Abstract
A strict regulation of contractility in the uterus and fallopian tube is essential for various reproductive functions. The uterus contributes, through either increased contractility or periods of relative quiescence, to: (i) expulsion of menstrual debris, (ii) sperm transport, (iii) adequate embryo placement during implantation, (iv) enlarging its capacity during pregnancy and (v) parturition. The dominant cell population of the uterine wall consists of smooth muscle cells that contain the contractile apparatus responsible for the generation of contractile force. Recent interest has focused on a new population of cells located throughout the myometrium on the borders of smooth muscle bundles. These cells are similar to interstitial cells of Cajal (ICC) in the gut that are responsible for the generation of electrical slow waves that control peristalsis. A precise role for myometrial Cajal-like interstitial cells (m-ICLC) has not been identified. m-ICLC express the c-kit receptor, involved in creating and maintaining the ICC phenotype in the gastrointestinal tract. However, both acute and prolonged inhibition of this receptor with the c-kit antagonist imatinib mesylate does not appear to affect the spontaneous contractility of myometrium. Calcium imaging of live tissue slices suggests that contractile signalling starts on the borders of smooth muscle bundles where m-ICLC are located and recently the possible role of extracellular ATP signalling from m-ICLC has been studied. This manuscript reviews the evidence regarding tissue-level signalling in the myometrium with a particular emphasis on the anatomical and possible functional aspects of m-ICLC as new elements of the contractile mechanisms in the uterus.
Keywords: interstitial cells, myometrial contractility, c-kit
Introduction
Nomenclature
Uterine contractions and the ‘functional syncytium theory’
Interstitial cells of Cajal in the gastrointestinal and urinary tracts
Are there m-ICLC in myometrium?
The effect of c-kit inhibition on myometrial contractility
Electrophysiology of m-ICLC
Imaging of tissue level signals in myometrium
Emerging concepts – the possible role of extracellular ATP in myometrial contractility
Where do m-ICLC fit in with a model for the generation of myometrial contractions?
Future research directions
Conclusion
Introduction
Advances in the fields of molecular biology have improved our knowledge of the cellular mechanisms controlling contractility of smooth muscle cells in the myometrium. The processes involved in the increase of intracellular calcium and the subsequent activation of the contractile apparatus of the cell are understood in great detail. Unfortunately, this increased understanding has not translated into similar advances in clinical treatments. The ability of clinicians to manipulate uterine contractions, particularly in cases of premature birth, remains limited. As an alternative to inhibiting the cellular processes responsible for generating the contractile forces, it may be possible to prevent contractility at the tissue level by manipulating the signals that generate the contractions in the first place and coordinate them throughout the myometrium. Regrettably, our understanding of these signalling mechanisms is very limited.
The muscular wall of the uterus, the myometrium, is an example of smooth muscle. Other examples of smooth muscles are found in the gastrointestinal tract, urological tract and in blood vessels and all of them have in common the ability to generate contractions autonomously. In these tissues, cells called interstitial cells (ICs) are found consistently. In the gastrointestinal tract, experimental evidence has revealed that these ICs, termed interstitial cells of Cajal (ICC), are crucial in the generation and coordination of the muscular activity that results in peristalsis (the wave-like contractions that propel the luminal contents). Similar evidence regarding ICs in the myometrium has until recently been lacking. It is quite evident that ‘Cajal-like’ ICs, if they were found to be present in the myometrium, would have the potential to fulfil a role similar to those cells found in the gastrointestinal tract for signal generation and coordination. If this were to be the case, exploration of their function may be key to furthering our understanding of tissue-level signalling in the myometrium. This review aims to summarize the published evidence to date and tries to incorporate this evidence as a new model for the development of myometrial contractions.
Nomenclature
It is important to consider at the outset the nomenclature of ‘interstitial’ cells so as to avoid potential confusion. The classification of ICs remains a matter of debate with no general agreement on terminology both in the gut and in the bladder. This could reflect the possibility that ‘ICs’ are a heterogenous population of cells that may have a variety of functions. For example, in the pelvi–calyceal junction of the urinary tract, ‘atypical smooth muscle cells,’ so called because of morphological similarities to smooth muscle cells, show spontaneous electrical activity [1]. Cells located in the renal pelvis also show spontaneous electrical activity, but possess a morphology more typical of ICs. In the urinary bladder, ICs are located in the suburothelial region, on the surface of smooth muscle bundles and in the fibromuscular septum. It is believed that these locations could be associated with different roles for ICs such as sensation and motor coordination. In addition, the expression of c-kit differs between these regions with c-kit positive ICs located in the detrusor and c-kit negative ICs in the suburothelial region [2].
One consistent area of confusion is the designation of these cells either as fibroblasts, myofibroblasts or fibroblastic-type cells. This is particularly the case in the bladder [3]. Fibroblasts are cells of similar morphology to ICs and their function is to generate connective tissue matrix, specifically collagen. In order to synthesize collagen, they must possess an enzyme called prolyl 4-hydroxylase. Immunohistochemical labels are available for the identification of this enzyme, and when used on myometrium, they identify cells dispersed evenly throughout the tissue that are separate from myometrial ICs [4]. A similar pattern of c-kit positive ICs and c-kit negative prolyl 4-hydroxylase positive fibroblasts has been observed in the human gut [5]. Other criteria for the differentiation of fibroblasts from myometrial ICs will be presented later in this review (see section ‘Are there ICC or ICLC in myometrium?’) and are summarized in Table 1. The distinction between fibroblasts and myometrial ICs is important, as their functions are likely to be very different. If research into myometrial contractility is to advance, then non-smooth muscle cells that have the appearance of fibroblasts (i.e. myometrial ICs) should not be labelled as ‘fibroblasts’ and ignored, as they have been in the past [6, 7].
Table 1.
