based on the capacity of rat or mouse aortic explants to form new vessels in gels of collagen, fibrin or basement membrane [11]. The angiogenic outgrowth produced by the aortic rings consists of a mixed population of native cells that interact through paracrine mechanisms under chemically defined culture conditions. As such the aortic ring model bridges the gap between in vivo and in vitro models of angiogenesis, combining advantages of both systems. The purpose of this paper is to review the literature on this unique model and illustrate how the use of aortic cultures has contributed to our current understanding of the angiogenic process and the development of novel anti-angiogenic drugs.
Early history of the aortic ring model
The original observation that aortic rings have the capacity to produce microvessels in vitro dates back to the early 1980s when I was working in the research laboratory of Joseph Leighton at the Medical College of Pennsylvania in Philadelphia [170]. Leighton (Fig. 1), whose primary interest was cancer, was attempting to reproduce in vitro physiological gradients of oxygen and nutrient diffusion and had developed ingenious models for the three-dimensional growth of cancer cells. I was interested in the biology of blood vessels, a passion I had developed in medical school while working with an animal model of arterial injury and repair [213]. Intrigued by my fondness for endothelial cells, Leighton introduced me to the old tissue culture literature that described formation of capillaries in vitro. As I reviewed these pioneering papers, I learned that Warren Lewis in the 1930s had carefully documented formation of blood vessels in plasma clot cultures of chick embryo skin explants [133]. Leighton himself had observed capillary sprouting from different tissue explants but had never published these observations. He had long been interested in the mechanisms by which tumour aggregates replicate and spread, and suspected that the tumour stroma was involved in this process [127, 128]. My request to work in a vascular biology project rekindled Leighton’s interest in this area, and led to our first project in the field of angiogenesis.
Fig 1.

Joseph Leighton, physician and cancer researcher (1921–1999).
Among the tissues potentially capable of an angiogenic response we chose the rat aorta which Leighton had already cultured in the past, without ever characterizing the nature of its outgrowth. To my great excitement, rings of rat aorta embedded in plasma clot and cultured in medium supplemented with foetal bovine serum generated a florid angiogenic response [170]. This model proved to be ideal to study the influence of the fibrovascular stroma on the growth and spread of cancer cells.
In the meantime Judah Folkman’s hypothesis that tumour growth is angiogenesis dependent, originally formulated in 1971 [62], was beginning to take hold and an increasing number of investigators were joining this field. Folkman’s group successfully isolated microvascular endothelial cells from bovine adrenal glands and demonstrated that these cells were capable of forming capillary tubes in vitro[64]. Shortly thereafter, Shing, Folkman and Klagsbrun reported the isolation and purification of the first tumour angiogenic factor from a chondrosarcoma [209]. This molecule turned out to be basic fibroblast growth factor (bFGF) which Denis Gospodarowicz had purified earlier from the bovine pituitary gland [78]. Thus, the birth of the aortic ring model occurred at a very propitious time when the angiogenesis field was entering the age of molecular biology and new discoveries were creating a need for assays that could characterize the activity, potency and mechanism of action of angiogenic regulators.
Morphology of the angiogenic response
We originally cultured aortic rings in plasma clots with serum-supplemented growth medium [170]. Clots were obtained by mixing the supernatant of homogenized chick embryos with a chick plasma solution. In later studies we grew aortic rings under serum-free conditions in gels of purified bovine or human fibrin or rat interstitial collagen [34, 70, 168, 174]. The elimination of serum reduced the number of non-endothelial mesenchymal cells, greatly facilitating the observation and measurement of the angiogenic outgrowths [168]. The development of a whole mount staining method further simplified the morphological analysis of the cultures, obviating the need for the labour-intensive techniques initially used to prepare histological sections [245]. The following description reflects our current understanding of how vessels develop and are remodelled in aortic cultures. This knowledge is based on studies conducted over time in our laboratory and by others with constantly improving methodologies.
Aortic cultures are composed of a mixed population of endothelial cells, pericytes, fibroblasts, macrophages and dendritic cells (Fig. 2). The first cells to migrate out of the aortic rings after 1–2 days are fibroblasts and macrophages. Fibroblasts appear as spindly or tripolar cells with finely tapered cytoplasmic processes. Macrophages are characteristically rounded and have a granular and vacuolated cytoplasm with short pseudopods. Both macrophages and fibroblasts originate from the adventitia. Macrophages accumulate at the edges of resection where the aortic wall has been transected for the preparation of the rings ([70] and unpublished observations).
Fig 2.
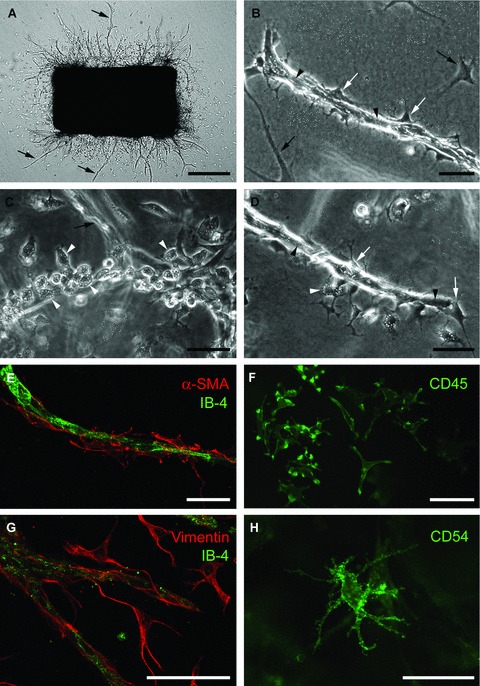
Aortic ring model of angiogenesis: cellular composition of angiogenic outgrowths. (A) Serum-free collagen gel culture of rat aorta photographed at day 6 (microvessels marked by arrows). (B) Microvessel composed of an inner core of endothelial cells (arrowheads) and surrounding pericytes (white arrows); black arrows indicate fibroblasts. (C) Linear cluster of macrophages (white arrowheads), with characteristically vacuolated and granular cytoplasm, at the root of an out-of-focus endothelial sprout (arrow). (D) Microvessel composed of endothelial cells (black arrowheads) and pericytes (white arrows) with surrounding macrophages (white arrowheads). (E) Confocal image of microvessel double stained with endothelial (isolectin-B4, IB-4) and pericyte (anti-α smooth muscle cell actin) markers. (F) Immunofluorescent image of macrophages stained for CD45. (G) Confocal image of aortic cultures double stained for endothelial cells (IB-4) and fibroblasts (vimentin). (H) Immunofluorescent image of dendritic cell stained for CD54 (ICAM). Magnification bars = 500 μm (A), 50 μm (B–H).
Endothelial sprouts first appear at the severed edges of the explants after 2–3 days of culture. They can be distinguished from fibroblasts by their thicker appearance and cohesive pattern of growth [168]. The neovessel tips are made of highly migratory cells which probe the surrounding matrix with filopodia-like processes. Stains for cell proliferation markers such as Ki-67 or phospho-histone demonstrate that endothelial tip cells migrate without dividing whereas trailing endothelial cells actively proliferate [73]. Endothelial cell proliferation is critical for optimal angiogenic responses but not necessary for minimal sprouting which can occur exclusively through endothelial migration when cultures are treated with mitomycin C, an inhibitor of cell mitosis [162, 170]. As the outgrowth expands and matures, vessels develop a visible lumen [168, 170]. Over time neovessels become surrounded by pericytes, which migrate and proliferate along the endothelium. Pericytes are readily recognized by their dendritic morphology and close association with the endothelium [174] (Fig. 2).
Neovessels express the endothelial markers von Willebrand factor (vWF), CD31 and Tie2 [169, 245], bind the Griffonia Simplicifolia isolectin-B4 [245], and take up DiI-acetylated LDL [208]. Pericytes are specifically highlighted by immunostaining the cultures for α-smooth muscle actin (α-SMA), desmin, calponin or the proteoglycan NG2 [98, 168, 245]. Fibroblasts stain for vimentin, which is also expressed in pericytes and endothelial cells [34]. Some fibroblasts also express α-SMA (myofibroblasts). Macrophages react for CD45, CD68 and CD163 [12, 70]. Dendritic cells stain for CD45 and CD54 (ICAM) (Fig. 2).
Electron microscopy shows that immature sprouts are composed of solid endothelial cords. Primitive lumens formed through an intercellular mechanism appear as slit-like spaces sealed by junctional complexes. Lumens also develop by coalescence of cytoplasmic vacuoles in adjacent endothelial cells [168, 170]. Endothelial cells rest on a thin basement membrane which is best visible in serum-supplemented cultures [170]. Endothelial organelles include mitochondria, free ribosomes, rough endoplasmic reticulum, Golgi complex, pinocytotic vesicles and occasional lysosomes. Weibel–Palade bodies are rarely observed. Pericytes have an electron dense cytoplasm and abundant rough endoplasmic reticulum. Pericyte cell processes are focally connected with the endothelium through cell junctions [168, 170].
After sprouting, branching and forming networks for approximately 1 week, vessels stop growing and begin to regress. This phenomenon is particularly pronounced in serum-free collagen gel cultures. As neovessels are reabsorbed, the gel inside the aortic lumen and around the explants lyses, causing formation of a periaortic halo. Matrix degradation also occurs at a much finer level around each microvessel, causing endothelial cells to detach from the underlying substrate and one another. The angiogenic outgrowth becomes fragmented and is gradually reabsorbed. The neovessel roots retract and are pulled back into the stalks of the thicker vessels. The combination of collagen lysis, vessel fragmentation and vessel retraction ultimately leads to complete vascular regression [241].
Source of microvessels in aortic cultures
During the early 1980s the capacity to form vascular tubes was thought to be a specific feature of microvascular endothelial cells and not large vessel endothelium. Our observation with the aortic ring model suggested that large vessel endothelial cells were capable of sprouting as well [168]. Working with an in vitro collagen invasion assay, Montesano and Orci observed formation of microvessels in cultures of human umbilical vein and calf pulmonary artery endothelial cells [150] as previously reported by the same group for microvascular endothelial cells [149]. Meanwhile Diglio et al. reported that selective killing of the adventitia with ethanol abrogated angiogenesis in aortic cultures, but noticed that some ethanol treated rings retained the capacity to sprout [47]. The angiogenic response of these rings was completely eliminated only by killing the intimal endothelium. These authors concluded that both aortic adventitia and intima were needed for the angiogenic response.
To evaluate the capacity of the aortic endothelium to form neovessels we studied the angiogenic behaviour of aortic tubes that had been turned inside out prior to collagen embedding. This method directly exposed intimal cells to collagen while blocking the outgrowth of adventitial cells. The intimal endothelial cells that survived the eversion procedure reorganized into microvessels and sprouted into the collagen gel [164]. Using a similar approach Mori et al. noticed that aortic endothelial sprouting occurred at sites of intimal injury [152]. We later observed that isolated rat aortic endothelial cells formed capillary networks when cultured between two layers of collagen, as reported for microvascular endothelial cells [151], and sprouted following stimulation with angiogenic factors [175]. Moreover rat carotid artery explants failed to generate an angiogenic response when completely de-endothelialized with a balloon catheter whereas control carotid arteries with an intact intimal endothelium produced microvessels from their ends of resection (unpublished observations in collaboration with Michael Reidy, University of Washington).
Taken together these studies indicated that the intimal endothelium of the aorta and its collaterals had full angioformative capacity and was a major contributor to the angiogenic outgrowth. We later learned that the aortic adventitia was equally important because it contained macrophages which are a critical source of angiogenic stimuli [70] (see section below on macrophages and cytokines/chemokines). Adventitial microvessels participated in the angiogenic response if they were still present following the aortic dissection, though the intimal endothelium appeared to be the primary source of neovessels [47, 168]. Vascular outgrowths might have also originated from endothelial progenitor cells, as described for the embryonal aorta [6]. Although we found no evidence of these cells in the adult aorta [98], it is possible that immature endothelial cells with angioformative properties are present in the aortic intima [103]. It is also reasonable to postulate that the migration of these cells into the collagen matrix is promoted by mechanical forces generated by fibroblasts, which characteristically precede the angiogenic outgrowth, as recently proposed for the formation of neovessels in granulation tissue [114]. Finally, it is likely that non-sprouting morphogenetic events comparable to cell assembly processes described in other model of angiogenesis contribute to the formation of vascular structures in aortic cultures [8, 224]. A process comparable to non-sprouting angiogenesis occurs at the root of the angiogenic outgrowths where the aortic intimal endothelium reorganizes into vascular channels following exposure of its apical surface to collagen [164].
Stimulatory effect of angiogenesis on cancer spread
Our initial work focused on the interactions between microvessels and cancer cells. For these studies we co-cultured rat aortic rings with the rat bladder carcinoma cell line NBT-II-81. Aggregates of cancer cells cultured in plasma clot grew slowly by expansion forming spheroids with smooth outlines and necrotic centres. Aortic rings placed at 300–500 μm from the aggregates gave rise to branching vessels. When the vessel outgrowth contacted the tumour aggregates, cancer cells invaded the perivascular spaces, disrupted the endothelium and used the denuded vascular tunnels to spread into the plasma clot [171]. Normal bladder epithelium instead formed cystic structures and did not invade after being contacted by the angiogenic outgrowths [129]. Studies with isolated endothelial cells showed that cancer cells preferentially adhered to the subendothelial matrix compared to plasma clot or gelatin. This suggested that favourable adhesive gradients generated by sprouting microvessels were promoting the spread of cancer in our model [172].
In the early 1960s Pietro Gullino had observed that when normal renal tissue was replaced by cancer in an experimental animal model, the blood flow fell to very low levels even though the tumour was growing and becoming richly vascularized. He concluded that there was no direct correlation between the tumour promoting effect of angiogenesis and blood flow [80, 81]. Our observations with the aortic ring model, which lacks blood flow, corroborated Gullino’s observations indicating that angiogenesis could stimulate tumour spread not only by providing oxygen and nutrients but also by secreting molecules that facilitated the growth and invasive behaviour of cancer cells. Studies by other investigators later confirmed the hypothesis that cancer and endothelial cells can reciprocally influence each other’s behaviour in a paracrine or juxtacrine manner [188].
