Abstract
Systemic sclerosis (SSc) is a systemic autoimmune disease that is characterized by microangiopathy with progressive loss of capillaries and tissue fibrosis. Imatinib exerts potent anti-fibrotic effects and is currently evaluated in clinical trials. The aim of the present study was to exclude that the anti-fibrotic effects of imatinib are complicated by inhibitory effects on endothelial cell functions, which might augment vascular disease in SSc. Endothelial cells and mice were treated with pharmacologically relevant concentrations of imatinib. The expression of markers of vascular activation was assessed with real-time PCR. Proliferation was analysed with the cell counting experiments and the MTT assay. Apoptosis was quantified with caspase 3 assays, annexin V in vitro and with TUNEL staining in vivo. Migration was studied with scratch and transwell assays. Tube forming was investigated with the matrigel assay. Imatinib did not alter the expression of markers of vascular activation. Imatinib did not increase the percentage of annexin V positive cells or the activity of caspase 3. No reduction in proliferation or metabolic activity of endothelial cells was observed. Imatinib did not affect migration of endothelial cells and did not reduce the formation of capillary tubes. Consistent with the in vitro data, no difference in the number of apoptotic endothelial cells was observed in vivo in mice treated with imatinib. Imatinib does not inhibit activation, viability, proliferation, migration or tube forming of endothelial cells in vitro and in vivo. Thus, treatment with imatinib might not augment further endothelial cell damage in SSc.
Keywords: imatinib, scleroderma, sclerosis, endothelial cells, angiogenesis, vasculopathy, fibrosis, c-ab, PDGF, TGFβ
Introduction
Systemic sclerosis (SSc) is an autoimmune disease of unknown aetiology that affects the skin and various internal organs. The most obvious histological feature of SSc is a massive accumulation of extracellular matrix components such as collagens, glycosaminoglycans and fibronectin [1]. The resulting fibrosis leads frequently to dysfunction of the affected organs. Tissue fibrosis contributes significantly to the high morbidity and the increased mortality of SSc.
We and others demonstrated recently that imatinib mesylate (Gleevec®/Glivec®, Novartis, Basel, Switzerland) exerts potent anti-fibrotic effects [2, 3]. Imatinib is a small molecule inhibitor that binds to the ATP-binding pocket of abelson kinase (c-abl) and blocks efficiently its tyrosine kinase activity. C-abl is an important downstream signalling molecule of TGFβ that mediates the pro-fibrotic effects of TGFβ independent of Smad 3/4 [2]. In addition to its effects on c-abl, imatinib mesylate blocks the tzyrosine kinase activity of platelet-derived growth factor (PDGF) receptors [4]. Imatinib is widely used for the treatment of bcr-abl positive chronic myelogenous leukaemia and gastrointestinal stromal tumours. Clinical trials as well as post-marketing registries suggested that imatinib mesylate is well-tolerated by these patients [4]. Due to the potent anti-fibrotic effects in several animal models, its favourable pharmacokinetics, the good clinical experience in other diseases and first promising case reports [5], clinical trials investigating the anti-fibrotic potential of imatinib in SSc, are currently under way.
Besides fibrosis, a progressive microangiopathy is a characteristic feature of SSc. Increased apoptosis of microvascular endothelial cells as one of the first manifestations of vasculopathy occurs before tissue fibrosis becomes evident [1]. The ongoing endothelial cell damage results in a progressive loss of capillaries with decreased capillary blood flow, lack of nutrients and severe tissue hypoxia. The microangiopathy often manifests clinically as fingertip ulcers or even gangrene, and strongly contributes to the morbidity of SSc patients.
Many novel drugs with potent anti-fibrotic activity in pre-clinical models such as Src kinase inhibitors might interfere with endothelial cell function [6, 7]. Anti-angiogenic side effects might complicate the use of novel anti-fibrotic drugs in SSc patients as they might worsen the vascular manifestations of SSc. The aim of the present study was to determine whether imatinib mesylate interferes with endothelial cell function and might thereby aggravate the vascular disease in SSc.