Morphological aspects, semi-quantitative data concerning the ultrastructural elements (transmission electron microscopy) and specific markers of m-ICLC in comparison with archetypal enteric interstitial cells of Cajal (ICC) and fibroblasts [65–71]
| Interstitial cells of Cajal (ICC) | Myometrial interstitial Cajal-like cells | Fibroblasts | ||
|---|---|---|---|---|
| Cell shape | Oval or spindle-shaped cells | Spindle or stellate body | Polymorphic body | |
| Nucleus | Oval, mostly euchromatic | Oval, heterochromatic under nuclear membrane | Oval, euchromatic with 1–2 visible nucleoli | |
| Cytoplasm | Smooth ER | ++ | + | +− |
| Rough ER | + | + | +++ | |
| Golgi complex | + | + | +++ | |
| Mitochondria | +++ | ++ | + | |
| Intermediate filaments | ++ | + | + | |
| Microtubules | + | + | + | |
| Thin filaments | + | + | + | |
| Calcium releasing units | n.a. | present | n.a. | |
| Other structures | Caveolae | + | + | − |
| Basal lamina | 0 | +− | − | |
| Immunohisto-chemical markers | c-kit | Co-localization of c-kit, CD34 and connexin 43, lack of prolyl 4-hydroxlase | Prolyl 4-hydroxylase | |
| Intercellular contacts | Nerve endings | ++ | + | − |
| Blood vessels | n.a. | + | − | |
| Immune cells | n.a. | +++ | − | |
| Smooth muscle cells | + | + | − | |
| Other interstitial cells | + | + | − | |
| Gap junctions | + | + | − |
It is likely that there will be a need for a strict classification for ICs in the myometrium as more is discovered about their morphology, antigen expression, and most importantly function. The term ‘myometrial Cajal-like interstitial cells’ (m-ICLC), that has been proposed previously [8], is reasonable given their ultrastructural similarities with ICCs. However, it should be noted that a pacemaking role for m-ICLC similar to that of ICCs in the gut has not yet been demonstrated and so the term m-ICLC should not be taken to mean that these cells have the same role.
Uterine contractions and the ‘functional syncytium theory’
For parturition to occur, three processes take place: (i) The foetal membranes become weaker and eventually rupture, (ii) remodelling and ripening of the cervix takes place and (iii) regular and forceful uterine contractions become established [9]. These changes do not just occur at the time of parturition. During pregnancy the uterus evolves to prepare for the onset of labour, most notably in the case of the cervix that undergoes change throughout the pregnancy [10]. However, it is the development of forceful uterine contractions that is perhaps the most dramatic of these changes as it occurs over a relatively short span of time. This switch from uterine quiescence to an actively contracting uterus is thought to depend on the increased expression of a cassette of genes encoding a number of proteins known collectively as contraction associated proteins (CAP). These include the oxytocin receptor, prostaglandin receptors and the gap junction protein connexin 43 [11–15].
Research in the field of myometrial contractility has tended to focus on the cellular mechanisms involved in the coupling of membrane depolarization, via an action potential in the tissue, to the activation of the contractile apparatus within the smooth muscle cell. This excitation–contraction coupling is achieved by an elevation in intracellular calcium levels and is tightly controlled by a variety of factors [16]. These mechanisms are now understood in great detail but the process by which this cellular contractility is initiated in a coordinated fashion, so as to be able to generate contractions throughout the entire organ, is less understood. In fact, our knowledge of tissue-level signalling in the myometrium has not progressed significantly since the work of Robert Garfield, first published in 1977 [17]. He showed that connexin 43 gap junctions are seen in great numbers on the smooth myocyte membrane only at the time of parturition and this coincides with an increase in the electrical conductance of the tissue [18]. He also showed that the inhibition of connexin 43 pores by agents affecting the plasma membrane leads to an abolition of contractions. From these and other data, it was concluded that connexin 43 gap junctions expressed at the time of labour lead to the development of a ‘functional syncytium’ where all the smooth muscle cells become connected electrically [6]. This permits an action potential to pass throughout the tissue in all directions, so allowing coordinated contractions to take place. This concept was the focus of a great deal of research in the 1980s and 1990s and remains accepted by the majority of physiologists working in this field as the mechanism by which uterine contractions are coordinated.
However, the functional syncytium model has been challenged recently. In a comprehensive article, theoretical and experimental evidence was presented regarding tissue-level signalling in the myometrium [19]. This evidence argued that a functional syncytium is not consistent with the development of the classic 40- to 60-sec duration bell-shaped contraction profile of the myometrium. Instead it should produce a contraction of rapid onset lasting for approximately 20 sec. It also showed that an action potential is observed only at the beginning of each myometrial contraction with the remaining myocytes being recruited in a second phase of non-electrical signalling. Again, one would expect all myocytes to be recruited synchronously in a ‘functional syncytium’. In order to resolve these issues, a ‘biphasic’ model was proposed in which a rapid signal is conducted through the myometrium followed by a slower phase of recruitment of the remaining majority of smooth muscle cells via a ‘calcium wave’[20, 21]. Although this biphasic model appears to fit with the experimental findings, it does not as yet offer a complete explanation for the following reasons. First, the mechanism for the generation of the action potential has not been identified. Secondly, in this biphasic model, the action potential travels through the myometrium only stimulating a few cells in each bundle and leaving the remaining majority of these cells to be stimulated by intercellular calcium waves. The reason for this selective recruitment of smooth muscle cells, rather than the expected recruitment of all cells as predicted in the functional syncytium model, has not been addressed. Thirdly, and finally, the model does not address the presence of organized and co-ordinated contractions of similar profiles at times other than in labour, such as those contractions occurring throughout pregnancy (Braxton–Hicks contractions [22]) and contractions in the non-pregnant uterus [23, 24]. It is proposed later in this article that ICs may provide a mechanism that can explain these inconsistencies and support a biphasic model. However, before considering the case of the myometrium, it is pertinent to consider first the evidence regarding ICs in other contractile muscular organs.
Interstitial cells of Cajal in the gastrointestinal and urinary tracts
Experimental evidence using smooth muscle from the gut has strongly suggested that ICC act as both pacemakers and signal transducers for electrical activity [25, 26]. ICCs are characterized by the finding of c-kit, a membrane bound tyrosine kinase essential for normal functioning of the cell. ICCs also have specific ultrastructural morphological features evident on transmission electron microscopy (TEM) that together are considered the ‘gold standard’ for the identification of these cells [27]. These cells have an ovoid or spindle-shaped body, a clear nucleus, two or more processes, several mitochondria evenly dispersed, well developed smooth endoplasmic reticulum and many caveolae. Moreover, all of them are in close contact with smooth muscle cells, nerve endings and to each other. The cell-to-cell contacts with the smooth muscle cells and to each other are often gap junctions. As a result of all these characteristics, these cells fulfil the ideal conditions to play a role in eliciting and co-ordinating muscle activity. Functional evidence from studies of White-Spotting mice that do not have ICCs in the myenteric region of the small intestine [28], and from physiological studies using neutralizing antibodies directed against the c-kit receptor in gastrointestinal smooth muscle [29, 30], has shown that ICCs are essential for normal contractility of the muscle. Studies have also implicated abnormal function of ICCs in several diseases such as diabetic gastroparesis [31], post-operative ileus [32], Hirschsprungs disease [33] and gastrointestinal dysmotility following repair of gastroschisis in the neonate [34, 35].