Regulation of angiogenesis by the extracellular matrix
Our observation that the microvascular extracellular matrix (ECM) was involved in the spread of cancer cells was made at a time when ECM molecules were found to be key regulators of endothelial cell behaviour and capillary morphogenesis [66]. Intrigued by the adhesive properties of the endothelial ECM [172], we set out to study its composition in the aortic ring model. Immunohistochemical analysis of plasma clot cultures showed that immature endothelial sprouts were enveloped by fibronectin and type V collagen fibrils, and by patchy deposits of laminin and type IV collagen. As microvessels matured they became surrounded by abundant laminin and type IV collagen, interstitial collagens types I and III, and type V collagen [166]. Treatment with ascorbic acid enhanced the synthesis and perivascular accumulation of collagen. Microvessels formed in the absence of ascorbic acid became markedly dilated, whereas microvessels treated with ascorbic acid remained small throughout the angiogenic process [160]. Inhibition of perivascular collagen accumulation with cis-hydroxyproline, a proline analogue that interfered with collagen secretion, blocked the angiogenic response. This effect was reversed by supplementing the cultures with L-proline, which restored endogenous collagen synthesis and secretion [160].
To investigate the role of the ECM in angiogenesis, we cultured aortic rings in basement membrane-like gels or collagen gels supplemented with purified basement membrane molecules. Hynda Kleinman and coworkers had developed a method to obtain a basement membrane-like gel composed of laminin, type IV collagen, heparan sulphate and entactin from a mouse sarcoma cell line [117]. Using this matrix, which was later termed Matrigel, they were able to induce rapid reorganization of endothelial cells into networks of ‘tube-like’ structures [123]. We found that aortic rings cultured in Matrigel produced polygonal networks of highly branched endothelial cords. Luminal spaces and DNA synthesis were however significantly reduced in Matrigel compared to plasma clot, fibrin or collagen [169]. These observations suggested that premature exposure of aortic outgrowths to high concentrations of basement membrane components interfered with vessel growth and maturation which instead occurred unimpeded in interstitial collagen and fibrin gels.
To define the effect of individual basement membrane molecules in angiogenesis we cultured aortic rings in gels of interstitial collagen supplemented with fibronectin, laminin, entactin or type IV collagen. Each of these molecules caused a dose-dependent elongation of microvessels [26, 162, 163]. Fibronectin-treated microvessels grew longer through an increased migratory recruitment of endothelial cells. This effect did not require cell proliferation because it occurred also in cultures treated with mitomycin C, an inhibitor of cell mitosis [162]. Our results were consistent with earlier studies indicating that fibronectin promoted endothelial cell migration [7, 144] and that endothelial cell proliferation was not required for in vivo angiogenic sprouting [212].
In addition to promoting vascular elongation, type IV collagen and the laminin–entactin complex had a bimodal effect on vascular proliferation. Intermediate concentrations of these molecules stimulated angiogenesis whereas low concentrations were ineffective and high concentrations were anti-angiogenic [26, 163]. In keeping with these observations Kleinman’s group later identified 13 synthetic laminin peptides with pro-angiogenic effects in the aortic ring model [140]. Others demonstrated that oncothain, a peptide from the NC1 domain of type IV collagen inhibited aortic angiogenesis, probably through adhesive mechanisms [207].
The few vessels that formed in aortic cultures supplemented with high doses of type IV collagen or laminin–entactin were highly stable and survived longer than control microvessels [26, 163]. Because the high doses of type IV collagen and laminin–entactin needed to promote vessel survival were similar to the natural concentration of these molecules in vivo, our results strongly suggested that mature basement membranes provided stabilizing signals to microvessels.
Autoradiographic studies of aortic cultures labelled with 35SO4 showed that the microvascular ECM contained sulphated proteoglycans (unpublished observations). When examined by electron microscopy following Ruthenium red staining, the proteoglycan-rich glycocalyx of microvessels appeared as a fluffy and electron dense material that uniformly coated endothelial surfaces, except at sites of tight junctions [170]. The sulphated component of the subendothelial matrix was later found to contain collagen XVIII, a heparan sulphate-collagen hybrid molecule whose proteolytic degradation generates endostatin, an inhibitor of angiogenesis [148, 180]. Li and Olsen found that aortic rings from collagen XVIII deficient mice generated twice as many microvessels as control explants [134], unless the cultures were treated with recombinant endostatin which reduced angiogenesis to control levels. Collagen XVIII probably interfered with cell adhesion by competing with fibronectin for heparan sulphate groups on the cell surface. The increased adhesiveness of collagen XVIII-deficient endothelial cells to endogenous fibronectin seemingly promoted angiogenesis through enhanced vessel stabilization and reduced vascular regression [134]. The anti-angiogenic effect of endostatin was confirmed in a separate study in which mouse aortic rings were transduced with an adenoviral vector carrying the endostatin gene [85]. Inhibition of angiogenesis in aortic cultures was also obtained by overexpressing angiostatin, an anti-angiogenic molecule derived from proteolytic degradation of plasminogen [181].
DeanneLee and coworkers recently demonstrated that microvessels in aortic cultures express syndecan-1, a cell membrane protein that binds to ECM molecules via heparan sulphate proteoglycan side chains. Synstatin, a synthetic peptide that interferes with syndecan-1 function, inhibited bFGF- and VEGF-induced angiogenesis [18].
Role of integrin receptors in aortic angiogenesis
In 1987, Ruoslahti and Piershbacher identified the arginine–glycine–aspartic acid (RGD) amino acid sequence as the cell attachment domain of fibronectin [199]. The RGD motif was also found to mediate cell adhesion to vitronectin, fibrinogen and vWF [45]. RGD peptides interfered with cell adhesion by competing with natural RGD-containing molecules for cell receptor binding, causing detachment of endothelial cells [36]. Because sprouting endothelial cells produced RGD containing molecules [162], we hypothesized that angiogenesis could be an RGD-dependent process. Treatment of collagen gel cultures with a GRGDS peptide potently inhibited angiogenesis, whereas no effect was obtained with a GRGES control peptide. The effect of GRGDS was nontoxic and reversible because the angiogenic response of the aortic rings was restored following withdrawal of the drug [161]. The GRGDS peptide also induced vascular regression when added to the cultures after the angiogenic growth phase [162]. These observations implicated for the first time RGD sensitive integrin receptors as regulators of angiogenesis. These integrins probably mediated the attachment of endothelial cells not only to native fibronectin but also to degraded type I collagen and laminin whose cryptic RGD sequences could be unmasked by proteolysis [15, 39, 42].
Putative candidates of the RGD sensitive subfamily of integrin receptors included α5β1, α8β1, αVβ1, αVβ3, αVβ5, αVβ6 and αVβ8[102, 246]. Although progress was initially delayed by the limited availability of neutralizing reagents, knowledge of how integrins regulate angiogenesis in the aortic ring model has advanced significantly in recent years. Using double immunofluorescence microscopy to localize integrin receptors in specific cell types we found that endothelial cells and pericytes expressed both β1 and β3 integrins [34]. Angiogenesis in collagen was blocked with an anti-β1 antibody but not with an anti-β3 antibody. Likewise vascular regression was accelerated by blocking β1 but not β3 integrins. In keeping with these observations aortic rings from β3 deficient mice cultured in collagen or Matrigel produced the same angiogenic response as normal rings [39, 139, 193]. β3 integrins, however, were found to participate in angiogenesis under selected culture conditions. We observed that antibody blockade of both β1 and β3 integrins was needed to effectively inhibit aortic angiogenesis and promote vascular regression when aortic rings were embedded in fibrin gels [34]. Using a >600 fold higher concentration of anti-β3 integrin antibody (20 mg/ml) than the one tested in our study (30 μg/ml) Hayashi et al. were able to reduce by ∼50% angiogenesis in fibrin gel cultures [90]. Interestingly, aortic rings from mice expressing a mutated β3 integrin failed to form capillaries in response to VEGF, a key regulator of aortic angiogenesis (see below), or serum in Matrigel culture. The difference in angiogenic behaviour between β3 deficient aortic rings (normal angiogenesis, see above) and aortic rings with mutated β3 integrin (reduced angiogenesis), was attributed to the overexpression of VEGFR2 that occurred following β3 integrin ablation [192, 193]. Stimulation of VEGFR2 expression and angiogenesis was recently obtained also by treating VEGF-stimulated aortic cultures with low (nanomolar) concentrations of synthetic αVβ3/αVβ5 inhibitors [191].
A number of studies have attempted to identify the α partners of the β integrin receptors involved in aortic angiogenesis. Suda et al. observed that ‘migrating cells’ at the periphery of mouse foetal aortic outgrowths expressed α1 and α2 integrins whereas cells near the aortic explants were reactive for α3 integrins [217]. Using immunoelectron microscopy they were able to localize the α3 integrin to the membrane of periendothelial cells. Treatment with the synthetic peptide KDGEA, which competes with collagen for α2β1 integrin binding, significantly decreased cell migration. Zhang et al. found that aortic rings from α2 integrin deficient mice embedded in Matrigel produced the same number of vessels as control rings in response to VEGFA or VEGFC, but had a significantly enhanced angiogenic response to placental growth factor (PlGF) [238]. This paradoxical effect was attributed to the increased expression of the PlGF receptor VEGFR1 in the α2 null mice.
Trikha et al. reported that treatment of bFGF-stimulated collagen cultures of rat aorta with an antibody that bound all αV integrins reduced angiogenesis to ∼30% of control values [225]. Similarly Sassoli and coworkers obtained inhibition of angiogenesis in bFGF- and VEGF-stimulated collagen cultures with specific anti-αVβ3 and anti-αVβ5 antibodies [201]. These results suggest that bFGF and VEGF, which up-regulate expression of αVβ3 and αVβ5 integrins [51, 67], caused aortic microvessels to become dependent on these integrin subtypes [67].
Studies with isolated cells showed that the laminin integrins α3β1 and α6β1 promoted basement membrane assembly, pericyte association and tube stabilization [43] whereas the integrin α6β4 was involved in angiogenic sprouting [177]. These integrins closely associate with CD151, a tetraspanin family member that modulates endothelial cell spreading, migration and sprouting [93]. The angiogenic response of aortic rings from CD151 null mice in Matrigel was reduced by ∼50%. These changes correlated with significant defects in endothelial cell migration, spreading, sprouting and signalling, particularly on a laminin substrate [222].
Paracrine regulation of angiogenesis: role of endogenous growth factors
My first experiments with the aortic ring model [170, 171] had shown that the aortic wall produced neovessels in response to injury. The presence of serum in the growth medium was however a confounding variable to the analysis of this phenomenon. The same consideration applied to the plasma clot which we were using as a substrate for angiogenic sprouting [170, 171]. We serendipitously overcame this limitation in a collaborative project with George Tuszynski, who was studying the cancer promoting properties of thrombospondin-1 (TSP-1), a platelet-derived protein [228]. Tuszynski asked us to test the angiogenic activity of TSP-1, but serum which contained abundant TSP-1, could not be used for this study. We therefore decided to culture the aortic explants without serum in gels of purified collagen or fibrin.
The serum-free modification of the aortic ring model was made possible by the work of Richard Ham and Gordon Sato who had separately developed methods to optimize the composition of culture media for the growth of mammalian cells [87, 91]. Following their pioneering studies, serum-free growth media had been used to culture a variety of isolated cell types but no to study angiogenesis. For the TSP-1 experiments we decided to use MCDB131, a growth medium optimized for microvascular endothelial cells [118] which had become commercially available under the trade name of endothelial basal medium. To our surprise, control cultures without serum or TSP-1, produced an angiogenic outgrowth that we could score by counting the number of microvessels. For the first time we were able to reproduce angiogenesis ex vivo under chemically defined culture conditions [168]. Similar observations were independently made by Kawasaki et al.[112]. Using the serum-free aortic ring assay we demonstrated that TSP-1 had the capacity to stimulate angiogenesis when bound to the ECM, and that this effect was mediated by myofibroblasts [173].
The discovery that the injured aortic wall produced vessels in the absence exogenous growth factors set us on a quest for the endogenous regulators of this process. In 1987 Gospodarowicz’ group had reported that endothelial cells expressed bFGF, a potent inducer of endothelial cell proliferation and angiogenesis, and proposed that endothelial bFGF could function as an autocrine stimulator of angiogenesis [203]. Artery associated bFGF was also found in smooth muscle cells and macrophages [137, 218]. Because injury was believed to be the main mechanism of bFGF release [41], we suggested that bFGF was one of the autocrine/paracrine regulators of angiogenesis in our model. Immunochemical studies showed that the aortic wall expressed bFGF and rapidly released it following the injury of the dissection procedure [230]. bFGF-containing aorta conditioned medium and purified bFGF increased both the number and length of microvessels sprouting from the explants (Fig. 3). The bFGF stimulatory effect was most pronounced during the second week of culture when release of bFGF was minimal. The angiogenic response of aortic rings was reduced by 40% with a neutralizing anti-bFGF antibody [230]. Inhibition of angiogenesis was later obtained also with platelet factor-4 (PF-4) which blocks bFGF binding to endothelial cells [84].
Fig 3.
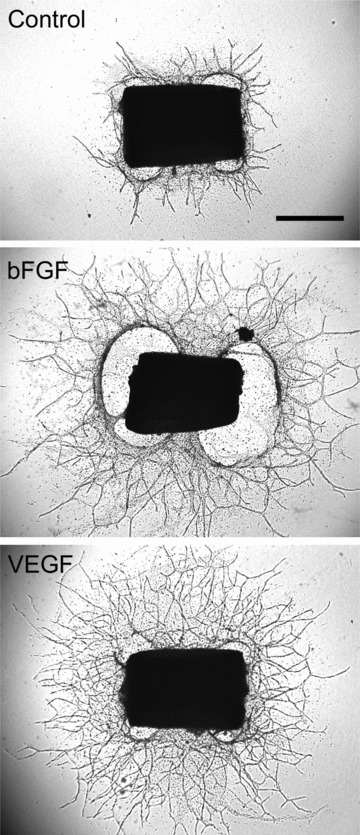
Angiogenesis in the aortic ring model: The angiogenic response of aortic rings in serum-free collagen gel culture can be stimulated with exogenous angiogenic factors. Shown here are a control aortic ring and rings treated with bFGF (25 ng/ml) or VEGF (10 ng/ml). Magnification bar: 1000 μm.