Material and methods
Cell culture
Immortalized human microvascular endothelial (HMEC-1) cells were cultured as described previously [8].
Incubation with imatinib mesylate
HMEC-1 cells were incubated with imatinib mesylate in concentrations from 0.001 to 1.0 μg/ml for 24–96 hrs. These concentrations correspond to the mean plasma peak and trough concentrations in human beings after administration of standard doses of imatinib [2, 3].
Caspase 3 activity assay
The activities of caspase-3-like proteases were determined using the EnzChek caspase-3 assay kit (Invitrogen, Karlsruhe, Germany) as described [9].
Quantification of apoptotic cells by staining for annexin V
HMEC-1 cells were stained with fluorescein isothiocyanate-labelled annexin V (BD Bioscience, Heidelberg, Germany). Staining with annexin V in the absence of calcium was used for control. The number of cells positive for annexin V was quantified with the FACS Calibur flow cytometer (Becton Dickinson, Heidelberg, Germany).
Microtitre tetrazolium (MTT) assay
The metabolic activity of HMEC-1 cells incubated with imatinib for 24–96 hrs was assessed using the MTT [3, (4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide] method. Fresh medium and imatinib mesylate were added every other day. Untreated HMEC were used as controls.
Quantitative real-time PCR
Total RNA was isolated with the NucleoSpin RNA II extraction system (Machery-Nagel, Düren, Germany) and reverse transcribed into complementary DNA (cDNA) with random hexamers. Gene expression was quantified by TaqMan or SYBR Green real-time PCR using the ABI Prism 7300 Sequence Detection System (Applied Biosystems, Forster City, CA, USA) as previously described [3]. Specific primer pairs for each gene are given in the supplementary text.
Generation of proliferation curves
HMEC-1 cells were seeded onto 6-well plates at a density of 1.3 × 104 cells/well and incubated with imatinib at concentrations from 0.1 to 1.0 μg/ml. Fresh medium and imatinib mesylate were added every other day. Untreated HMEC were used as controls. After 48, 72 and 96 hrs, cells were detached using trypsin and counted with a BD FACS Calibur flow cytometer.
Scratch assay
Confluent monolayers of HMEC-1 cells were wounded by scratching the surface uniformly with a pipette tip. The migration of cells into the scratched area was photographically monitored using the Spot Insight QE camera. Shortening of the distance between the two borders of the scratch was determined at three defined sites per sample after 0, 24 and 48 hrs.
Chemotaxis assay
The effect of imatinib on the chemotaxis of HMEC-1 cells was determined with a transwell chemotaxis assay using 24-well cell culture inserts with the bottom sealed by an 8-μm pore polycarbonate-filter (Becton Dickinson). The number of cells that migrated through the filter to the lower compartment was quantified after 36 hrs with a FACS Calibur flow cytometer (Becton Dickinson).
In vitro capillary morphogenesis assay
To assess the influence of imatinib on the formation of tubes, an in vitro capillary morphogenesis assay was performed as described previously [10]. Tube forming was analysed after 24 and 48 hrs. Representative images were obtained using an Axiovert 25 microscope and a Spot Insight QE camera (Zeiss, Jena, Germany).
Treatment of mice with bleomycin
To investigate whether treatment of fibrosis with imatinib induces apoptosis of endothelial cells under fibrotic conditions in vivo, dermal fibrosis was induced in female 6-week-old C3H/HeJ mice (Sankyo, Tokyo, Japan) by local injection of bleomycin as described [3]. Two subgroups were additionally treated with imatinib mesylate in pharmacologically relevant doses of 50 mg/kg/d or 150 mg/kg/d.
Terminal uridine deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay
Paraffin-embedded tissue sections from lesional skin of mice challenged with bleomycin were used to quantify the number of apoptotic endothelial cells. TUNEL staining was performed using the in situ cell death detection kit (Roche, Mannheim, Germany) as recommended by the manufacturer including positive and negative controls.
Statistics
Data are expressed as mean ± standard error of the mean. The Wilcoxon signed rank test for non-related samples were used for statistical analyses. A P-value of less than 0.05 was considered statistical significant.