The morphological and functional role of ICs in the urinary tract has also been the subject of interest recently [36, 37]. Anatomically, ICs are predominantly located on the boundary of smooth muscle bundles throughout the detrusor muscle [38]. NO-sensitive cGMP-producing ICs have been identified in the detrusor muscle of the mouse bladder. Activation of these cells was shown to alter the pattern of muscarinic-induced phasic activity implying a functional role for IC in the bladder [39]. Hashitani studied electrical signalling and calcium transients in guinea-pig detrusor and showed that action potentials preceded the rise in intracellular calcium. These rises in intracellular calcium were seen to spread as a calcium wave from the boundary of the smooth muscle bundle where ICs are located [40]. In a later study using microelectrodes, a direct role for signal initiation by IC was not confirmed and it was considered that IC might instead modulate signalling rather than generate the signals [41]. A recent study on rabbit bladder suggested that ICs could be an important source of PGs involved in the regulation of spontaneous rhythmic contraction of the bladder [42].
It can be seen that ICs appear essential for smooth muscle contractility in the gastrointestinal tract and probably in the genitourinary tract. ICs have also been identified in other smooth muscle organs such as in the wall of several blood vessels [43, 44], gall bladder [45, 46] and fallopian tube [47, 48]. ICs, recently called interstitial Cajal-like cells (ICLC), also exist in pancreas [49], prostate [50–52], penis [53], mammary gland [54–56] and myocardium [57–60].
Are there m-ICLC in myometrium?
The presence of non-smooth muscle cells (possibly m-ICLC) in the myometrium has been documented by a number of authors [7, 61], but the first article addressing myometrial ICs in detail was published in 2005 [62]. This study identified myometrial ICs using an antibody for the intermediate filament vimentin, a marker for cells of mesenchymal origin (this includes both m-ICLC and fibroblasts). These vimentin-positive cells were negative for c-kit using standard light microscopy immunohistochemistry. Ultrastructurally, myometrial ICs contained numerous mitochondria, caveolae and intermediate filaments, but did not contain myofilaments and were non-contractile. Whilst this paper remains the most important reference to date, a number of criticisms can be made of the methodology used. For example, the use of the vimentin antibody does not discriminate between m-ICLC and fibroblasts.
In the following year, another group published evidence regarding myometrial ICs which they termed m-ICLC, using a variety of immunohistochemical techniques, electron microscopy and electrophysiology [8]. First of all, they searched for m-ICLC in human myometrial tissue and cell cultures using classical staining methods used by Cajal himself when he described the cells bearing his name (Figs. 1–3A). In vitro, myometrial ICs possessed very long cytoplasmic processes which were found by staining with Janus green B to contain numerous mitochondria (Figs 3B and 4A). These cells represented approximately 7% of the total cell number when counting these cells on semi-thin sections stained with toluidin blue, considered by the authors as relevant for preliminary identification of m-ICLC (Fig. 4B). This technique is easy to perform and permits the general characterization of myometrial ICs. Ultrastructurally, two to three characteristic processes were observed and these prolongations were very long and thin (60 and <0.5 μm, respectively) (Fig. 5). The cells expressed c-kit when examined by immunofluorescence (Fig. 6) and they connected with each other and with smooth muscle cells by gap junctions. Based on ultrastructural features, a set of criteria was assembled that allowed the differentiation of m-ICLC from fibroblasts:
close contact with target nerve bundles and smooth muscle cells,
characteristic cytoplasmic processes,
gap junctions with smooth muscle cells or with each other,
basal lamina occasionally present,
caveolae 2–4% of cytoplasmic volume; ∼0.5 caveolae/μm of cell membrane length,
mitochondria 5–10% of cytoplasmic volume,
endoplasmic reticulum about 1–2%, either smooth or rough,
cytoskeleton consisting of intermediate and thin filaments, as well as microtubules and
myosin thick filaments undetectable.
Fig 1.
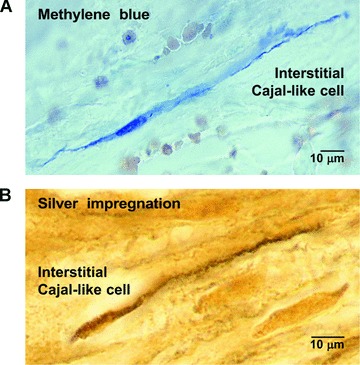
Human myometrium. (A) Methylene blue vital staining, before cryofixation (cryosectioning). Note the selective affinity of an m-ICLC for the blue dye. (B) Silver impregnation after fixation and paraffin embedding. A pyriform m-ICLC cell with a very long, moniliform process. Original magnification: 1000×. Reprinted from Ref. [78], with permission from Elsevier.
Fig 3.
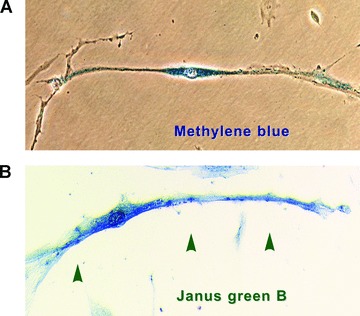
Vital staining methods (efficient in pointing out the very long processes) performed on m-ICLC in culture. Primary confluent cultures (day 8) from pregnant human myometrium. (A) Representative m-ICLC with high affinity for methylene blue. (B) m-ICLC stained with Janus green B, a marker for mitochondria in living cells. Note the positive mitchondria in m-ICLC dilations (arrows) original magnification 40×. Modified from Ref. [8], with permission from FCMM.
Fig 4.
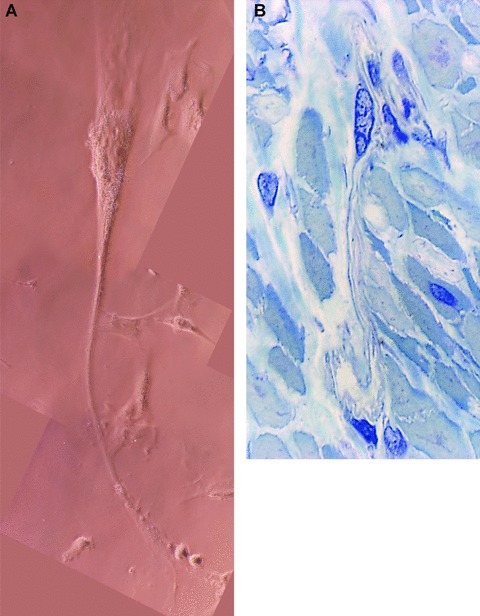
(A) Human myometrium. Primary semi-confluent cell culture (day 4), phase contrast microscopy. Photographic reconstruction of an m-ICLC with a very long, moniliform cytoplasmic processes (arrows) emerging from cell body. Higher magnification (40×). (B) Human pregnant myometrium (39 weeks of gestation). Semi-thin sections (0.5- to –1-μm thick) of uterine muscular layer embedded in Epon resin and stained with toluidine blue. One may observe the very long process of m-ICLC squeezing between obliquely cut smooth muscle cells. Original magnification 100×.