While we were studying mechanisms of angiogenesis in the aortic ring model, three groups independently identified a novel endothelial growth factor in both neoplastic and normal cells [40, 131, 204]. This molecule originally described as a vascular permeability factor by Harold Dvorak and collaborators was later found by Napoleone Ferrara’s group to be a specific angiogenic regulator and termed vascular endothelial growth factor (VEGF) [33, 58, 59]. In our laboratory we found that aortic rings produced the alternatively spliced VEGF isoforms 164 and 188 and expressed the VEGF receptor flk-1 (VEGFR2). Rakic et al. later reported that the angiogenic outgrowths produced primarily VEGF120 whereas aortic explants express VEGF 164 and 120 and to a less extent VEGF188 [189]. Protein levels of VEGF in aorta conditioned medium decreased during the second week of culture when the explants became quiescent and microvessels stopped growing [165]. Exogenous VEGF potently stimulated angiogenesis in aortic cultures (Fig. 3) [165, 167], whereas an anti-VEGF blocking antibody inhibited angiogenesis by 70%[165]. These experiments established VEGF as a key regulator of angiogenesis in the aortic ring model.
Subsequent studies showed that VEGF production in cells of the vessel wall was stimulated by nitric oxide donors and suppressed by nitric oxide synthase (NOS) inhibitors [49]. Aortic rings harvested from endothelial NOS (eNOS) deficient mice and cultured in Matrigel exhibited a 44% reduction in angiogenesis and a 68% reduction in DNA synthesis compared to control explants [125]. Expression of VEGF was found to be regulated also by prostaglandin E2 (PGE2) [20] which is angiogenic in vivo[65]. As a result, aortic rings from mice deficient in EP2, one of PGE2 receptors, produced fewer vessels than control aortas [110].
Aortic cultures also produced PDGF, a potent mural cell mitogen and chemotactic factor [159, 197] which Risau and collaborators had found to be angiogenic as well [195]. In the aortic ring model endogenous PDGF was required for pericyte recruitment (see below) but not for endothelial sprouting [57, 159]. Exogenous PDGF molecules however dose dependently potentiated the angiogenic response of the aortic rings. PDGF-BB was as effective as VEGF and more potent than PDGF-AA, as reported earlier by Risau and collaborators [167, 195]. Unlike VEGF which was stimulatory since the beginning of the experiment, PDGF molecules promoted angiogenesis during the second week of culture when vessels were regressing in unstimulated cultures. PDGF-stimulated cultures contained many fibroblast-like cells which increased progressively in number over time. These findings suggested that the pro-angiogenic effect of PDGF was indirectly mediated by endothelial growth factors produced by PDGF-stimulated connective tissue cells. Consistent with this interpretation we found that fibroblasts and smooth muscle cells had the capacity to promote angiogenesis in cultures of isolated aortic endothelial cells or from aortic rings [173–175, 231]. More recently Li et al. demonstrated that the angiogenic response of the aortic rings was stimulated by PDGF-CC. They also showed that the angiogenic effect of PDGF-CC was suppressed by concurrent treatment with an anti-VEGF antibody [135].
Paracrine regulation of angiogenesis: role of resident macrophages and inflammatory cytokines and chemokines
In 2004, the Rat genome Sequencing Project Consortium completed a decade long study aimed at deciphering the rat genome [74]. Meanwhile microarray analysis of cDNA had become a powerful method to detect genes dysregulated in physiological or pathological conditions [202]. To further define genetic events occurring in the aortic wall prior to angiogenic sprouting, we applied the microarray analysis method to rat aortic rings treated with angiogenic factors. For this study, aortic explants were made quiescent by a 14-day pre-incubation step in serum-free medium prior to collagen embedding. This procedure abrogated the spontaneous angiogenic activity of the rings while preserving their capacity to sprout in response to angiogenic factors or re-injury of the vessel wall. This approach produced angiogenic ‘on’ and ‘off’ conditions that enabled us to identify transcriptional events induced by angiogenic factors prior to endothelial sprouting (Fig. 4). We stimulated quiescent aortic rings with VEGF or angiopoietin-1 (Ang-1). Ang-1 had been identified by Yancopoulos’ group as the ligand of the tyrosine kinase receptor Tie2 and a key regulator of angiogenic sprouting, vessel branching and vessel maturation [44, 119, 219, 220]. Microarray analysis of quiescent aortic rings treated with VEGF or Ang-1 were confirmed by real time polymerase chain reaction and ELISA for selected genes.
Fig 4.
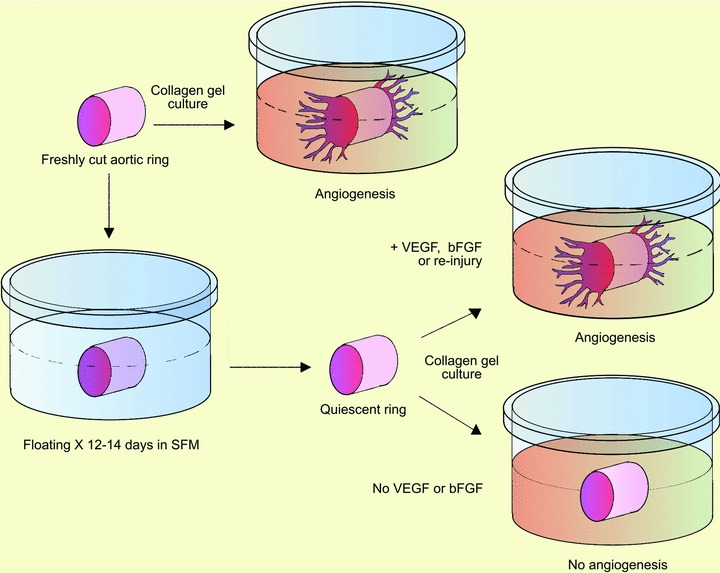
Standard and modified aortic ring model of angiogenesis with quiescent aortic rings. Freshly cut rings of rat aorta embedded in collagen gel generate outgrowths of branching microvessels. Aortic rings lose their spontaneous angioformative properties and become angiogenically quiescent if kept in serum-free growth medium for ∼2 weeks prior to collagen embedding. The medium is changed three times a week during this time to deplete the cultures of endogenous growth factors. Quiescent aortic rings remain viable and produce an angiogenic response comparable to that of freshly cut rings when they are re-injured or treated with exogenous angiogenic factors.
Of the more than 31,200 transcripts analysed, ∼3.5% were dysregulated by VEGF and Ang-1 [12]. Approximately one fourth of these transcripts corresponded to known genes, and the largest group of these was related to the immune system. Up to 10 cytokines and chemokines were up-regulated by VEGF and/or Ang-1. Five of these – GRO-1, interleukin (IL)-1β, MIP-1α, MIP-2 and tumour necrosis factor (TNF)-α– were stimulated by both factors. A cocktail of these VEGF- and Ang-1 induced cytokines and chemokines stimulated the angiogenic response of freshly cut aortic rings and was synergistic with a low dose of VEGF (5 ng/ml) which was otherwise minimally angiogenic when used by itself. In addition, abrogation of GRO-1 and MIP-2 signalling through pharmacological inhibition or genetic disruption of the G-coupled receptor CXCR2 significantly reduced the spontaneous angiogenic response of the aortic rings [70]. The pattern of immune gene up-regulation in VEGF- and Ang-1-treated aortic cultures mimicked activation of Toll-like receptors (TLRs) which cells of the innate immune system use to respond to microbial pathogens and endogenous molecules released by injured cells [221, 226]. In addition VEGF induced expression of TLR2 whereas Ang-1 stimulated expression of CD14, a TLR4 coreceptor [12]. The angiogenic response of the aortic rings and the endogenous production of VEGF were stimulated by treating the cultures with TLR ligands (unpublished observations). In a separate study concurrent treatment of aortic cultures with VEGF and IL-8, the human equivalent of GRO-1, produced an additive angiogenic effect [153]. Synergistic stimulation of angiogenesis in vivo was attained by combining a low non-angiogenic dose of VEGF with stromal derived factor-1 (SDF-1), a VEGF-inducible chemotactic factor for leucocytes and pro-angiogenic accessory cells of bone marrow orign [79, 122, 200]. SDF-1 also promoted the spontaneous angiogenic response of aortic rings in collagen gel culture [200].
We observed that quiescent aortic rings produced neovessels in response to VEGF but lost their ability to respond to the cytokine/chemokine cocktail that was instead angiogenic in cultures of freshly cut explants [70]. This change in angiogenic behaviour correlated with depletion of adventitial macrophages, and near complete loss of endogenous VEGF production in response to the cytokines/chemokines [70]. To investigate the angiogenic role of resident macrophages, we treated prior to the assay freshly cut aortic rings with liposomal clodronate, a compound that is toxic to phagocytic cells [229]. Selective killing of macrophages markedly impaired the angiogenic response of the aortic rings and their ability to produce VEGF. A comparable effect was obtained by ablating macrophages with diphtheria toxin in aortic cultures from CD11bDTR transgenic mice [31]. Angiogenic sprouting and VEGF production were restored by co-culturing macrophage-depleted rings with exogenous macrophages of bone marrow origin [70].
Further studies with immunosuppressive compounds corroborated the hypothesis that endogenous stimulation of angiogenesis in the aortic ring model was regulated by the immune system. Aortic angiogenesis was inhibited with hydrocortisone [168], a commonly used anti-inflammatory drug, TGFβ1 [155, 167], an inhibitor of the immune system [37, 237] and deactivator of macrophage function [227], and rmob-1, the rat homologue of the human chemokine interferon γ inducible protein 10, IP-10, which inhibits bone marrow colony formation and angiogenesis [1, 9, 215]. Angiogenic sprouting in aortic cultures was also blocked with IL-4 and IL-13 [70], both of which inhibit the pro-inflammatory response of macrophages [88] and with adenoviral vectors carrying the IL-4 or IL-13 genes [82, 83]. Pre-treating the aortic explants with macrophage colony stimulating factor (M-CSF/CSF-1) prior to preparation of the rings promoted angiogenesis whereas treatment with anti-CSF1 antibody significantly impaired the angiogenic response [70]. More recently we found that granulocyte macrophage colony stimulating factor (GM-CSF) stimulates the angiogenic response of the aortic rings (unpublished observations). Others reported that MCP-1, a potent monocyte/macrophage chemokine, promoted aortic angiogenesis while an anti-MCP1 antibody or a synthetic peptide that blocked MCP1 binding to its receptor CCR2 abrogated the MCP-1 effect [115].
Recently McGonigle and coworkers reported that osteprotegerin (OPG), a soluble TNF receptor family molecule involved in skeletal and immune system function and development [17, 107], stimulated aortic angiogenesis in collagen culture, and found that this effect was blocked by pre-incubating OPG with its ligands receptor activator of NF-κB ligand (RANKL) and TNF-related apoptosis inducing ligand (TRAIL). They also observed that RANKL but not TRAIL potently inhibited basal and VEGF-stimulated angiogenesis. OPG promoted endothelial proliferation whereas RANKL had antiproliferative and pro-apoptotic effects [146].
Expression and function of neural related genes
Studies conducted during the past decade showed that developing nerves and blood vessels share molecular mechanisms of cellular path finding and morphogenesis [154, 205]. Several axonal guidance ligand/receptor systems were found to regulate angiogenesis: Ephrin/Eph, Neuropilin/Semaphorins, Notch/Delta, Netrin/UNC5, Neogenin and DCC (deleted in colorectal cancer) and Slits/Roundabouts.
Our microarray studies showed that aortic rings up-regulate numerous neural related genes prior to angiogenesis [12]. Michael Klagsbrun’s group found that semaphorin 3A, also known as collapsin-1, competed with VEGF for binding to neuropilin-1 (NRP1) a VEGFR2 coreceptor, and acting through NRP1 inhibited the motility of endothelial cells, as previously reported for neuronal axons. Using the aortic ring assay they demonstrated that semaphorin 3A blocked endothelial sprouting by 80–90%[147]. Studies from other laboratories implicated the Slit/Roundabout (Robo) system in angiogenesis [68, 126]. Slits are large secreted proteins that bind to Robo transmembrane receptors inducing a repulsive signal during neuronal migration. One of these ligands, Slit2, was shown to either inhibit or stimulate endothelial cell migration by different investigators [185, 206, 233]. An endothelial specific Robo protein termed Robo4 was specifically localized to areas of angiogenesis [101]. Robo4-Fc, a soluble chimeric receptor, potently inhibited the angiogenic response of aortic rings. This effect correlated with the ability of Robo4-Fc to inhibit endothelial cell migration and proliferation in vitro and angiogenesis in vivo[216].
Studies with genetically modified mice showed that Ephrins and their Eph receptors regulated angiogenesis in different systems [3, 71, 234]. Ephrins are cell membrane-bound ligands which signal through Eph receptors at sites of cell–cell interactions. Ephrins and Eph receptors are divided into two classes, A and B. Ephrins A bind to Eph A receptors while Ephrins B bind to Eph B receptors, but there is significant promiscuity of receptor-ligand interactions within each class [69]. Blockade of Ephrin A signalling with soluble EphA2 receptor (EphA2-Fc) dose dependently inhibited angiogenesis in aortic cultures [48]. Simultaneous treatment with EphA2-Fc and the VEGFR2 inhibitor CEP-5214 had additive effects resulting in 90–95% inhibition of aortic angiogenesis. Conversely treatment with EphrinB1-Fc or Ephrin B2-Fc promoted the angiogenic response of the aortic rings [120, 141].