Results
Confirmation of the potent biologic effects of imatinib
To demonstrate that imatinib exerts potent biological effects in the concentrations used in the study, dermal fibroblasts were incubated with imatinib in concentrations ranging from 0.001–1.0 μg/ml. Imatinib potently decreased the levels of mRNA for col 1a1 and fibronectin-1. Similarly, significant anti-fibrotic effects with reduced accumulation of collagen were observed in mice treated with imatinib in doses of 50 mg/kg/d and 150 mg/kg/d (data not shown). Thus, imatinib exerts potent biologic effects in the concentrations used in the study.
Effects of imatinib on the viability and proliferation of endothelial cells
The effect of imatinib on apoptosis of microvascular endothelial cells was analysed by quantification of caspase 3 activity and staining for annexin V. After incubation with imatinib mesylate in concentrations from 0.001 to 1.0 μg/ml, no changes of the activity of caspase 3 were detectable (Fig. 1A). Incubation of HMEC-1 cells with increasing concentrations of imatinib did also not increase the number of annexin V positive, apoptotic cells (Fig. 1B). To investigate whether imatinib augments pre-existing endothelial cell damage, HMEC-1 cells were serum starved and then incubated with imatinib. As under normal culture conditions, imatinib did neither induce activation of caspase 3 nor increase the number of apoptotic cells.
Fig 1.
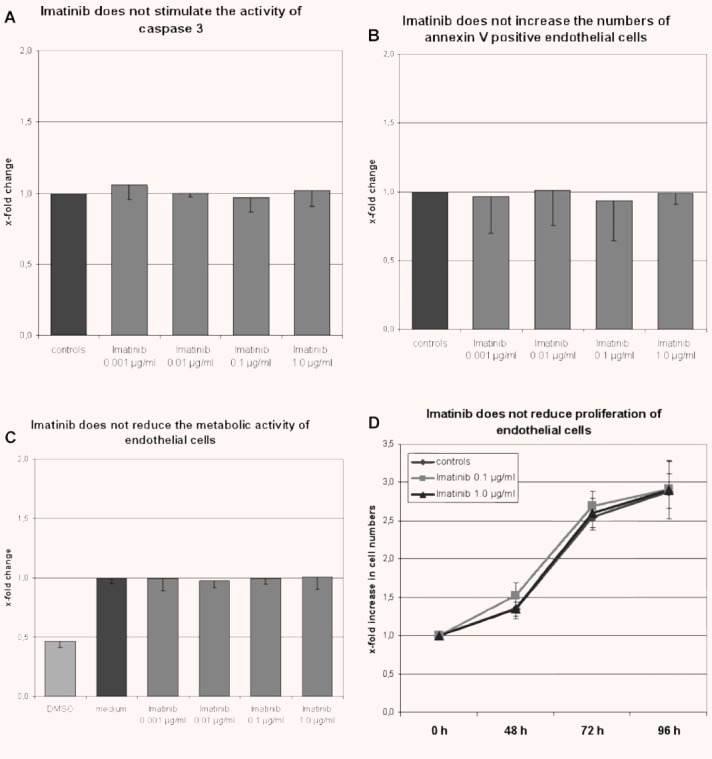
Imatinib does not induce apoptosis, reduce the metabolic activity or decrease proliferation of endothelial cells. (A and B) Incubation with imatinib in concentrations from 0.001 to 1.0 μg/ml for 24 hrs did not increase the activity of caspase 3 (n= 3) (A) or the number of annexin V positive HMEC-1 cells (n= 9) (B). (C) Incubation with imatinib in pharmacologically relevant concentrations for 48 hrs did not reduce the metabolic activity of HMEC-1 cells compared with cells incubated with medium only (n= 5). In contrast, incubation with 50% DMSO profoundly reduced the metabolic activity. (D) Imatinib did not reduce proliferation of HMEC-1 cells as analysed by direct cell counting (n= 6).