Fig 5.
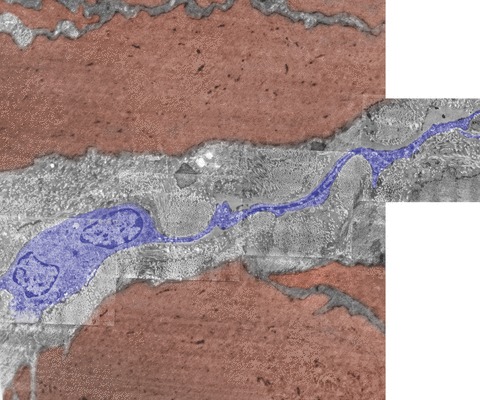
Electron micrograph of human non-pregnant myometrium. Note the presence of an m-ICLC (blue) with a long thin cytoplasmic process that suddenly comes out from the cellular body and runs between smooth myocytes (brown). One may observe, on the length of the cellular process, the presence of cytoplasmic dilations housing mitochondria. Original magnification, ×9,100. TEM (photographic reconstruction) illustrating a thin (wontedly below 0.1-μm thick) cytoplasmic process of m-ICLC expanded from the cell body.
Fig 6.
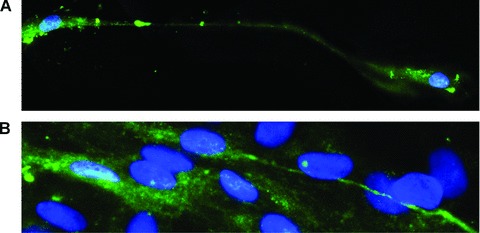
Human myometrium cells in culture (the second passage); immunofluorescence labelling for c-kit; FITC-conjugated secondary antibodies (green) were used to visualize the reaction (green), m-ICLC that display characteristic morphologic feature (long, moniliform processes), express c-kit and contact adjacent cells; Hoechst 33342 (blue) for nuclear counterstaining. Original magnification 60×. Reprinted from Ref. [8], with permission from FCMM.
Fig 2.
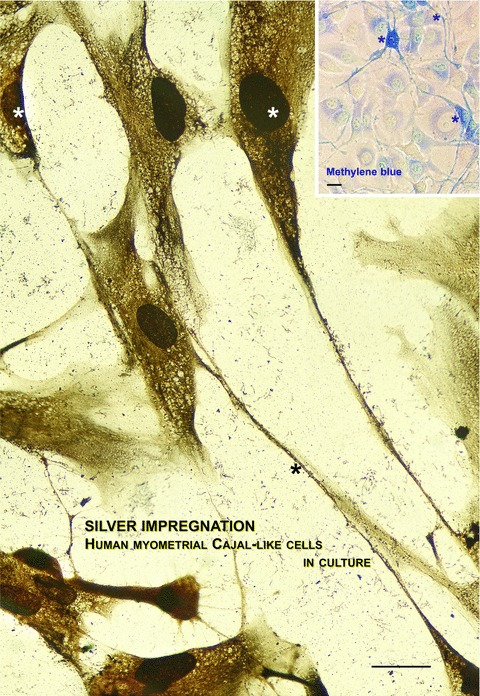
Photographic reconstruction of human m-ICLC in culture establishing contacts with smooth muscle cells. In compliance with our experience, silver impregnation is one of the choice methods for revealing the typical moniliform aspect of m-ICLC in culture. Inset displaying the same interlaced distribution of m-ICLC (*) by methylene blue vital staining. Both methods reveal weaker stained myocytes. Scale bar = 10 μm. Reprinted from Ref. [63], with permission from FCMM.
The possible spatial relationships between myometrial ICs and other cells in the myometrium are represented in Fig. 7.
Fig 7.
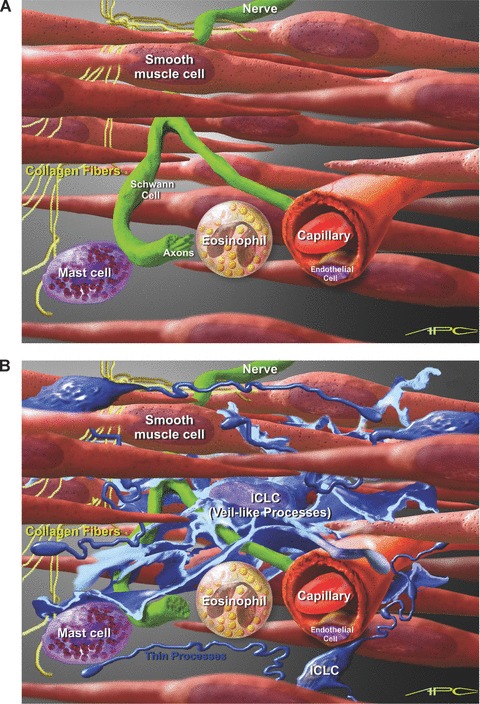
Artistic view of possible interconnectivity of m-ICLC in the uterine wall. (A) Myometrial background with smooth muscle cells, nerve fibers and some connective tissue cells. (B) Close proximity of m-ICLC processes with smooth muscle cells, unmyelinated nerves, capillaries, collagen fibres and immunoreactive cells. Scale bar – approximately 7 μm (erytrocyte’s diameter).
More recently, the same group showed evidence that oestrogen and progesterone receptors are located in the nuclei of m-ICLC [63] (Fig. 8). The authors concluded by hypothesising that myometrial ICs could act as ‘hormonal “sensors”, possibly participating in the regulation of human myometrial contractions (via gap junctions and/or by paracrine signalling)’.
Fig 8.