Neural development is regulated by netrins, bifunctional proteins capable of attracting or repelling axons. The netrin effects are mediated by receptors of the DCC and uncoordinated 5 (UNC5) families. DCC receptors include DCC and Neogenin whereas the UNC5 family comprises four members UNC5A to UNC5D [2, 130, 132]. DCC receptors mediate attractive signals whereas repulsive signals are regulated by UNC5-DCC receptor heterodimers or UNC5 dimers [56, 92, 113]. Using transgenic mice expressing LacZ under the control of the UNC5b promoter, Lerrivee and coworkers identified expression of UNC5b in sprouting microvessels. When they subjected collagen cultures of mouse aorta to gradients of netrin-1 they observed filopodial retraction in endothelial sprouts from UNC5B+/+ and UNC5b+/– aortas but not in sprouts from UNC5b–/– aortas [124]. Conversely Nguyen and Cai reported that aortic discs embedded in fibrin produced more vessels when treated with netrin-1 and that this effect was abrogated with an anti-DCC antibody [157]. Although it remains unclear if netrin-1 is pro- or anti-angiogenic, these studies suggest that netrins and their receptors are potential regulators of aortic angiogenesis.
Neuropeptide Y (NPY), a sympathetic vasoconstrictor molecule released during nerve activation and ischemia, stimulated endothelial migration, proliferation and angiogenesis by activating receptors Y1, Y2 and Y5 [145, 247]. When tested in the aortic ring model NPY markedly stimulated endothelial sprouting from mouse aortic rings cultured in Matrigel. The pro-angiogenic effect of NPY was significantly reduced in aortic cultures from aged animals probably due to reduced expression of the receptor Y2 and the NPY converting enzyme [116].
Signal transduction pathways
Our studies implicated bFGF and VEGF as key regulators of angiogenesis in aortic cultures [165, 230]. Because bFGF can induce secretion of VEGF [19], its rapid release from pre-existing aortic depots after injury [230] may be one of the earliest events responsible for the induction of VEGF production and angiogenesis in aortic cultures. Support to this hypothesis was provided by a study on the angiogenic role of Net (Elk-3/Sap-2/ERP), a transcription factor that is converted from a negative to a positive regulator through phosphorylation by Ras, Src and MAPK signalling. bFGF-induced endothelial sprouting was severely impaired in aortic cultures from Net deficient mice. This defect was corrected with exogenous VEGF, suggesting that impaired production of VEGF was responsible for the decreased sprouting [239]. Zhu et al. found that bFGF and VEGF production could be induced by H-2g, a glucose analogue of blood group antigen H [240], through the NF-κB pathway. H-2g-mediated stimulation of aortic angiogenesis in Matrigel cultures was inhibited by AG490, a JAK2 inhibitor and LY294002, a PI3 kinase inhibitor. Similar effects were obtained with antisense oligonucleotides (ODN) against these signalling molecules. The angiogenic effect of H-2g was also abrogated by treating the cultures with a decoy NF-κB ODN but not with a scrambled control ODN.
Studies aimed at developing synthetic inhibitors of VEGFR tyrosine kinases for therapeutic applications further implicated VEGF in the angiogenic response of the aortic rings. Wood and coworkers obtained dose-dependent inhibition of aortic angiogenesis in fibrin gel culture with the VEGFR2 inhibitor PTK787/ZK 222584 [235]. Ruggeri and collaborators observed that the VEGF-R2 signalling inhibitor CEP-5214 (20 nm) significantly reduced the sprouting of aortic rings in collagen gels [198]. They also found that simultaneous inhibition of both VEGFR and EphA2 pathways caused near complete inhibition of angiogenesis [48]. Anti-angiogenic effects were obtained with JNJ-17029259, a tyrosine kinase inhibitor that blocked VEGFR2 phosphorylation and MAPK signalling [53]. Furthermore transduction of aortic rings with an adenovirus carrying HCPTPA, a tyrosine phosphatase that binds to VEGFR2, caused inhibition of angiogenesis due to impairment of VEGF-mediated VEGFR2 autophosphorylation and MAPK activation [100]. Aortic angiogenesis was also blocked by AZM475271 a src kinase inhibitor required for the phosphorylation of Net [239] and the transduction of VEGF signals [52, 104, 186].
In our laboratory we found that intracellular signalling downstream of the VEGF/VEGFR system was characterized by biphasic phosphorylation of p44/42 MAPK and Akt, with peaks at 15 min. and 4–24 hrs. Both pathways were important for VEGFR signalling because the spontaneous angiogenic response of aortic rings was blocked with specific inhibitors of p44/42 MAPK (U0126) and Akt/PI3-kinase (LY294002)[244]. p44/42 MAPK and Akt were also activated by Ang-1, but only transiently at 15 min. Ang-1 promoted angiogenesis in collagen gel cultures of freshly cut aortic rings, but unlike VEGF did not significantly induce sprouting from quiescent rings. This suggested that sustained phosphorylation of p44/42 MAPK (U0126) and Akt/PI3-kinase were needed to induce aortic angiogenesis. Huang and coworkers observed that transduction of aortic rings with an adenovirus carrying the PTEN gene, a phosphatase that inhibits PI3-kinase signalling, caused shortening of vascular sprouts in Matrigel culture whereas the dominant negative form of PTEN promoted the formation of longer vessels [99]. VEGF and Ang-1 also activated p38 MAPK, but inhibitors of this pathway interfered with pericyte recruitment without significantly affecting endothelial sprouting [242] (see below).
Takeshita et al. reported that VEGF signalling downstream of PI3 kinase/Akt involved activation of γ-secretase activity and cleavage of Notch-1, a transmembrane receptor implicated in the regulation of embryonal angiogenesis [121, 136]. Endothelial sprouting was reduced by 80% in cultures of aortic rings from haploinsufficient Notch 1+/– mice [223]. In a separate study Paris et al. found that the γ-secretase inhibitor DAPT dose-dependently inhibited angiogenic sprouting in aortic cultures. The same authors observed that aortic angiogenesis was also blocked by β-secretase inhibitors [184]. Both γ- and β-secretase are involved in the production of the β amyloid component of plaques in Alzheimer’s patients. In addition to Notch and the Notch ligands Delta and jagged, γ-secretase also mediates the cleavage of E-cadherin and N-cadherin [184].
Bos and coworkers reported that the angiogenic response of aortic rings from mice deficient in Rap1b, a GTPase that modulates cell migration, differentiation and growth, was significant reduced compared to that of normal control rings, and was not restored by exogenous VEGF or bFGF [27]. These findings correlated with decreased in vivo neovascularization in Rap1b-deficient mice and suggested that VEGF and bFGF signalling in the aortic ring model was regulated by Rap1b [38].
Pericyte origin and recruitment mechanisms
An intriguing feature of the aortic outgrowths was the presence of pericytes, which are characteristically found in the microcirculation. Where did these pericytes originate from? Using cultures of everted aortic tubes we found that in microvessels sprouted directly from the intimal layer were enveloped by pericytes [164]. Mural cells isolated from the intimal aspect of the aorta exhibited polygonal shapes, proliferated rapidly, and grew as monolayers. Conversely medial-derived mural cells were spindly and grew slowly in ‘hills and valleys’, as described for vascular smooth muscle cells [76]. Intimal derived mural cells produced abundant laminin and type IV collagen and contracted collagen gels in response to endothelin-1, features that were not prominent in medial derived mural cells [232]. Intimal-derived but not medial-derived mural cells transformed into pericytes when co-cultured with networks of endothelial tubes in collagen gels [174]. These intimal-derived pericyte precursor cells (PPC) expressed Tie2 and responded to Ang-1 and Ang-2 by migrating and producing matrix metalloproteinases (MMP)-2 [106]. We successfully isolated PPC also by culturing non-endothelial cells obtained by enzymatic digestion of the rat aorta in a medium originally developed for neural stem cells [22]. These PPC were anchorage independent, proliferated in suspension forming spheroidal colonies and expressed CD34, Tie2, NG2 and the PDGF receptor molecules α and β. Upon exposure to serum, PPC attached to the culture dish, lost expression of CD34, and acquired smooth muscle cell markers such as α-SMA, calponin and desmin. PPC isolated from suspension cultures responded to PDGF BB and generated pericytes when co-cultured with rat aorta outgrowths or capillary networks formed by isolated endothelial cells [98].
The possibility offered by the aortic ring model to directly visualize pericytes in the living cultures prompted us to study the molecular mechanisms of pericyte recruitment (Fig. 5). We were able to reduce the number of pericytes with a neutralizing antibody against PDGF-B, which mediates pericyte recruitment during embryonal and postnatal angiogenesis [21, 72], by neutralizing angiopoietin function with Tie2-Fc [106], by blocking the p38 MAPK pathway [242] and by treating the cultures with heparin [174]. Conversely, we observed increased pericyte numbers in cultures treated with Ang-1 or Ang-2 [106] or with an adenoviral vector carrying the MMK6 gene, an upstream activator of p38 MAPK [242]. We observed an increased recruitment of pericytes also in aortic cultures treated with MCP-1 [10] an Ang-1-induced chemokine [12]. Paik et al. observed that siRNA-mediated knock down of N-cadherin, a cell adhesion molecule involved in endothelial-pericyte interactions markedly reduced the pericyte coating of microvessels in aortic cultures and suppressed the mural cell stimulatory effect of sphingosine-1-phosphate, a platelet-derived mediator of mural cell recruitment [183].
Fig 5.
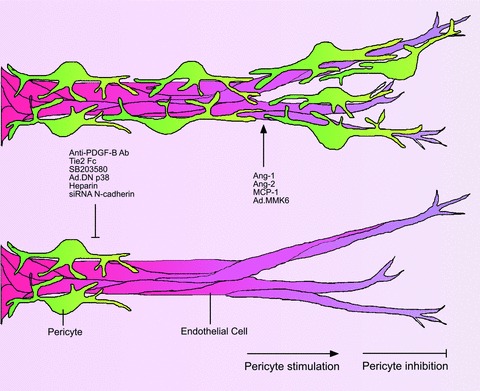
Modulation of pericyte recruitment in the aortic ring model. The number of pericytes recruited by microvessels in the aortic ring model can be increased by treating the cultures with the angiogenic regulators Ang1 and Ang-2 or the chemokine MCP-1. Enhanced pericyte recruitment is also obtained by transducing the aortic cultures with an adenoviral vector carrying MKK6, an upstream activator of p38 MAPK. Pericyte recruitment is inhibited by blocking PDGF B (anti-PDGF-B Ab), Ang-1 (Tie2 Fc) or the p38 MAPK pathway (AdDN p38). Reduction in pericytes is also obtained with heparin or by siRNA-mediated knockdown of the cell adhesion molecule N-cadherin.
Role of proteolytic enzymes in angiogenesis and vascular regression
Aortic ring cultures produce a broad spectrum of matrix proteinases and inhibitors of proteolysis including plasminogen activators (PA), MMPs, PA inhibitor (PAI-1) and tissue inhibitors of MMPs (TIMPs) [16, 30, 89, 241]. When we first cultured aortic rings in fibrin gels under serum-free conditions, we observed that endothelial sprouting was precluded by rapid (overnight) and extensive lysis of the fibrin. Blocking fibrinolysis with the serine protease inhibitor ɛ-aminocaproic acid preserved the fibrin scaffold and allowed endothelial cells to sprout freely into the fibrin gel [168]. Consistent with this observation, Devy and collaborators observed that aortic rings from plasminogen deficient mice maintained their capacity to sprout, though angiogenesis in these cultures was delayed [39, 46]. Likewise single deficiency of tissue type PA activator (tPA), uPA or uPAR, as well as combined deficiencies of uPA and tPA, did not significantly affect the angiogenic response of aortic explants [46, 95]. However, up-regulated expression of uPA through adenoviral transduction markedly inhibited microvessel growth [143]. Defective angiogenesis was also observed in cultures of aortic rings from mice lacking PA inhibitor-1 (PAI-1). This angiogenic defect could be reversed by adding recombinant PAI-1 to the culture medium or by transducing the explants with an adenovirus carrying the PAI-1 gene. Exogenous PAI-1 added to cultures of PAI-1 deficient aortic rings was stimulatory at low concentrations (<100 ng/ml) and inhibitory when used at higher dose (>1000 ng/ml). Transduction studies with specific mutants showed that PAI-1 promotes angiogenesis through its anti-proteolytic activity and not through its ability to interact with vitronectin [46].
Aortic rings cultured in collagen gel lysed the matrix over time. Using antibodies against a specific fragment of cleaved type I collagen or denatured collagen Stephen Weiss’ group identified collagen degradation products in the ECM of neovessel sprouts [39]. Studies from our laboratory and by others demonstrated that collagen lysis in aortic cultures was mediated by MMPs. The most abundant MMPs found in aortic cultures were MMP-2 and MMP-9. MMP-2 was abundantly secreted in the culture medium whereas MMP-9 was predominantly found in the gel and specifically in sprouting microvessels [89, 142, 241]. Aortic cultures also produced MMP-3, MMP-10, MMP-11, MMP-13 and MT1-MMP (MMP-14) [30].Treatment with the broad spectrum MMP inhibitors BB-94 (batimastat), BB-2516 (matimastat) or GM6001 blocked the angiogenic response of the aortic rings in collagen gel cultures [39, 241]. However, the angiogenic response of aortic rings was not affected by isolated or combined genetic disruption of MMP-2 or MMP-9 [13, 39, 142]. Similarly, aortic explants from mice deficient in CD44, an MMP-9 cell surface partner, retained their ability to sprout [96].
Weiss and coworkers demonstrated that that cellular invasion of collagen matrices required membrane type (MT) MMPs, which focus lytic activity to the invadopdia of migrating cells [236], and not soluble MMPs [96]. Disruption of the MT1-MMP gene caused complete abrogation of aortic angiogenesis in collagen [39]. Reduction of angiogenesis was also obtained by treating normal aortic rings with TIMP-2, which inhibited MT1-MMP, but not with TIMP-1, a weak inhibitor of MT1-MMP [75]. A similar effect was obtained by transducing aortic rings with a retrovirus carrying the TIMP-2 gene [86]. The MT1-MMP requirement was matrix dependent because MT1-MMP deficient rings sprouted normally in fibrin gel [39]. However, angiogenesis in fibrin was inhibited by TIMP-2 and TIMP-3, which can block other MT-MMPs besides MT1-MMP [97]. In addition aortic rings from TIMP-3 deficient mice had a significantly enhanced angiogenic response in fibrin gels compared with normal aortic rings [108]. One possible explanation for these results is that the expression and requirement of proteolytic enzymes by aortic outgrowths is influenced by the external matrix and/or growth factor milieu in which vessels sprout. Burbridge et al. observed that collagen induced overexpression by aortic rings of MT1-MMP, MMP-13, MMP-10 and MMP-9 whereas fibrin up-regulated MMP-2 and MMP-3 [30]. In addition, bFGF stimulated the production of MMP-2, MMP-3, MMP-9, MMP-10, MMP-11 and MMP-13 whereas VEGF induced overexpression of MMP-2 only. Furthermore sprouting of MMP-2 deficient aortas in Matrigel following stimulation with bFGF was reduced to 40% of control whereas no differences were observed when cultures were treated with serum instead of bFGF [111].