Potential effects of imatinib mesylate on the metabolic activity and proliferation of endothelial cells were analysed by MTT assay and by generating proliferation curves. No differences in metabolic activity were observed with the MTT assay between HMEC-1 cells treated with imatinib for 24–96 hrs and untreated controls (Fig. 1C). Incubation with imatinib in pharmacologically relevant concentrations for up to 96 hrs also did not alter cell counts (Fig. 1D). Imatinib did also not increase the time period needed to reach 100% confluence.
Expression of markers for vascular activation by imatinib-treated endothelial cells
ICAM-1, VCAM-1, endothelin-1 and VEGF are differentially expressed in SSc and are common markers of endothelial cell activation. Incubation of HMEC-1 cells with imatinib did not change the expression of ICAM-1, VCAM-1, endothelin-1 and VEGF (data not shown), suggesting that imatinib might not alter the activation of endothelial cells.
HMEC-1 cells expressed mRNA for PDGF recptor α (PDGFRα), PDGFRβ and c-kit, which are established targets of imatinib. Incubation with imatinib did not alter the expression of these genes in HMEC-1 cells (data not shown).
Effects of imatinib on migration, chemotaxis and tube forming
To assess whether imatinib reduces the migration of endothelial cells, scratch assays were performed. No differences in the distance between the two borders of the scratch were observed in HMEC-1 cells incubated with imatinib compared with untreated controls after 24 hrs and 48 hrs (Fig. 2A and B). The time until closure of the scratch was also not prolonged.
Fig 2.
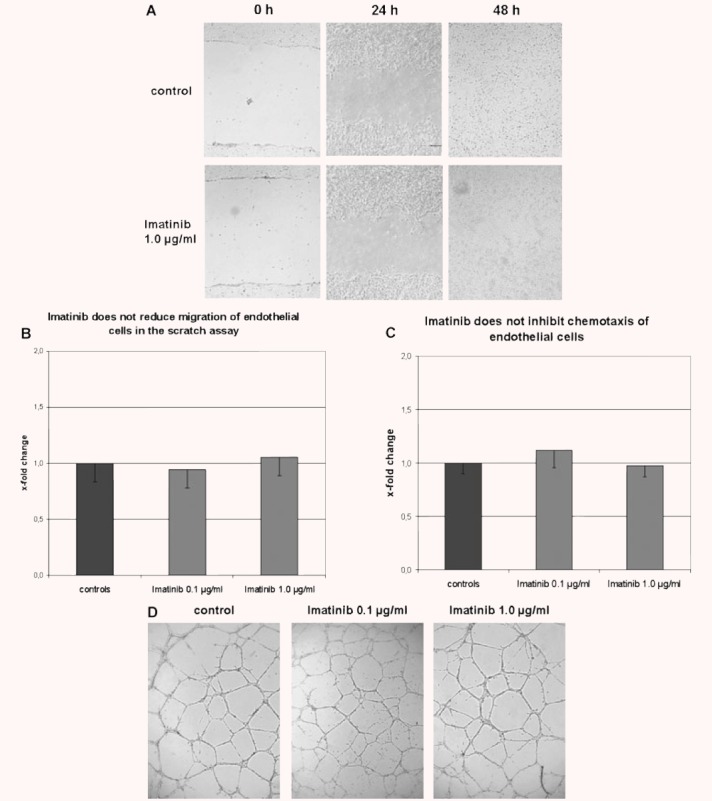
Imatinib does not affect migration, chemotaxis or tube formation of endothelial cells. (A and B) Imatinib does not reduce cell migration in the scratch assay. The distances between the borders of the scratch were not increased in imatinib-treated HMEC-1 cells at various time-points. Representative images taken after 48 hrs from untreated cells and HMEC-1 cells incubated with 0.1 μg/ml and 1.0 μg/ml imatinib are shown at 100-fold magnification in (A) and summarized in (B) (n= 6). (C) Imatinib does not inhibit chemotaxis of HMEC-1 cells in the transwell assay. The number of HMEC-1 cells that migrated through the 8-μm pores did not differ between HMEC-1 cells incubated with imatinib and controls (n= 8). (D) Imatinib does not inhibit the formation of capillary tubes. Imatinib did not reduce the number of tubes, the maximal length of the capillary tubes or the number of branching points. Representative images from controls and HMEC-1 cells treated with imatinib in concentrations of 0.1 μg/ml and 1.0 μg/ml are shown at 50-fold magnification (n= 15).