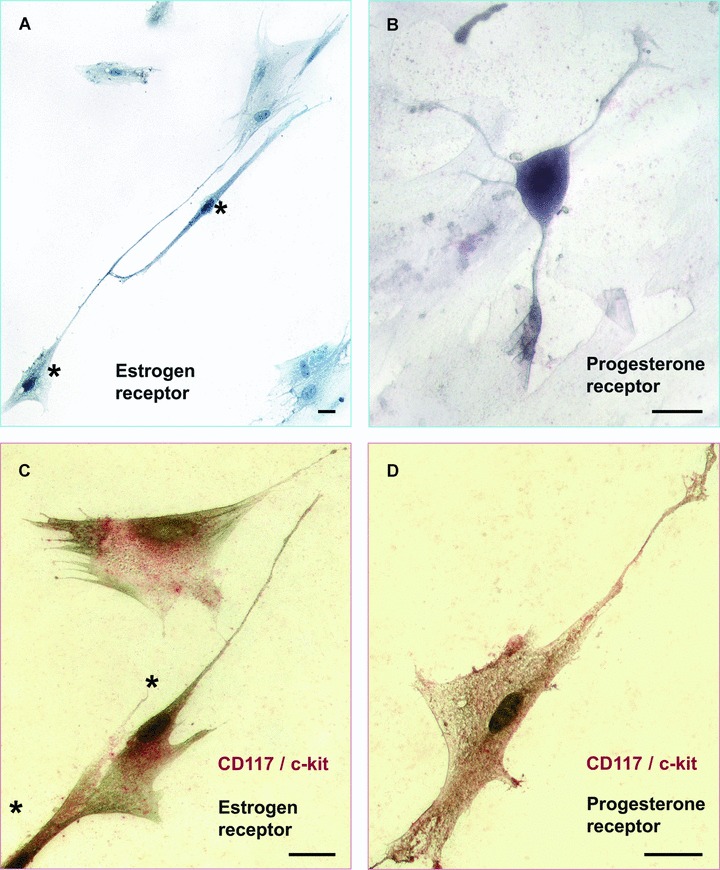
Human myometrial cell culture, fourth passage. Immunocytochemical staining for the estrogen and progesterone receptors. (A) Immunocytochemical detection of estrogen receptor – dark stained nuclei (*), counterstaining with methyl green for negative nuclei. (B) ICLC stained positive for progesterone receptor. (C) Double staining (*) for CD117/c-kit (red) and estrogen receptor (black). (D) Double staining for CD117/c-kit (red) and progesterone receptor (black). Scale bar = 10 μm.
A further publication has supported the observation that m-ICLC are c-kit positive [64]. m-ICLC were again seen to be located mainly on the boundaries of smooth muscle bundles throughout the myometrium and are present in myometrial biopsies taken throughout reproductive life. Also, it was observed that m-ICLC possess connexin 43 in its unphosphorylated form distributed evenly throughout the cell membrane. The strong and specific expression of this form of connexin 43 may be useful in future experiments for the isolation and identification of m-ICLC and the authors postulated that the pattern of staining could represent the presence of connexin 43 hemichannels (see the section ‘Emerging concepts’).
All these data are summarized in Table 1.
The effect of c-kit inhibition on myometrial contractility
c-kit is a transmembrane receptor that has been shown to be essential for the development and the maintenance of the IC phenotype in smooth muscle from the gastrointestinal tract [72, 73]. As m-ICLC express c-kit, the next logical step for a number of investigators was to explore the effect of c-kit receptor inhibition on myometrial contractility.
A c-kit receptor antagonist called imatinib mesylate (Glivec) [74] has been used to study the effects of c-kit inhibition on the in vitro contractility of myometrium from term pregnant rabbits. Only amplitude and not frequency was affected following the addition of imatinib and this occurred only at the highest dose studied (100 μM), whereas a dose of 10 μM had no effect [75]. This study was repeated with human myometrium obtained from term elective caesarean sections and again only the amplitude and not the frequency was affected (unpublished observation – Hutchings). In contrast, a different study using human myometrium obtained from benign non-pregnant hysterectomy specimens showed that the administration of imatinib led to a reduction in both amplitude and frequency with the eventual abolition of contractions after prolonged exposure (up to 180 min for 2 μM imatinib). Spontaneous contractions were restored after 30 min of washing with physiological saline. In a third study, imatinib decreased the frequency and amplitude of longitudinal but not circular contractions of the mouse uterus [76].
Data from studies of muscle from other organs have also led to conflicting conclusions. Three studies observed that imatinib leads to a decrease in contractile amplitude in guinea pig [77] and rat [2] detrusor muscle and in human intestine [78]. In another study, the addition of imatinib to smooth muscle from the gastrointestinal tract of mice abolished muscular activity in a dose-dependant manner from a concentration of between 9–27 μM up to a dose of 81 μM. The effect was reversed when the agent was washed from the tissue [79]. Following abolition of the contractions, the muscle was seen to be able to respond to depolarization following addition of potassium chloride.
Two observations are evident from the results of these experiments. First, the doses of imatinib that were used are very high compared with either the IC50 concentrations (between 0.1 and 1 μM) or the serum levels achieved in humans receiving the drug (approximately 4 μM for peak levels and 2 μM for trough values [74]). This raises the possibility that any effect seen with these high doses could be as a result of non-specific effects rather than as a consequence of c-kit inhibition. Secondly, the results from the different studies fall into two broad groups. Either only amplitude is decreased at high doses of imatinib or imatinib has effects on both frequency and amplitude and leads to the eventual abolition of contractions. Perhaps these differences could be explained by differences in species or tissue type, but regarding the two human myometrial studies, there are no obvious differences in methodology that could account for the conflicting results. Popescu’s team used non-pregnant myometrium (Fig. 9), but why this differs from pregnant myometrium is not easy to explain.
Fig 9.
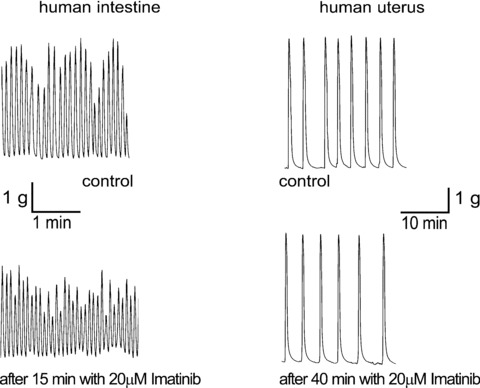
Representative traces of myometrial human smooth muscle inhibition by imatinib 20 μM. Reprinted from Ref. [78], with permission from Elsevier.