When we first analysed the role of MMPs in aortic angiogenesis we noticed that the few microvessels formed in the presence of synthetic MMP inhibitors survived longer than those of untreated controls. MMP inhibitors added to the growth medium after the growth phase potently inhibited collagen lysis and vascular regression, and promoted vascular survival (Fig. 6) [241]. We recently found that TIMP-2, TIMP-3 and TIMP-4, which are anti-angiogenic, stabilized neovessels if added to the culture medium after the growth phase. TIMP-1, which is not anti-angiogenic, similarly promoted vessel survival. Conversely aortic rings from TIMP-1 or TIMP-2 deficient mice regressed at a faster rate than normal control rings [13].
Fig 6.
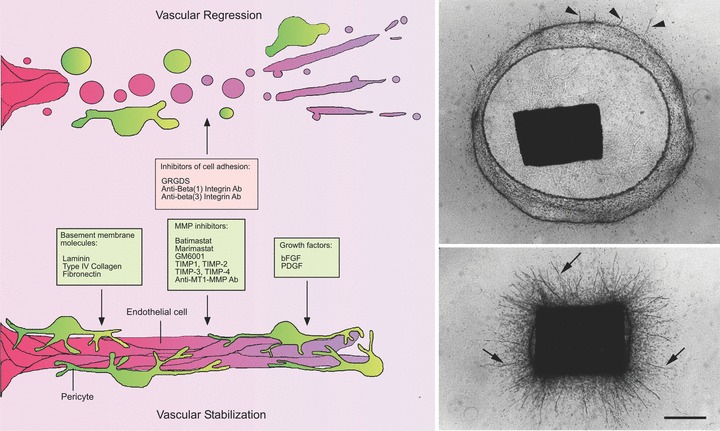
Vascular regression in the aortic ring model. Serum-free cultures of rat aorta can be used to study mechanisms of vascular regression following angiogenesis. Aorta-derived microvessels spontaneously regress after the first week of growth. Vascular regression is characterized by cell detachment from the ECM, vessel fragmentation and retraction of the remaining stumps. Molecules that interfere with cell attachment to the ECM such as RGD peptides and antibodies against β1 integrin or β3 integrins have the capacity to accelerate vascular regression. The anti-β1 integrin antibody induces vascular regression in collagen. The anti-β3 antibody has no effect in collagen but it synergizes with the anti-β1 integrin antibody to promote vascular regression in fibrin. Vessel survival can be extended by supplementing gels of interstitial collagen with basement membrane molecules and by blocking MMP activity with synthetic inhibitors, TIMPs or anti-MT1-MMP antibody. Vessel survival is also promoted by bFGF or PDGF. Photomicrographs show a control culture (upper panel) in an advanced stage of collagen lysis and vessel regression (residual short vessel stumps marked by arrowheads) and a parallel culture (lower panel) treated with the MMP inhibitor Marimastat to block collagen lysis and promote vascular survival (vessels marked by arrows). Magnification bar = 800 μm.
Influence of genetic background and aging on angiogenic response
When we first studied the angiogenic behaviour of the mouse aorta we noticed significant interassay variability. Unlike rat aorta cultures which were prepared from Fisher 344 rats only, mouse aorta cultures had different genetic backgrounds. To determine if this variable could account for the inconsistent angiogenic response of mouse aortic rings, we compared four different strains of mice for their ability to sprout in response to bFGF or VEGF [243]. Aortic rings from 129/SVJ mice produced a much stronger and sustained angiogenic response to bFGF than those from C57BL/6 or BALB/c mice which were in turn more angiogenic than rings from FVB mice. C57BL/6 mouse aortic rings produced more microvessels in response to VEGF compared to rings from 129/SVJ, BALB/c or FVB mice, which were capable of only a limited response. In a separate study Rohan et al. made comparable observations and identified the 129 mice as the strongest responders to bFGF [196].
Aortic sprouting in collagen was significantly influenced by the age of the animals. Aortic rings from young mice stimulated with bFGF or VEGF produced microvessels faster, more uniformly and in greater number than aortic rings from older mice. As a result the angiogenic response of aortic rings from 10-month-old mice to VEGF was ∼22% of that from 1-month-old animals [243]. Comparable results were obtained by Shimada and collaborators who observed reduced angiogenesis and eNOS release in Matrigel culture by aortic rings from genetically modified mice with disrupted anti-aging klotho gene [208]. In a modified mouse aortic ring assay VEGF-induced angiogenic sprouting was reduced by 40% in aged animals compared to controls [190]. Recently Facchetti et al. found that aortic rings from 24-month-old rats produced 90% fewer vessels than rings from 3.5-month-old animals in collagen gel culture. Interestingly, fasting dietary regimens boosted the angiogenic response of rings from old animals by 45–63%[55].
Adaptation of the aortic ring model to different species and vessel types
Originally developed to study the angiogenic response of the rat aorta, the aortic ring model was later applied to other species and vessel types (Table 1). The adaptation of this model to the mouse aorta enabled investigators to study the angiogenic role of proteins whose expression had been silenced or up-regulated through genetic manipulation. Human vessels were used to test the activity of pro-angiogenic and anti-angiogenic regulators prior to clinical trials.
Table 1.
Vessel types used to study angiogenesis ex vivo
| Vessel type | Species | Biomatrix | References |
|---|---|---|---|
| Aorta | Rat | Collagen, fibrin, Matrigel | [168–170] |
| Aorta | Mouse | Collagen | [46, 243] |
| Aorta | Chicken | Matrigel | [156] |
| Aorta | Rabbit | Collagen | [35] |
| Aorta | Cow, Dog | Matrigel | [214] |
| Aorta | Human | Collagen | [6] |
| Carotid artery | Pig, cow | Matrigel | [214] |
| Carotid artery | Rat | Collagen | Nicosia, unpublished observations |
| Saphenous vein | Human | Fibrin, Matrigel | [94] |
| Vena cava | Rat | Collagen | [176] |
| Thoracic duct | Rat, mouse | Collagen | [29, 158] |
In our laboratory we observed that mouse aortic rings were unable to produce an angiogenic response in serum-free medium. We later found that this deficiency was due to the small size of the rings and the excessive dilution of the growth factors produced by the explants. Mouse aortic rings sprouted only if treated with exogenous bFGF, VEGF or 2.5% autologous serum [46, 243]. We overcame this limitation by reducing the volume of the medium from 400 to 150 μl and the size of the culture well from 17 mm (4- or 24-well culture dishes) to 6.4 mm (96-well culture plates), levels of endogenous growth factors including VEGF increased several fold and mouse aortic rings generated vessel sprouts without the need for exogenous growth factors or serum [11]. This approach also enhanced the spontaneous angiogenic response of rat aortic rings. Akimoto and coworkers observed that addition of serum, VEGF or bFGF to cultures of mouse embryonic aorta failed to significantly stimulate angiogenesis in room air. Mouse embryonic rings however increased expression of VEGFR2 and FGFR1, thereby becoming responsive to VEGF or bFGF, when cultured under hypoxic conditions (5% O2) [4, 5].
Stiffey-Wilusz et al. reported that square pieces of porcine carotid arteries embedded in Matrigel and cultured in growth medium supplemented with EGF, bovine hypothalamic extract and 2% serum generated an angiogenic response that was inhibited with the MMP inhibitor Batimastat, 2-methoxyestradiol or suramin. They also observed angiogenic sprouting in Matrigel cultures of aortic or carotid explants from cows or dogs, but porcine carotid arteries proved to be the most reliable material for this study. Porcine and bovine arteries obtained from an abattoir remained angiogenically viable after being kept on ice for 24 hrs [214].
Results comparable to the rat aortic ring model were obtained by culturing explants of rabbit thoracic aorta in collagen gel under serum free conditions. Normal aortic explants from control rabbits or atherosclerotic explants from hypercholesterolaemic animals produced angiogenic outgrowths whereas lesion-free explants from hypercholesterolaemic rabbits exhibited a markedly reduced angiogenic growth. Microvessels formed in cultures of atherosclerotic aortas were surrounded by foamy macrophages and became more numerous than control vessels over time. Angiogenesis in this system was stimulated by bFGF and blocked by anti-bFGF antibody or oxidized LDL [35].
Auerbach and collaborators adapted the aortic ring model to the chick embryo. This assay developed to test thalidomide, which had limited effects in rodents, used explants of aortic arches. Rings placed on Matrigel generated vessel-like structures within 24–48 hrs. Angiogenesis in this model was stimulated by bFGF and inhibited by endostatin [156].
Hiran and coworkers showed that fragments of human saphenous vein produce neovessel sprouts in Matrigel or fibrin. They used this assay to test the effect of the angiogenic inhibitor endostatin and to evaluate the role of the α6β4 integrin which was expressed in mature vessels and down regulated in sprouting endothelial cells [94]. Enzymatic removal of the intimal endothelium significantly impaired the angiogenic response of the saphenous vein [138].
Alessandri et al. used the aortic ring assay to study the angioformative behaviour of aortic explants from 11- to 12-week-old aborted human embryos. Aortic rings embedded in collagen gels produced branching capillary-like structures from immature endothelial precursor cells localized in the outer layer of the aortic wall [6]. Undifferentiated CD34+ and CD31– mesenchymal cells isolated from the embryonal aortas and cultured on collagen-fibronectin, differentiated into CD31+ and vWF+ endothelial cells and formed networks of capillary-like structures on Matrigel. This study showed that the human embryonic aorta is a rich source of endothelial progenitor cells that have the capacity to form neovessels de novo through a vasculogenic process.
In our laboratory we found that explants of rat inferior vena cava cultured in collagen under serum-free conditions produced microvascular outgrowths which were dose-dependently stimulated by VEGF and bFGF. VEGF and the VEGF/bFGF combination also promoted pericyte recruitment. The spontaneous angiogenic response of the vena cava was significantly reduced by blocking endogenous VEGF with a neutralizing antibody. Neovessels of vena cava origin were longer and had fewer pericytes than aorta-derived vessels. Aortic and venous explants co-embedded in the same gel stimulated each other’s growth and formed anastomosing networks of microvessels [176].
Recently Agnes Noel’s group developed a quantifiable three-dimensional culture system for the study of mouse lymphangiogenesis ex vivo. This study confirmed and expanded my earlier observation that rat thoracic ducts have the capacity to sprout and form lymphatic-like channels in plasma clot culture [158]. In their assay, Noel and collaborators demonstrated that mouse thoracic duct fragments embedded in a collagen gel formed lymphatic capillaries and that this process was stimulated by serum, low oxygen levels (5%), PDGF-BB and the lymphangiogenic factor VEGF-C. Interestingly, bFGF or PlGF had no direct stimulatory effects. Lymphangiogenesis was inhibited by blocking the VEGFC receptor VEGFR3 but not with the bFGF inhibitor suramin [29].
Limitations of the aortic ring assay
When compared with in vivo models of angiogenesis, the aortic ring assay has no blood flow, a limitation shared with all other in vitro and ex vivo models of angiogenesis. As a result, the role in angiogenesis of haemodynamic factors and mechanochemical forces cannot be studied in this system [210]. Aortic explants contain macrophages and dendritic cells, but lack other immune cell types, which can however be added to the cultures. The same consideration applies to platelets which are major carriers of both angiogenic and anti-angiogenic factors [28, 105]. Manual counts of microvessels is the most rapid method to measure the angiogenic response in the aortic cultures [11, 168]. This approach allows the observer to follow the angiogenic response over time and to generate curves of microvascular growth and regression. Proper training and knowledge of scoring criteria reduces intra- and inter-observer variability to negligible levels. However, when angiogenic stimulation with exogenous growth factors results in very high numbers, the margin of error becomes high, and the counting process becomes tedious and time consuming. Counting vessels manually is also not practical when the assay is being used for high-throughput screening of drugs. This limitation can be overcome by computer aided analysis of digital images. A number of approaches have been developed to perform image analysis of aortic cultures and to specifically measure vascular area, vessel length and branching patterns. Some of these methods allow automated batch processing of many digital images per hour [23–25, 178]. The angiogenic response of the aortic rings to agonists or antagonists represents the net result of complex paracrine interactions between endothelial cells, macrophages, pericytes, dendritic cells and fibroblasts. While the presence of different cell types renders the aortic ring model a more physiological system compared to in vitro models with isolated endothelial cells, it also ads complexity to the interpretation of data and poses additional challenges to the investigator. Finally, the arterial nature of the aortic explants represents a potential limitation because neovessels in vivo characteristically originate from the venous side of the vascular bed. Although results obtained with the aortic ring assay consistently correlate with in vivo observations, more studies are needed to comparatively evaluate the angiogenic response of arterial and venous vessels. The recent adaptation of the aortic ring assay to veins [94, 138, 176] provides the opportunity to bridge this gap and evaluate in a chemically defined culture environment the angiogenic response of arteries and veins to the same angiogenic regulators.