To study the effects of imatinib on chemotaxis of endothelial cells, a microchemotaxis assay was performed. Imatinib did not reduce the number of migrated HMEC-1 cells (Fig. 2C).
Next, we analysed whether imatinib inhibits tube formation using an in vitro capillary morphogenesis assay. Imatinib did not reduce the number of tubes, the maximal length of the capillary tubes or the number of branching points (Fig. 2), suggesting that imatinib does not affect tube formation in vitro.
Apoptosis of endothelial cells in mice treated with imatinib
To evaluate whether imatinib induces apoptosis of endothelial cells in vivo under fibrotic conditions, the number of apoptotic endothelial cells in mice challenged with bleomycin were quantified by TUNEL staining. Treatment of mice with imatinib in doses of 50 mg/kg/d for 4 weeks did not increase the number of TUNEL positive apoptotic endothelial cells compared with bleomycin alone (Fig. 3). Similarly, no increase in apoptotic endothelial cells was detectable in mice treated with imatinib at 150 mg/kg/d.
Fig 3.
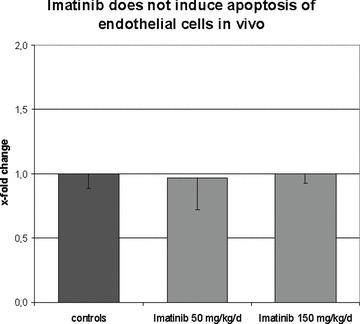
Imatinib does not induce apoptosis of endothelial cells in vivo. The number of apoptotic endothelial cells upon treatment with imatinib in vivo was quantified in the mouse model of bleomycin-induced dermal fibrosis. Treatment with imatinib at doses of 50 mg/kg/d and 150 mg/kg/d did not increase the number of apoptotic endothelial cells (n= 6 for each group).
Discussion
In the present study, we demonstrate that imatinib does not induce apoptosis or inhibit proliferation of microvascular endothelial cells in vitro and in vivo. Imatinib did also not affect activation of endothelial cells, reduce migration and chemotaxis or decrease the formation of capillary tubes. Together, these data suggest that imatinib does not affect major functions of endothelial cells. The lack of vascular side effects of imatinib observed herein is supported by first case reports describing the use of imatinib in patients with SSc and related fibrosing disorders [5, 11, 12]. All patients reported in these case series experienced a strong regression of fibrosis and tolerated imatinib well. No vascular side effects such as exacerbation of Raynaud’s phenomenon or increased frequency of fingertip ulcers were reported. Thus, treatment with imatinib might not be complicated by vascular side effects under physiological conditions. In contrast to the results obtained in our study, imatinib increased apoptosis, reduced migration and increased intercellular permeability in the EA.hy 926 endothelial cell line [13]. However, the concentrations of imatinib used in this study were significantly higher than in our study and exceed by far the concentrations needed for anti-fibrotic effects and the peak concentrations in human plasma after standard doses.
In SSc, ongoing damage of endothelial cells occurs via several distinct mechanisms. Cross-reactivity with antibodies against cytomegalovirus, anti-endothelial cell autoantibodies and microparticles has been implicated in the vascular pathogenesis of SSc [1]. Although we did not observe inhibitory effects of imatinib on major functions of endothelial cells, we cannot exclude that imatinib might inhibit functions of endothelial cells that have previously been affected by autoantibodies or microparticles. However, imatinib did not increase apoptosis of serum starved, pre-damaged HMEC-1 cells, indicating that imatinib does not enhance pre-existing damage of endothelial cells in general. Furthermore, first case reports of SSc patients treated with imatinib did not report exacerbations of vascular manifestations of SSc [5]. Another limitation of our study is that we worked with immortalized HMEC-1 cells. Although the phenotype of these cells resembles closely that of primary microvascular endothelial cells [8], these cells might still be less sensitive to imatinib than primary cells.