Data from studies of the gastrointestinal tract has suggested that c-kit inhibition has a delayed effect taking up to 48 hrs to abolish electrical ‘slow-wave’ activity and to disrupt IC networks as imaged by immunohistochemistry [72]. In order to test for a delayed action on myometrium, the effect of a prolonged administration of imatinib of up to 14 days on myometrial contractility has been investigated in a study using pregnant rabbits [4]. However, no effect was observed on gestation at delivery and no difference in myometrial contractility in vitro was observed between treated and control rabbits. In summary, it does not appear that c-kit receptor inhibition has promise as a potential therapeutic target for the clinical manipulation of uterine contractions. Nor can it be used readily as a research tool for the study of myometrial IC function.
Electrophysiology of m-ICLC
The accepted mechanism for the generation of contractile force in the myometrium is through an action potential giving rise to elevated free cytosolic calcium levels [8]. In theory, if m-ICLC are the pacemaking cells in the myometrium, these cells should be found to be electrically active. Several studies have approached this question by employing direct electrophysiological measurements.
In the first study, isolated myometrial ICs were shown to possess a stable resting membrane potential of −58 ± 7 mV compared to −65 ± 13 mV seen with smooth muscle cells. Outward currents were not observed implying these cells were not capable of generating spontaneous action potentials [62]. The authors concluded that m-ICLC were not likely to be pacemaking cells in view of their inability to generate ‘slow waves’ of spontaneous electrical activity. In contrast, another study showed that isolated myometrial ICs exhibited spontaneous electrical activity with field potentials of 62.4 ± 7.22 mV of short duration (1.197 ± 0.04ms) [8] (Fig. 10). Therefore, it is unclear from these contrasting studies as to whether or not isolated m-ICLC are electrically active.
Fig 10.
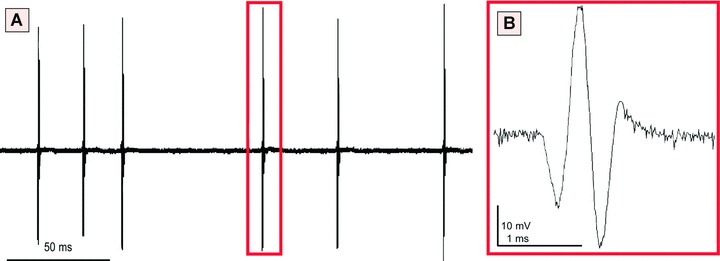
Extracellular single-unit recording performed on m-ICLC cultures (human pregnant myometrium), at the third passage. (A) Notice the spontaneous electrical activity in MIC. (B) Spontaneous field potential pattern. Reprinted from Ref. [8], with permission from FCMM.
The use of isolated ICs for the study of spontaneous electrical activity may need to be questioned. In an interesting series of experiments using smooth muscle from the guinea pig stomach, it has been shown that isolated cells that are not spontaneously electrically active can nevertheless be made to function as electrical pacemakers [80]. For this to happen, the cells need to be capable of generating spontaneous intracellular calcium oscillations that then become amplified by the fact that the cells are joined together by cell–cell contacts to form a network of ‘coupled oscillators’. These amplified calcium oscillations are then sufficient to generate action potentials virtually simultaneously over relatively large distances. Therefore, future study of pacemaking activity in the myometrium and related tissues may need to incorporate the idea that cells behave differently when in tissue as opposed to in isolation.
Imaging of tissue level signals in myometrium
Myometrial smooth muscle cells contract in response to an elevation in intracellular free calcium. Consequently, as an alternative to electrophysiological methods, calcium imaging may be used to observe the spatial and temporal pattern of the spread of contractility. In parallel with the idea expressed in the previous section that the study of tissue is crucial for the study of tissue-level signalling in the myometrium, Shmygol and colleagues developed a novel technique involving thin slices of live myometrial tissue loaded with Fluo-4. With this technique, they have been able to demonstrate synchronous large rises in intracellular calcium within smooth muscle bundles [81]. Prior to the start of these calcium waves, fluctuations in intracellular calcium are seen in cells within the fibromuscular septum in exactly the same location as that of m-ICLC. These findings are important in that the site of origin and subsequent spread of the signal can literally be seen. This is the first evidence of its kind in the myometrium and supports the possibility that the signals for myometrial contractions originate from m-ICLC.
Emerging concepts – the possible role of extracellular ATP in myometrial contractility
If it is indeed the case that m-ICLC generate the signal for the initiation of contractions, then the mechanisms involved in this process may be important targets for therapeutic interventions that aim to alter uterine contractility (e.g. in the case of premature birth). One recent hypothesis is that the unphosphorylated connexin 43 expressed on m-ICLC represents the presence of connexin hemichannels rather than gap junctions [4]. Hemichannels are free connexins, transmembrane hexamer proteins whose central pore is able to open under certain conditions to release molecules involved in cell–cell communication [82]. ATP is the molecule implicated most frequently [83, 84], and a recent study using myometrial biopsies from elective caesarean sections has shown that extracellular ATP appears crucial for the initiation of contractions and regulation of their frequency [85]. Multiple purinergic receptors are present in the myometrium, and early contractility studies have suggested that the G-protein coupled P2Y2 receptor is likely to be responsible for this action (unpublished data – Hutchings). Therefore, signalling via extracellular ATP and purinergic receptors represents one of the possible mechanisms by which m-ICLC may act as pacemakers. Further study of this mechanism and others like it may offer novel therapeutic strategies for the control of uterine contractility.
Where do m-ICLC fit in with a model for the generation of myometrial contractions?
A possible schematic model for the generation of myometrial contractions is presented in Fig. 11. The anatomical arrangement of smooth muscle bundles with m-ICLC located on the surfaces of these bundles is consistent with the immunohistochemical observations described above. It is proposed that interconnected m-ICLC provide a synchronized first phase signal throughout the myometrium in keeping with the first phase of Young’s biphasic theory. The mechanism for this synchronization could be similar to that of ‘coupled oscillators’ proposed by Van Helden (see the section ‘Electrophysiology of myometrial interstitial cells’). m-ICLC then stimulate neighbouring smooth muscle cells to contract with the subsequent calcium wave travelling at a relatively lower velocity towards the centre of the smooth muscle bundle (as seen with calcium imaging of myometrial sections) [81]. This calcium wave would also be consistent with the second slower phase in the biphasic theory.
Fig 11.