Summary and conclusion
More than a quarter century after we first reported that explants of rat aorta have the capacity to generate vessels ex vivo[170] the aortic ring model has become one the most commonly used assays of angiogenesis. Many factors have contributed to its popularity including reproducibility, cost effectiveness, ease of use and good correlation with in vivo studies. An important milestone in the establishment of this model was the discovery that endothelial sprouting from the aortic explants does not require serum or exogenous growth factors and is driven by endogenous mechanisms triggered by the injury of the dissection procedure [112, 168]. This fundamental observation has enabled investigators to analyse mechanisms of angiogenesis in a chemically defined culture environment in which native macrophages, dendritic cells, fibroblasts, pericytes and endothelial cells interact through paracrine and juxtacrine mechanisms comparable to those operating in the live animal. The relative ease with which the soluble and solid phases of the culture system can be modified has facilitated the molecular analysis of specific steps of the angiogenic process from the induction of endothelial sprouting to the recruitment of pericytes and the reabsorption (regression) of the newly formed vasculature. These studies have helped define the role in angiogenesis of many growth factors, inflammatory cytokines and chemokines, axonal guidance molecules, tyrosine kinase receptors, integrin receptors, ECM molecules, MMPs, as well signal transduction pathways and transcription factors (Fig. 7). Co-cultures of aortic rings with different cell types have provided novel insights on the angiogenic role of fibroblasts, smooth muscle cells, macrophages, platelets and cancer cells [28, 70, 168, 174, 231, 232]. The self-limited nature of the angiogenic process and the induction of angiogenic quiescence have facilitated the analysis of the angiogenic cascade from its earliest inductive events to its final resolution through vascular regression. Studies with aortic rings from young and old animals and different animal strains have demonstrated that the angiogenic process is greatly influenced by age and genetic background. The adaptation of the aortic ring model to different species and vessel types has broadened its use to genetically modified mice and human beings. Finally the development of methods to virally transduce the aortic rings has facilitated the study of genes involved in the regulation of the angiogenic process.
Fig 7.
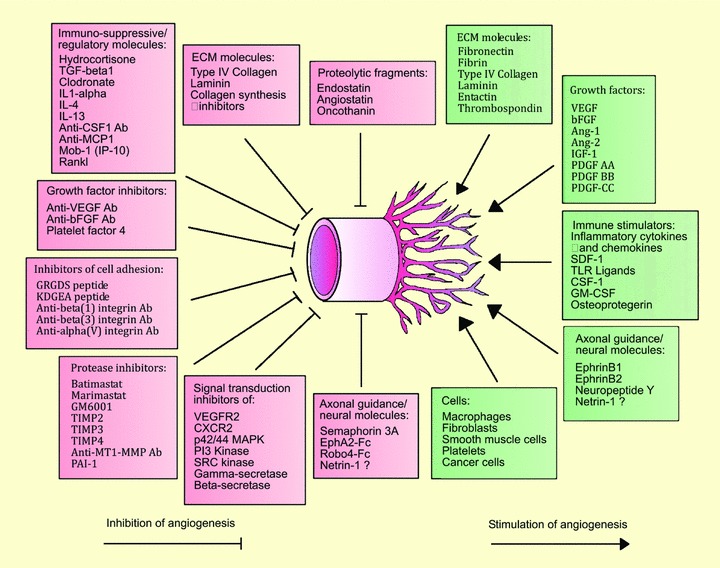
The aortic ring model has been extensively used to test the activity of angiogenic regulators. This drawing lists many of the stimulators and inhibitors of aortic angiogenesis that have been reported to date. Note: ECM molecules such as laminin and type IV collagen have the capacity to promote angiogenesis, but become inhibitory at concentrations comparable to those found in mature basement membranes. Netrin-1 has been reported to inhibit or stimulate angiogenesis by different groups. PAI-1 inhibits angiogenesis when administered to aortic cultures at high dose but it is also required for vessel growth because angiogenesis is impaired in cultures of PAI-1 deficient mouse aortas.
In 2004, more than 30 years after Judah Folkman proposed anti-angiogenic therapy as a cancer treatment [62], bevacizumab (Avastin), an anti-VEGF monoclonal antibody, was approved by the FDA as adjuvant therapy for metastatic colorectal cancer [60].
Two years later anti-VEGF therapy with ranibizumab (Lucentis) became the treatment of choice for wet age-related macular degeneration, an angiogenesis-dependent cause of vision loss in the elderly [187]. These successful clinical applications of angiogenic research have proven that angiogenesis-dependent disorders in human beings can be halted or even reversed by blocking the activity of a single angiogenic factor. Implementation of anti-angiogenic therapy has however revealed pitfalls and new challenges. For example existing anti-VEGF therapies have caused side effects such as hypertension, thrombotic microangiopathy and proteinuria due to inhibition of the non-angiogenic functions of VEGF [54, 109]. In addition this type of treatment has slowed down the progression of cancer without inducing tumour dormancy as originally suggested. It has also become clear that experimental tumours may develop resistance to anti-VEGF therapy due to production of other angiogenic factors [211]. Furthermore anti-VEGF therapy has recently been shown to elicit tumour adaptation and progression to greater invasiveness and metastatic behaviour following an initial anti-tumour growth effect [50, 182]. These observations have revealed gaps in our understanding of the angiogenic process, underscoring the need for additional studies aimed at better characterizing the molecular mechanisms of angiogenesis. To that end the aortic ring model represents a powerful tool for the dissection of the angiogenic cascade and the identification of novel molecular targets for therapeutic intervention in cancer and other angiogenesis-dependent disorders.
Acknowledgments
I wish to thank all the postdoctoral fellows, technicians and collaborators who over the years have contributed to our studies on the aortic ring model and whose names are cited in the reference list. I am particularly grateful to Alfred C. Aplin, whose molecular expertise has been essential for the advances we made in recent years and who has graciously helped me with the references and the figures of this paper. I am also indebted to the W. W. Smith Charitable Trust, which funded my first studies, and the National Institutes of Health (HL52585) and the Department of Veterans affairs for their continuous support.
References
- 1.Abdullah F, Ovadia P, Feuerstein G, et al. The novel chemokine mob-1: involvement in adult respiratory distress syndrome. Surgery. 1997;122:303–12. doi: 10.1016/s0039-6060(97)90022-2. [DOI] [PubMed] [Google Scholar]
- 2.Ackerman SL, Kozak LP, Przyborski SA, et al. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–42. doi: 10.1038/386838a0. [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akimoto T, Hammerman MR. Fibroblast growth factor 2 promotes microvessel formation from mouse embryonic aorta. Am J Physiol Cell Physiol. 2003;284:C371–7. doi: 10.1152/ajpcell.00193.2002. [DOI] [PubMed] [Google Scholar]
- 5.Akimoto T, Liapis H, Hammerman MR. Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol Regul Integr Comp Physiol. 2002;283:R487–95. doi: 10.1152/ajpregu.00699.2001. [DOI] [PubMed] [Google Scholar]
- 6.Alessandri G, Girelli M, Taccagni G, et al. Human vasculogenesis ex vivo: embryonal aorta as a tool for isolation of endothelial cell progenitors. Lab Invest. 2001;81:875–85. doi: 10.1038/labinvest.3780296. [DOI] [PubMed] [Google Scholar]
- 7.Alessandri G, Raju KS, Gullino PM. Interaction of gangliosides with fibronectin in the mobilization of capillary endothelium. Possible influence on the growth of metastasis. Invasion Metastasis. 1986;6:145–65. [PubMed] [Google Scholar]
- 8.Anghelina M, Krishnan P, Moldovan L, et al. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair: conversion of cell columns into fibrovascular bundles. Am J Pathol. 2006;168:529–41. doi: 10.2353/ajpath.2006.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aplin AC, Fogel E, Nicosia RF. 2009. Ang-1 and MCP-1 cooperate in pericyte recruitment during angiogenesis, abstr. Experimental Biology, April 18–22, 2009, New Orleans, LA.
- 11.Aplin AC, Fogel E, Zorzi P, et al. The aortic ring model of angiogenesis. Methods Enzymol. 2008;443:119–36. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 12.Aplin AC, Gelati M, Fogel E, et al. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics. 2006;27:20–8. doi: 10.1152/physiolgenomics.00048.2006. [DOI] [PubMed] [Google Scholar]
- 13.Aplin AC, Zhu WH, Fogel E, et al. Vascular regression and survival are differentially regulated by MT1-MMP and TIMPs in the aortic ring model of angiogenesis. Am J Physiol Cell Physiol. 2009;297:C471–80. doi: 10.1152/ajpcell.00019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auerbach R, Lewis R, Shinners B, et al. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 15.Aumailley M, Gerl M, Sonnenberg A, et al. Identification of the Arg-Gly-Asp sequence in laminin A chain as a latent cell-binding site being exposed in fragment P1. FEBS Lett. 1990;262:82–6. doi: 10.1016/0014-5793(90)80159-g. [DOI] [PubMed] [Google Scholar]
- 16.Bacharach E, Itin A, Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Sci USA. 1992;89:10686–90. doi: 10.1073/pnas.89.22.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud’huin M, Lamoureux F, Duplomb L, et al. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–50. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauvais DM, Ell BJ, McWhorter AR, et al. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belgore F, Lip GY, Blann AD. Basic fibrobrast growth factor induces the secretion of vascular endothelial growth factor by human aortic smooth muscle cells but not by endothelial cells. Eur J Clin Invest. 2003;33:833–9. doi: 10.1046/j.1365-2362.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- 20.Ben Av P, Crofford LJ, Wilder RL, et al. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83–7. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- 21.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005:115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 22.Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993:18–29. doi: 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Blacher S, Devy L, Burbridge MF, et al. Improved quantification of angiogenesis in the rat aortic ring assay. Angiogenesis. 2001;4:133–42. doi: 10.1023/a:1012251229631. [DOI] [PubMed] [Google Scholar]
- 24.Blatt RJ, Clark AN, Courtney J, et al. Automated quantitative analysis of angiogenesis in the rat aorta model using Image-Pro Plus 4.1. Comput Methods Programs Biomed. 2004;75:75–9. doi: 10.1016/j.cmpb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Boettcher M, Gloe T, De Wit C. Semiautomatic quantification of angiogenesis. J Surg Res. 2009 doi: 10.1016/j.jss.2008.12.009. ; doi: 10.1016/j.jss.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Bonanno E, Iurlaro M, Madri JA, et al. Type IV collagen modulates angiogenesis and neovessel survival in the rat aorta model. In vitro Cell Dev Biol Anim. 2000;36:336–40. doi: 10.1290/1071-2690(2000)036<0336:TICMAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Bos JL, De Bruyn K, Enserink J, et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–6. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- 28.Brill A, Dashevsky O, Rivo J, et al. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–8. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Bruyere F, Melen-Lamalle L, Blacher S, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–7. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- 30.Burbridge MF, Coge F, Galizzi JP, et al. The role of the matrix metalloproteinases during in vitro vessel formation. Angiogenesis. 2002;5:215–26. doi: 10.1023/a:1023889805133. [DOI] [PubMed] [Google Scholar]
- 31.Cailhier JF, Partolina M, Vuthoori S, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–42. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 34.Carnevale E, Fogel E, Aplin AC, et al. Regulation of postangiogenic neovessel survival by beta1 and beta3 integrins in collagen and fibrin matrices. J Vasc Res. 2007;44:40–50. doi: 10.1159/000097976. [DOI] [PubMed] [Google Scholar]
- 35.Chen CH, Cartwright J, Jr, Li Z, et al. Inhibitory effects of hypercholesterolemia and ox-LDL on angiogenesis-like endothelial growth in rabbit aortic explants. Essential role of basic fibroblast growth factor. Arterioscler Thromb Vasc Biol. 1997;17:1303–12. doi: 10.1161/01.atv.17.7.1303. [DOI] [PubMed] [Google Scholar]
- 36.Chen CS, Hawiger J. Reactivity of synthetic peptide analogs of adhesive proteins in regard to the interaction of human endothelial cells with extracellular matrix. Blood. 1991;77:2200–6. [PubMed] [Google Scholar]
- 37.Christ M, McCartney-Francis NL, Kulkarni AB, et al. Immune dysregulation in TGF-beta 1-deficient mice. J Immunol. 1994;153:1936–46. [PubMed] [Google Scholar]
- 38.Chrzanowska-Wodnicka M, Kraus AE, Gale D, et al. Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood. 2008;111:2647–56. doi: 10.1182/blood-2007-08-109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun TH, Sabeh F, Ota I, et al. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–67. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–8. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Amore PA. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990;9:227–38. doi: 10.1007/BF00046362. [DOI] [PubMed] [Google Scholar]
- 42.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 43.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 44.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–9. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 45.Dedhar S, Ruoslahti E, Pierschbacher MD. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987;104:585–93. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devy L, Blacher S, Grignet-Debrus C, et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2002;16:147–54. doi: 10.1096/fj.01-0552com. [DOI] [PubMed] [Google Scholar]
- 47.Diglio CA, Grammas P, Giacomelli F, et al. Angiogenesis in rat aorta ring explant cultures. Lab Invest. 1989;60:523–31. [PubMed] [Google Scholar]
- 48.Dobrzanski P, Hunter K, Jones-Bolin S, et al. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004;64:910–9. doi: 10.1158/0008-5472.can-3430-2. [DOI] [PubMed] [Google Scholar]
- 49.Dulak J, Jozkowicz A, Dembinska-Kiec A, et al. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659–66. doi: 10.1161/01.atv.20.3.659. [DOI] [PubMed] [Google Scholar]
- 50.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eliceiri BP, Cheresh DA. Role of alpha v integrins during angiogenesis. Cancer J. 2000;6:S245–9. [PubMed] [Google Scholar]
- 52.Eliceiri BP, Paul R, Schwartzberg PL, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 53.Emanuel S, Gruninger RH, Fuentes-Pesquera A, et al. A vascular endothelial growth factor receptor-2 kinase inhibitor potentiates the activity of the conventional chemotherapeutic agents paclitaxel and doxorubicin in tumor xenograft models. Mol Pharmacol. 2004;66:635–47. doi: 10.1124/mol.104.000638. [DOI] [PubMed] [Google Scholar]
- 54.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–36. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Facchetti F, Monzani E, Cavallini G, et al. Effect of a caloric restriction regimen on the angiogenic capacity of aorta and on the expression of endothelin-1 during ageing. Exp Gerontol. 2007;42:662–7. doi: 10.1016/j.exger.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Fazeli A, Dickinson SL, Hermiston ML, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 57.Ferns GA, Raines EW, Sprugel KH, et al. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253:1129–32. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am J Physiol Cell Physiol. 2001;280:C1358–66. doi: 10.1152/ajpcell.2001.280.6.C1358. [DOI] [PubMed] [Google Scholar]