Although we did not observe toxic effects on endothelial cells in vitro and in our mouse model in vivo, we cannot exclude anti-angiogenic effects in other settings via inhibition of pericytes. PDGF signalling is crucial for several functions of pericytes and inhibition of the tyrosine kinase activity of PDGFR by imatinib-reduced migration, proliferation and survival of pericytes [14–16]. Another concern might the potential risk of cardiotoxicity as a small number of patients developed cardiomyopathy while on imatinib [17, 18].
In summary, we demonstrate that imatinib does not inhibit major functions of endothelial cells. Thus, based on pre-clinical models, the anti-fibrotic activity of imatinib does not seem to be accompanied by unwanted side effects on endothelial cells and imatinib might worsen the pre-existing vascular damage in SSc.
Acknowledgments
We thank Maria Halter for excellent technical assistance. Grant support for this study was provided by the Interdisciplinary Center of Clinical Research (IZKF) in Erlangen (grant A20), the Career Support Award of Medicine of the Ernst Jung Foundation 2007 and the ARTICULUM fellowship.
References
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–16. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Distler JH, Jungel A, Huber LC, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311–22. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 4.Savage DG, Antman KH. Imatinib mesylate–a new oral targeted therapy. N Engl J Med. 2002;346:683–93. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 5.Sfikakis PP, Gorgoulis VG, Katsiari CG, et al. Rheumatology. Oxford University Press; 2008. Imatinib for the treatment of refractory, diffuse systemic sclerosis. : Oxford. [DOI] [PubMed] [Google Scholar]
- 6.Park SI, Shah AN, Zhang J, et al. Regulation of angiogenesis and vascular permeability by Src family kinases: opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets. 2007;11:1207–17. doi: 10.1517/14728222.11.9.1207. [DOI] [PubMed] [Google Scholar]
- 7.Skhirtladze C, Distler O, Dees C, et al. Src kinases in systemic sclerosis: central roles in fibroblast activation and in skin fibrosis. Arthritis Rheum. 2008;58:1475–84. doi: 10.1002/art.23436. [DOI] [PubMed] [Google Scholar]
- 8.Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–90. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 9.Distler JH, Huber LC, Hueber AJ, et al. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005;10:731–41. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 10.Serrati S, Cinelli M, Margheri F, et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol. 2006;210:240–8. doi: 10.1002/path.2048. [DOI] [PubMed] [Google Scholar]
- 11.Distler J, Manger B, Spriewald B, et al. Treatment of pulmonary fibrosis with 20 weeks of treatment with imatinib in a patient with mixed connective tissue disease. Arthritis Rheum. 2008;58:2219–24. doi: 10.1002/art.23694. [DOI] [PubMed] [Google Scholar]
- 12.Kay J, High W. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 2008;58:2538–42. doi: 10.1002/art.23696. [DOI] [PubMed] [Google Scholar]
- 13.Vrekoussis T, Stathopoulos EN, De Giorgi U, et al. Modulation of vascular endothelium by imatinib: a study on the EA.hy 926 endothelial cell line. J Chemother. 2006;18:56–65. doi: 10.1179/joc.2006.18.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Perros F, Montani D, Dorfmuller P, et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–8. doi: 10.1164/rccm.200707-1037OC. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar VS, Shiwen X, Bostrom M, et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–65. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha A, Azevedo I, Soares R. Anti-angiogenic effects of imatinib target smooth muscle cells but not endothelial cells. Angiogenesis. 2007;10:279–86. doi: 10.1007/s10456-007-9080-2. [DOI] [PubMed] [Google Scholar]
- 17.Distler JH, Distler O. Cardiotoxicity of imatinib mesylate: an extremely rare phenomenon or a major side effect. Ann Rheum Dis. 2007;66:836. doi: 10.1136/ard.2006.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkela R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]