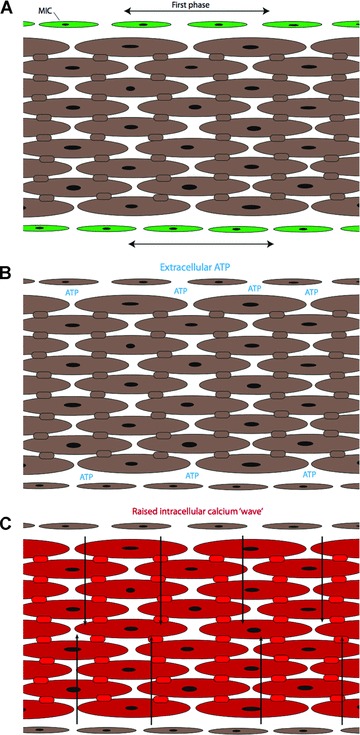
Schematic representation of a smooth muscle bundle. (A) Myometrial ICs (green) produce a synchronous signal along the border of the smooth muscle bundles. (B) ATP and possibly other messenger molecules released by myometrial ICs provide the stimulus for the generation of a contraction. (C) The contraction starts from the border of the smooth muscle bundle and passes towards the centre via a calcium wave (arrows) transmitted through gap junctions.
Future research directions
The model proposed in the previous section fits with existing data regarding tissue-level signalling, but evidently many gaps in our current understanding remain. In order to validate the model, further work is warranted. Specifically, the method of synchronization of m-ICLC, the regulation of their spontaneous activity, the precise role of extracellular ATP and the nature of the signal from m-ICLC to smooth muscle cell need to be established.
It should be re-emphasized that the study of tissue-level signalling is likely to be more successful and informative if actual tissue rather than cell culture or isolated cells are investigated. With this in mind, the development of techniques such as the use of thin slices of live myometrium, as used by Shmygol and colleagues, can be seen to be of critical importance.
Conclusion
After many years of research directed at cellular mechanisms responsible for the development of uterine contractions, interest is now being directed to the emerging field of tissue-level signalling and the potential role of m-ICLC. Further research in this direction holds the promise of a better understanding of the mechanisms controlling myometrial contractility and consequently of developing therapeutic strategies for the treatment of conditions such as premature birth.
Acknowledgments
This review article is based on a thesis entitled ‘Myometrial ICs, extracellular ATP and uterine pacemaking’, submitted by GH for the award of Doctor in medical sciences. GH would like to acknowledge the support of Professors Jan Deprest and Dirk de Ridder of the Catholic University of Leuven, Belgium.
References
- 1.Lang RJ, Klemm MF. Interstitial cell of Cajal-like cells in the upper urinary tract. J Cell Mol Med. 2005;9:543–56. doi: 10.1111/j.1582-4934.2005.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gevaert T. Acta biomedical Lovaniensia. Leuven University Press; 2007. Autonomous contractile activity in bladder as a new concept in neuro-urology: a study in rat and mouse Thesis 394, [Google Scholar]
- 3.Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int. 2006;97:29–32. doi: 10.1111/j.1464-410X.2006.05818.x. [DOI] [PubMed] [Google Scholar]
- 4.Hutchings G, Gevaert T, Deprest J, et al. Immunohistochemistry using an antibody to unphosphorylated connexin 43 to identify human myometrial interstitial cells. Reprod Biol Endocrinol. 2008;6:43. doi: 10.1186/1477-7827-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderwinden JM, Rumessen JJ, De Laet MH, et al. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest. 1999;79:59–65. [PubMed] [Google Scholar]
- 6.Garfield RE, Ali M, Yallampalli C, et al. Role of gap junctions and nitric oxide in control of myometrial contractility. Semin Perinatol. 1995;19:41–51. doi: 10.1016/s0146-0005(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 7.Yoshino M, Wang SY, Kao CY. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J Gen Physiol. 1997;110:565–77. doi: 10.1085/jgp.110.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciontea SM, Radu E, Regalia T, et al. C-kit immunopositive interstitial cells (Cajal-type) in human myometrium. J Cell Mol Med. 2005;9:407–20. doi: 10.1111/j.1582-4934.2005.tb00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith R. Parturition. N Engl J Med. 2007;356:271–83. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 10.Word RA, Li XH, Hnat M, et al. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey JT, Puttaramu R, Word RA, et al. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab. 2009;94:421–7. doi: 10.1210/jc.2008-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fetalvero KM, Zhang P, Shyu M, et al. Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition. J Clin Invest. 2008;118:3966–79. doi: 10.1172/JCI33800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taggart MJ, Europe-Finner GN, Mitchell BF. Possible dual roles for prostacyclin in human pregnancy and labor. J Clin Invest. 2008;118:3829–32. doi: 10.1172/JCI37785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Morgan KG, Jones CJ, et al. Myometrial mechanoadaptation during pregnancy: implications for smooth muscle plasticity and remodeling. J Cell Mol Med. 2008;12:1360–73. doi: 10.1111/j.1582-4934.2008.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong D, Lu X, Wang HX, et al. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biol Reprod. 2009;80:1099–106. doi: 10.1095/biolreprod.108.071969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wray S, Jones K, Kupittayanant S, et al. Calcium signaling and uterine contractility. J Soc Gynecol Investig. 2003;10:252–64. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 17.Garfield RE, Sims S, Daniel EE. Gap junctions: their presence and necessity in myometrium during parturition. Science. 1977;198:958–60. doi: 10.1126/science.929182. [DOI] [PubMed] [Google Scholar]
- 18.Sims SM, Daniel EE, Garfield RE. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J Gen Physiol. 1982;80:353–75. doi: 10.1085/jgp.80.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young RC. Myocytes, myometrium, and uterine contractions. Ann N Y Acad Sci. 2007;1101:72–84. doi: 10.1196/annals.1389.038. [DOI] [PubMed] [Google Scholar]
- 20.Young RC, Zhang P. Tissue-level bioelectrical signals as the trigger for uterine contractions in human pregnancy. J Soc Gynecol Investig. 2004;11:478–82. doi: 10.1016/j.jsgi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Young RC, Zhang P. Inhibition of in vitro contractions of human myometrium by mibefradil, a T-type calcium channel blocker: support for a model using excitation-contraction coupling, and autocrine and paracrine signalling mechanisms. J Soc Gynecol Investig. 2005;12:e7–12. doi: 10.1016/j.jsgi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull AC, Anderson AB. Uterine contractility and oxytocin sensitivity during human pregnancy in relation to the onset of labour. J Obstet Gynaecol Br Commonw. 1968;75:278–88. doi: 10.1111/j.1471-0528.1968.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 23.Bulletti C, De Ziegler D, Polli V, et al. Uterine contractility during the menstrual cycle. Hum Reprod. 2000;15(Suppl. 1):81–9. doi: 10.1093/humrep/15.suppl_1.81. [DOI] [PubMed] [Google Scholar]