- 59.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–42. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 60.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–5. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 61.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol. 2006:157–212. doi: 10.1007/3-540-36028-x_6. [DOI] [PubMed] [Google Scholar]
- 62.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 63.Folkman J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov. 2007;6:273–86. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 64.Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551–6. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 65.Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–8. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- 66.Form DM, Pratt BM, Madri JA. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986;55:521–30. [PubMed] [Google Scholar]
- 67.Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 68.Fujiwara M, Ghazizadeh M, Kawanami O. Potential role of the Slit/Robo signal pathway in angiogenesis. Vasc Med. 2006;11:115–21. doi: 10.1191/1358863x06vm658ra. [DOI] [PubMed] [Google Scholar]
- 69.Gale NW, Holland SJ, Valenzuela DM, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 70.Gelati M, Aplin AC, Fogel E, et al. The angiogenic response of the aorta to injury and inflammatory cytokines requires macrophages. J Immunol. 2008;181:5711–9. doi: 10.4049/jimmunol.181.8.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gerety SS, Wang HU, Chen ZF, et al. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 72.Gerhardt H, Betsholtz C. How do endothelial cells orientate. EXS. 2005:3–15. doi: 10.1007/3-7643-7311-3_1. [DOI] [PubMed] [Google Scholar]
- 73.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibbs RA, Weinstock GM, Metzker ML, et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- 75.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–47. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gimbrone MA, Jr, Cotran RS. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975;33:16–27. [PubMed] [Google Scholar]
- 77.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172–83. doi: 10.1016/j.mvr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975;250:2515–20. [PubMed] [Google Scholar]
- 79.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 80.Gullino PM. Angiogenesis and neoplasia. N Engl J Med. 1981;305:884–5. doi: 10.1056/NEJM198110083051508. [DOI] [PubMed] [Google Scholar]
- 81.Gullino PM, Grantham FH. Studies on the exchange of fluids between host and tumor. III. Regulation of blood flow in hepatomas and other rat tumors. J Natl Cancer Inst. 1962;28:211–29. [PubMed] [Google Scholar]
- 82.Haas CS, Amin MA, Allen BB, et al. Inhibition of angiogenesis by interleukin-4 gene therapy in rat adjuvant-induced arthritis. Arthritis Rheum. 2006;54:2402–14. doi: 10.1002/art.22034. [DOI] [PubMed] [Google Scholar]
- 83.Haas CS, Amin MA, Ruth JH, et al. In vivo inhibition of angiogenesis by interleukin-13 gene therapy in a rat model of rheumatoid arthritis. Arthritis Rheum. 2007;56:2535–48. doi: 10.1002/art.22823. [DOI] [PubMed] [Google Scholar]
- 84.Hagedorn M, Zilberberg L, Lozano RM, et al. A short peptide domain of platelet factor 4 blocks angiogenic key events induced by FGF-2. FASEB J. 2001;15:550–2. doi: 10.1096/fj.00-0285fje. [DOI] [PubMed] [Google Scholar]
- 85.Hajitou A, Grignet C, Devy L, et al. The antitumoral effect of endostatin and angiostatin is associated with a down-regulation of vascular endothelial growth factor expression in tumor cells. FASEB J. 2002;16:1802–4. doi: 10.1096/fj.02-0109fje. [DOI] [PubMed] [Google Scholar]
- 86.Hajitou A, Sounni NE, Devy L, et al. Down-regulation of vascular endothelial growth factor by tissue inhibitor of metalloproteinase-2: effect on in vivo mammary tumor growth and angiogenesis. Cancer Res. 2001;61:3450–7. [PubMed] [Google Scholar]
- 87.Ham RG, McKeehan WL. Development of improved media and culture conditions for clonal growth of normal diploid cells. In vitro. 1978;14:11–22. doi: 10.1007/BF02618170. [DOI] [PubMed] [Google Scholar]
- 88.Hart PH, Bonder CS, Balogh J, et al. Differential responses of human monocytes and macrophages to IL-4 and IL-13. J Leukoc Biol. 1999;66:575–8. [PubMed] [Google Scholar]
- 89.Hata-Sugi N, Kawase-Kageyama R, Wakabayashi T. Characterization of rat aortic fragment within collagen gel as an angiogenesis model; capillary morphology may reflect the action mechanisms of angiogenesis inhibitors. Biol Pharm Bull. 2002;25:446–51. doi: 10.1248/bpb.25.446. [DOI] [PubMed] [Google Scholar]
- 90.Hayashi H, Sano H, Seo S, et al. The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression. J Biol Chem. 2008;283:23791–800. doi: 10.1074/jbc.M800190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayashi I, Sato GH. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976;259:132–4. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- 92.Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- 93.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 94.Hiran TS, Mazurkiewicz JE, Kreienberg P, et al. Endothelial expression of the alpha6beta4 integrin is negatively regulated during angiogenesis. J Cell Sci. 2003;116:3771–81. doi: 10.1242/jcs.00681. [DOI] [PubMed] [Google Scholar]
- 95.Hiraoka N, Allen E, Apel IJ, et al. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–77. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- 96.Hotary K, Allen E, Punturieri A, et al. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–23. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hotary KB, Yana I, Sabeh F, et al. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195:295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Howson KM, Aplin AC, Gelati M, et al. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 99.Huang J, Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760–6. doi: 10.1074/jbc.M110219200. [DOI] [PubMed] [Google Scholar]
- 100.Huang L, Sankar S, Lin C, et al. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J Biol Chem. 1999;274:38183–8. doi: 10.1074/jbc.274.53.38183. [DOI] [PubMed] [Google Scholar]
- 101.Huminiecki L, Gorn M, Suchting S, et al. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–52. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- 102.Hynes RO, Bader BL. Targeted mutations in integrins and their ligands: their implications for vascular biology. Thromb Haemost. 1997;78:83–7. [PubMed] [Google Scholar]
- 103.Ingram DA, Mead LE, Moore DB, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 104.Ischenko I, Guba M, Yezhelyev M, et al. Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis. 2007;10:167–82. doi: 10.1007/s10456-007-9071-3. [DOI] [PubMed] [Google Scholar]
- 105.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iurlaro M, Scatena M, Zhu WH, et al. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–43. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 107.Izawa T, Ishimaru N, Moriyama K, et al. Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood. 2007;110:242–50. doi: 10.1182/blood-2006-11-059980. [DOI] [PubMed] [Google Scholar]
- 108.Janssen A, Hoellenriegel J, Fogarasi M, et al. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci. 2008;49:2812–22. doi: 10.1167/iovs.07-1444. [DOI] [PubMed] [Google Scholar]
- 109.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamiyama M, Pozzi A, Yang L, et al. EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene. 2006;25:7019–28. doi: 10.1038/sj.onc.1209694. [DOI] [PubMed] [Google Scholar]
- 111.Kato T, Kure T, Chang JH, et al. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–90. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- 112.Kawasaki S, Mori M, Awai M. Capillary growth of rat aortic segments cultured in collagen gel without serum. Acta Pathol Jpn. 1989;39:712–8. doi: 10.1111/j.1440-1827.1989.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 113.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–17. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 114.Kilarski WW, Samolov B, Petersson L, et al. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat Med. 2009;15:657–64. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 115.Kim MY, Byeon CW, Hong KH, et al. Inhibition of the angiogenesis by the MCP-1 (monocyte chemoattractant protein-1) binding peptide. FEBS Lett. 2005;579:1597–601. doi: 10.1016/j.febslet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 116.Kitlinska J, Lee EW, Movafagh S, et al. Neuropeptide Y-induced angiogenesis in aging. Peptides. 2002;23:71–7. doi: 10.1016/s0196-9781(01)00581-2. [DOI] [PubMed] [Google Scholar]
- 117.Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–8. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 118.Knedler A, Ham RG. Optimized medium for clonal growth of human microvascular endothelial cells with minimal serum. In vitro Cell Dev Biol. 1987;23:481–91. doi: 10.1007/BF02628418. [DOI] [PubMed] [Google Scholar]
- 119.Koblizek TI, Weiss C, Yancopoulos GD, et al. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 120.Kojima T, Chang JH, Azar DT. Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth factor-induced corneal angiogenesis. Am J Pathol. 2007;170:764–73. doi: 10.2353/ajpath.2007.060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krebs LT, Xue Y, Norton CR, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–52. [PMC free article] [PubMed] [Google Scholar]
- 122.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–72. [PubMed] [Google Scholar]
- 123.Kubota Y, Kleinman HK, Martin GR, et al. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–98. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Larrivee B, Freitas C, Trombe M, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–47. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee PC, Salyapongse AN, Bragdon GA, et al. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277:H1600–8. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 126.Legg JA, Herbert JM, Clissold P, et al. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 127.Leighton J. Aggregate replication, a factor in the growth of cancer. Science. 1959;129:466–7. doi: 10.1126/science.129.3347.466. [DOI] [PubMed] [Google Scholar]
- 128.Leighton J. Propagation of cancer: targets for future chemotherapy. Cancer Res. 1969;29:2457–65. [PubMed] [Google Scholar]
- 129.Leighton J, Tchao R, Nicosia RF, et al. Analysis of some tissue processes involved in the propagation of cancer using histophysiologic gradient culture. Prog Clin Biol Res. 1983;132C:51–62. [PubMed] [Google Scholar]
- 130.Leonardo ED, Hinck L, Masu M, et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–8. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- 131.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–9. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 132.Leung-Hagesteijn C, Spence AM, Stern BD, et al. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992;71:289–99. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- 133.Lewis WH. The outgrowth of endothelium and capillaries in tissue culture. Johns Hopkins Hosp Bull. 1931;48:242–53. [Google Scholar]
- 134.Li Q, Olsen BR. Increased angiogenic response in aortic explants of collagen XVIII/endostatin-null mice. Am J Pathol. 2004;165:415–24. doi: 10.1016/S0002-9440(10)63307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li X, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J Clin Invest. 2005;115:118–27. doi: 10.1172/JCI19189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Limbourg FP, Takeshita K, Radtke F, et al. Essential role of endothelial Notch1 in angiogenesis. Circulation. 2005;111:1826–32. doi: 10.1161/01.CIR.0000160870.93058.DD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lindner V, Lappi DA, Baird A, et al. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991;68:106–13. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- 138.Macpherson GR, Ng SS, Forbes SL, et al. Anti-angiogenic activity of human endostatin is HIF-1-independent in vitro and sensitive to timing of treatment in a human saphenous vein assay. Mol Cancer Ther. 2003;2:845–54. [PubMed] [Google Scholar]
- 139.Mahabeleshwar GH, Feng W, Phillips DR, et al. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Malinda KM, Nomizu M, Chung M, et al. Identification of laminin alpha1 and beta1 chain peptides active for endothelial cell adhesion, tube formation, and aortic sprouting. FASEB J. 1999;13:53–62. [PubMed] [Google Scholar]
- 141.Mansson-Broberg A, Siddiqui AJ, Genander M, et al. Modulation of ephrinB2 leads to increased angiogenesis in ischemic myocardium and endothelial cell proliferation. Biochem Biophys Res Commun. 2008;373:355–9. doi: 10.1016/j.bbrc.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 142.Masson V, De La Ballina LR, Munaut C, et al. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–6. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Masson V, Devy L, Grignet-Debrus C, et al. Mouse aortic ring assay: a new approach of the molecular genetics of angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McAuslan BR, Hannan GN, Reilly W, et al. Variant endothelial cells. Fibronectin as a transducer of signals for migration and neovascularisation. J Cell Physiol. 1980;104:177–86. doi: 10.1002/jcp.1041040207. [DOI] [PubMed] [Google Scholar]
- 145.McDermott BJ, Bell D. NPY and cardiac diseases. Curr Top Med Chem. 2007;7:1692–703. doi: 10.2174/156802607782340939. [DOI] [PubMed] [Google Scholar]
- 146.McGonigle JS, Giachelli CM, Scatena M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis. 2009;12:35–46. doi: 10.1007/s10456-008-9127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miao HQ, Soker S, Feiner L, et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–42. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miosge N, Simniok T, Sprysch P, et al. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J Histochem Cytochem. 2003;51:285–96. doi: 10.1177/002215540305100303. [DOI] [PubMed] [Google Scholar]
- 149.Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–77. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- 150.Montesano R, Orci L. Phorbol esters induce angiogenesis in vitro from large-vessel endothelial cells. J Cell Physiol. 1987;130:284–91. doi: 10.1002/jcp.1041300215. [DOI] [PubMed] [Google Scholar]
- 151.Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–52. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mori M, Sadahira Y, Kawasaki S, et al. Capillary growth from reversed rat aortic segments cultured in collagen gel. Acta Pathol Jpn. 1988;38:1503–12. doi: 10.1111/j.1440-1827.1988.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 153.Movahedi B, Gysemans C, Jacobs-Tulleneers-Thevissen D, et al. Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes. 2008;57:2128–36. doi: 10.2337/db07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mukouyama YS, Shin D, Britsch S, et al. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 155.Munaut C, Lorquet S, Pequeux C, et al. Hypoxia is responsible for soluble vascular endothelial growth factor receptor-1 (VEGFR-1) but not for soluble endoglin induction in villous trophoblast. Hum Reprod. 2008;23:1407–15. doi: 10.1093/humrep/den114. [DOI] [PubMed] [Google Scholar]
- 156.Muthukkaruppan VR. The chick embryo aortic arch assay: a new, rapid, quantifiable in vitro method for testing the efficacy of angiogenic and anti-angiogenic factors in a three-dimensional, serum-free organ culture system. Proc Am Assoc Cancer Res. 2000;41:65. [Google Scholar]
- 157.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103:6530–5. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nicosia RF. Angiogenesis and the formation of lymphaticlike channels in cultures of thoracic duct. In vitro Cell Dev Biol. 1987;23:167–74. doi: 10.1007/BF02623576. [DOI] [PubMed] [Google Scholar]
- 159.Nicosia RF. Endogenous regulation of angiogenesis in vitro, p. 285–296. In: Maragoudakis M, editor. Angiogenesis: models, modulators, and clinical applications. New York: Plenum Press; 1998. [Google Scholar]
- 160.Nicosia RF, Belser P, Bonanno E, et al. Regulation of angiogenesis in vitro by collagen metabolism. In vitro Cell Dev Biol. 1991;27A:961–6. doi: 10.1007/BF02631124. [DOI] [PubMed] [Google Scholar]
- 161.Nicosia RF, Bonanno E. Inhibition of angiogenesis in vitro by Arg-Gly-Asp-containing synthetic peptide. Am J Pathol. 1991;138:829–33. [PMC free article] [PubMed] [Google Scholar]
- 162.Nicosia RF, Bonanno E, Smith M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol. 1993;154:654–61. doi: 10.1002/jcp.1041540325. [DOI] [PubMed] [Google Scholar]
- 163.Nicosia RF, Bonanno E, Smith M, et al. Modulation of angiogenesis in vitro by laminin-entactin complex. Dev Biol. 1994;164:197–206. doi: 10.1006/dbio.1994.1191. [DOI] [PubMed] [Google Scholar]
- 164.Nicosia RF, Bonanno E, Villaschi S. Large-vessel endothelium switches to a microvascular phenotype during angiogenesis in collagen gel culture of rat aorta. Atherosclerosis. 1992;95:191–9. doi: 10.1016/0021-9150(92)90022-9. [DOI] [PubMed] [Google Scholar]
- 165.Nicosia RF, Lin YJ, Hazelton D, et al. Endogenous regulation of angiogenesis in the rat aorta model. Role of vascular endothelial growth factor. Am J Pathol. 1997;151:1379–86. [PMC free article] [PubMed] [Google Scholar]
- 166.Nicosia RF, Madri JA. The microvascular extracellular matrix. Developmental changes during angiogenesis in the aortic ring-plasma clot model. Am J Pathol. 1987;128:78–90. [PMC free article] [PubMed] [Google Scholar]
- 167.Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol. 1994;145:1023–9. [PMC free article] [PubMed] [Google Scholar]
- 168.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–22. [PubMed] [Google Scholar]
- 169.Nicosia RF, Ottinetti A. Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three-dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In vitro Cell Dev Biol. 1990;26:119–28. doi: 10.1007/BF02624102. [DOI] [PubMed] [Google Scholar]
- 170.Nicosia RF, Tchao R, Leighton J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In vitro. 1982;18:538–49. doi: 10.1007/BF02810077. [DOI] [PubMed] [Google Scholar]
- 171.Nicosia RF, Tchao R, Leighton J. Angiogenesis-dependent tumor spread in reinforced fibrin clot culture. Cancer Res. 1983;43:2159–66. [PubMed] [Google Scholar]
- 172.Nicosia RF, Tchao R, Leighton J. Interactions between newly formed endothelial channels and carcinoma cells in plasma clot culture. Clin Exp Metastasis. 1986;4:91–104. doi: 10.1007/BF00119076. [DOI] [PubMed] [Google Scholar]
- 173.Nicosia RF, Tuszynski GP. Matrix-bound thrombospondin promotes angiogenesis in vitro. J Cell Biol. 1994;124:183–93. doi: 10.1083/jcb.124.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nicosia RF, Villaschi S. Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab Invest. 1995;73:658–66. [PubMed] [Google Scholar]
- 175.Nicosia RF, Villaschi S, Smith M. Isolation and characterization of vasoformative endothelial cells from the rat aorta. In vitro Cell Dev Biol Anim. 1994;30A:394–9. doi: 10.1007/BF02634360. [DOI] [PubMed] [Google Scholar]
- 176.Nicosia RF, Zhu WH, Fogel E, et al. A new ex vivo model to study venous angiogenesis and arterio-venous anastomosis formation. J Vasc Res. 2005;42:111–9. doi: 10.1159/000083457. [DOI] [PubMed] [Google Scholar]
- 177.Nikolopoulos SN, Blaikie P, Yoshioka T, et al. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 178.Nissanov J, Tuman RW, Gruver LM, et al. Automatic vessel segmentation and quantification of the rat aortic ring assay of angiogenesis. Lab Invest. 1995;73:734–9. [PubMed] [Google Scholar]
- 179.Norrby K. In vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 181.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–28. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 182.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paik JH, Skoura A, Chae SS, et al. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Paris D, Quadros A, Patel N, et al. Inhibition of angiogenesis and tumor growth by beta and gamma-secretase inhibitors. Eur J Pharmacol. 2005;514:1–15. doi: 10.1016/j.ejphar.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 185.Park KW, Morrison CM, Sorensen LK, et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–67. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 186.Parker RC, Varmus HE, Bishop JM. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc Natl Acad Sci USA. 1981;78:5842–6. doi: 10.1073/pnas.78.9.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pieramici DJ, Avery RL. Ranibizumab: treatment in patients with neovascular age-related macular degeneration. Expert Opin Biol Ther. 2006;6:1237–45. doi: 10.1517/14712598.6.11.1237. [DOI] [PubMed] [Google Scholar]
- 188.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Eur J Cancer. 1996;32A:2438–50. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 189.Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–93. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 190.Reed MJ, Karres N, Eyman D, et al. Culture of murine aortic explants in 3-dimensional extracellular matrix: a novel, miniaturized assay of angiogenesis in vitro. Microvasc Res. 2007;73:248–52. doi: 10.1016/j.mvr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reynolds AR, Hart IR, Watson AR, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 192.Reynolds AR, Reynolds LE, Nagel TE, et al. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 193.Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 194.Ribatti D, Vacca A. Models for studying angiogenesis in vivo. Int J Biol Markers. 1999;14:207–13. doi: 10.1177/172460089901400403. [DOI] [PubMed] [Google Scholar]
- 195.Risau W, Drexler H, Mironov V, et al. Platelet-derived growth factor is angiogenic in vivo. Growth Factors. 1992;7:261–6. doi: 10.3109/08977199209046408. [DOI] [PubMed] [Google Scholar]
- 196.Rohan RM, Fernandez A, Udagawa T, et al. Genetic heterogeneity of angiogenesis in mice. FASEB J. 2000;14:871–6. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]
- 197.Ross R, Glomset J, Kariya B, et al. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA. 1974;71:1207–10. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruggeri B, Singh J, Gingrich D, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–91. [PubMed] [Google Scholar]
- 199.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 200.Salcedo R, Wasserman K, Young HA, et al. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999;154:1125–35. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sassoli PM, Emmell EL, Tam SH, et al. 7E3 F(ab’)2, an effective antagonist of rat alphaIIbbeta3 and alphavbeta3, blocks in vivo thrombus formation and in vitro angiogenesis. Thromb Haemost. 2001;85:896–902. [PubMed] [Google Scholar]
- 202.Schena M. Genome analysis with gene expression microarrays. Bioessays. 1996;18:427–31. doi: 10.1002/bies.950180513. [DOI] [PubMed] [Google Scholar]
- 203.Schweigerer L, Neufeld G, Friedman J, et al. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–9. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- 204.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 205.Serini G, Bussolino F. Common cues in vascular and axon guidance. Physiology. 2004;19:348–54. doi: 10.1152/physiol.00021.2004. [DOI] [PubMed] [Google Scholar]
- 206.Seth P, Lin Y, Hanai J, et al. Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem Biophys Res Commun. 2005;332:533–41. doi: 10.1016/j.bbrc.2005.03.250. [DOI] [PubMed] [Google Scholar]
- 207.Shahan T, Grant D, Tootell M, et al. Oncothanin, a peptide from the alpha3 chain of type IV collagen, modifies endothelial cell function and inhibits angiogenesis. Connect Tissue Res. 2004;45:151–63. doi: 10.1080/03008200490505923. [DOI] [PubMed] [Google Scholar]
- 208.Shimada T, Takeshita Y, Murohara T, et al. Angiogenesis and vasculogenesis are impaired in the precocious-aging klotho mouse. Circulation. 2004;110:1148–55. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- 209.Shing Y, Folkman J, Sullivan R, et al. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984;223:1296–9. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 210.Shiu YT, Weiss JA, Hoying JB, et al. The role of mechanical stresses in angiogenesis. Crit Rev Biomed Eng. 2005;33:431–510. doi: 10.1615/critrevbiomedeng.v33.i5.10. [DOI] [PubMed] [Google Scholar]
- 211.Shojaei F, Ferrara N. Role of the microenvironment in tumor growth and in refractoriness/resistance to anti-angiogenic therapies. Drug Resist Updat. 2008;11:219–30. doi: 10.1016/j.drup.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 212.Sholley MM, Ferguson GP, Seibel HR, et al. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest. 1984;51:624–34. [PubMed] [Google Scholar]
- 213.Spagnoli LG, Nicosia R, Villaschi S, et al. Smooth muscle cell response to endothelial injury. Prog Biochem Pharmacol. 1977;13:94–8. [PubMed] [Google Scholar]
- 214.Stiffey-Wilusz J, Boice JA, Ronan J, et al. An ex vivo angiogenesis assay utilizing commercial porcine carotid artery: modification of the rat aortic ring assay. Angiogenesis. 2001;4:3–9. doi: 10.1023/a:1016604327305. [DOI] [PubMed] [Google Scholar]
- 215.Strieter RM, Kunkel SL, Arenberg DA, et al. Interferon gamma-inducible protein 10 (IP-10), a member of the C-X-C chemokine family, is an inhibitor of angiogenesis. Biochem Biophys Res Commun. 1995;210:51–7. doi: 10.1006/bbrc.1995.1626. [DOI] [PubMed] [Google Scholar]
- 216.Suchting S, Heal P, Tahtis K, et al. Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J. 2005;19:121–3. doi: 10.1096/fj.04-1991fje. [DOI] [PubMed] [Google Scholar]
- 217.Suda H, Asami Y, Murata E, et al. Immuno-histochemical expression of alpha1, alpha2 and alpha3 integrin subunits during angiogenesis in vitro. Histol Histopathol. 2004;19:735–42. doi: 10.14670/HH-19.735. [DOI] [PubMed] [Google Scholar]
- 218.Sunderkotter C, Goebeler M, Schulze-Osthoff K, et al. Macrophage-derived angiogenesis factors. Pharmacol Ther. 1991;51:195–216. doi: 10.1016/0163-7258(91)90077-y. [DOI] [PubMed] [Google Scholar]
- 219.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 220.Suri C, McClain J, Thurston G, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–71. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 221.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 222.Takeda Y, Kazarov AR, Butterfield CE, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007;109:1524–32. doi: 10.1182/blood-2006-08-041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Takeshita K, Satoh M, Ii M, et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–8. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tigges U, Hyer EG, Scharf J, et al. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–32. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 225.Trikha M, Zhou Z, Nemeth JA, et al. CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–5. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 226.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–9. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 227.Tsunawaki S, Sporn M, Ding A, et al. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 228.Tuszynski GP, Gasic TB, Rothman VL, et al. Thrombospondin, a potentiator of tumor cell metastasis. Cancer Res. 1987;47:4130–3. [PubMed] [Google Scholar]
- 229.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 230.Villaschi S, Nicosia RF. Angiogenic role of endogenous basic fibroblast growth factor released by rat aorta after injury. Am J Pathol. 1993;143:181–90. [PMC free article] [PubMed] [Google Scholar]
- 231.Villaschi S, Nicosia RF. Paracrine interactions between fibroblasts and endothelial cells in a serum-free coculture model. Modulation of angiogenesis and collagen gel contraction. Lab Invest. 1994;71:291–9. [PubMed] [Google Scholar]
- 232.Villaschi S, Nicosia RF, Smith MR. Isolation of a morphologically and functionally distinct smooth muscle cell type from the intimal aspect of the normal rat aorta. Evidence for smooth muscle cell heterogeneity. In vitro Cell Dev Biol Anim. 1994;30A:589–95. doi: 10.1007/BF02631257. [DOI] [PubMed] [Google Scholar]
- 233.Wang B, Xiao Y, Ding BB, et al. Induction of tumor angiogenesis by Slit-Robo signaling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 234.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 235.Wood JM, Bold G, Buchdunger E, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–89. [PubMed] [Google Scholar]
- 236.Yana I, Sagara H, Takaki S, et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007;120:1607–14. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 237.Yaswen L, Kulkarni AB, Fredrickson T, et al. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood. 1996;87:1439–45. [PubMed] [Google Scholar]
- 238.Zhang Z, Ramirez NE, Yankeelov TE, et al. alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood. 2008;111:1980–8. doi: 10.1182/blood-2007-06-094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zheng H, Wasylyk C, Ayadi A, et al. The transcription factor Net regulates the angiogenic switch. Genes Dev. 2003;17:2283–97. doi: 10.1101/gad.272503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhu K, Amin MA, Zha Y, et al. Mechanism by which H-2g, a glucose analog of blood group H antigen, mediates angiogenesis. Blood. 2005;105:2343–9. doi: 10.1182/blood-2004-08-3140. [DOI] [PubMed] [Google Scholar]
- 241.Zhu WH, Guo X, Villaschi S, et al. Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest. 2000;80:545–55. doi: 10.1038/labinvest.3780060. [DOI] [PubMed] [Google Scholar]
- 242.Zhu WH, Han J, Nicosia RF. Requisite role of p38 MAPK in mural cell recruitment during angiogenesis in the rat aorta model. J Vasc Res. 2003;40:140–8. doi: 10.1159/000070711. [DOI] [PubMed] [Google Scholar]
- 243.Zhu WH, Iurlaro M, MacIntyre A, et al. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–9. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- 244.Zhu WH, MacIntyre A, Nicosia RF. Regulation of angiogenesis by vascular endothelial growth factor and angiopoietin-1 in the rat aorta model: distinct temporal patterns of intracellular signaling correlate with induction of angiogenic sprouting. Am J Pathol. 2002;161:823–30. doi: 10.1016/S0002-9440(10)64242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis. 2002;5:81–6. doi: 10.1023/a:1021509004829. [DOI] [PubMed] [Google Scholar]
- 246.Zhu X, Evans JP. Analysis of the roles of RGD-binding integrins, alpha(4)/alpha(9) integrins, alpha(6) integrins, and CD9 in the interaction of the fertilin beta (ADAM2) disintegrin domain with the mouse egg membrane. Biol Reprod. 2002;66:1193–202. doi: 10.1095/biolreprod66.4.1193. [DOI] [PubMed] [Google Scholar]
- 247.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Rose W, et al. Neuropeptide Y: a novel angiogenic factor from the sympathetic nerves and endothelium. Circ Res. 1998;83:187–95. doi: 10.1161/01.res.83.2.187. [DOI] [PubMed] [Google Scholar]