- 24.Bulletti C, De Ziegler D. Uterine contractility and embryo implantation. Curr Opin Obstet Gynecol. 2005;17:265–76. doi: 10.1097/01.gco.0000169104.85128.0e. [DOI] [PubMed] [Google Scholar]
- 25.Hirst GD, Ward SM. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J Physiol. 2003;550:337–46. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirst G, Edwards F. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- 27.Faussone Pellegrini MS, Thuneberg L. Guide to the identification of interstitial cells of Cajal. Microsc Res Tech. 1999;47:248–66. doi: 10.1002/(SICI)1097-0029(19991115)47:4<248::AID-JEMT4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 28.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond) 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ward SM, Harney SC, Bayguinoy JR, et al. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol (Lond) 1997;505:241–58. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torihashi S, Nishi K, Tokutomi Y, et al. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–8. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 31.Horvath VJ, Vittal H, Lorincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Yanagida H, Yanase H, Sanders KM, et al. Intestinal surgical resection disrupts electrical rhythmicity, neural responses, and interstitial cell networks. Gastroenterology. 2004;127:1748–59. doi: 10.1053/j.gastro.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 33.Rolle U, Piotrowska AP, Nemeth L, et al. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928–33. doi: 10.5858/2002-126-0928-ADOICO. [DOI] [PubMed] [Google Scholar]
- 34.Midrio P, Faussone-Pellegrini MS, Vannucchi MG, et al. Gastroschisis in the rat model is associated with a delayed maturation of intestinal pacemaker cells and smooth muscle cells. J Pediatr Surg. 2004;39:1541–7. doi: 10.1016/j.jpedsurg.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Vargun R, Aktug T, Heper A, et al. Effects of intrauterine treatment on interstitial cells of Cajal in gastroschisis. J Pediatr Surg. 2007;42:783–7. doi: 10.1016/j.jpedsurg.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 36.McHale NG, Hollywood MA, Sergeant GP, et al. Organization and function of ICC in the urinary tract. J Physiol. 2006;576:689–94. doi: 10.1113/jphysiol.2006.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang RJ, Tonta MA, Zoltkowski BZ, et al. Pyeloureteric peristalsis: role of atypical smooth muscle cells and interstitial cells of Cajal-like cells as pacemakers. J Physiol. 2006;576:695–705. doi: 10.1113/jphysiol.2006.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- 39.Lagou M, Drake MJ, Markerink-VAN Ittersum M, et al. Interstitial cells and phasic activity in the isolated mouse bladder. BJU Int. 2006;98:643–50. doi: 10.1111/j.1464-410X.2006.06255.x. [DOI] [PubMed] [Google Scholar]
- 40.Hashitani H, Fukuta H, Takano H, et al. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J Physiol. 2001;530:273–86. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–81. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins C, Klausner AP, Herrick B, et al. Potential for control of detrusor smooth muscle spontaneous rhythmic contraction by cyclooxygenase products released by interstitial cells of Cajal. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00714.x. ; doi: 10.1111/j.1582-4934.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harhun M, Szewczyk K, Laux H, et al. Interstitial cells from rat middle cerebral artery belong to smooth muscle cell type. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00567.x. ; doi: 10.1111/j.1582-4934.2008.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavoie B, Balemba OB, Nelson MT, et al. Morphological and physiological evidence for interstitial cell of Cajal-like cells in the guinea pig gallbladder. J Physiol. 2007;579:487–501. doi: 10.1113/jphysiol.2006.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinescu ME, Ardeleanu C, Gherghiceanu M, et al. Interstitial Cajal-like cells in human gallbladder. J Mol Histol. 2007;38:275–84. doi: 10.1007/s10735-007-9099-0. [DOI] [PubMed] [Google Scholar]
- 47.Popescu LM, Ciontea SM, Cretoiu D, et al. Novel type of interstitial cell (Cajal-like) in human fallopian tube. J Cell Mol Med. 2005;9:479–523. doi: 10.1111/j.1582-4934.2005.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popescu LM, Ciontea SM, Cretoiu D. Interstitial Cajal-like cells in human uterus and fallopian tube. Ann N Y Acad Sci. 2007;1101:139–65. doi: 10.1196/annals.1389.022. [DOI] [PubMed] [Google Scholar]
- 49.Popescu LM, Hinescu ME, Ionescu N, et al. Interstitial cells of Cajal in pancreas. J Cell Mol Med. 2005;9:169–90. doi: 10.1111/j.1582-4934.2005.tb00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van der Aa F, Roskams T, Blyweert W, et al. Interstitial cells in the human prostate: a new therapeutic target. Prostate. 2003;56:250–5. doi: 10.1002/pros.10264. [DOI] [PubMed] [Google Scholar]
- 51.Shafik A, Shafik I, El-Sibai O. Identification of c-kit-positive cells in the human prostate: the interstitial cells of Cajal. Arch Androl. 2005;51:345–51. doi: 10.1080/014850190944456. [DOI] [PubMed] [Google Scholar]
- 52.Exintaris B, Klemm MF, Lang RJ. Spontaneous slow wave and contractile activity of the guinea pig prostate. J Urol. 2002;168:315–22. [PubMed] [Google Scholar]
- 53.Shafik A. Study of interstitial cells in the penis: human study. J Sex Med. 2007;4:66–71. doi: 10.1111/j.1743-6109.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 54.Gherghiceanu M, Popescu LM. Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma.Transmission electron microscope (TEM) identification. J Cell Mol Med. 2005;9:893–910. doi: 10.1111/j.1582-4934.2005.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radu E, Regalia T, Ceafalan L, et al. Cajal-type cells from human mammary gland stroma: phenotype characteristics in cell culture. J Cell Mol Med. 2005;9:748–52. doi: 10.1111/j.1582-4934.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popescu LM, Andrei F, Hinescu ME. Snapshots of mammary gland interstitial cells: methylene-blue vital staining and c-kit immunopositivity. J Cell Mol Med. 2005;9:476–7. doi: 10.1111/j.1582-4934.2005.tb00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandache E, Popescu LM, Gherghiceanu M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: shed vesicles as intermediates. J Cell Mol Med. 2007;11:1175–84. doi: 10.1111/j.1582-4934.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suciu L, Popescu LM, Regalia T, et al. Epicardium: Interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009;13:771–7. doi: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shafik A, El-Sibai O, Shafik I. Identification of c-kit-positive cells in the uterus. Int J Gynaecol Obstet. 2004;87:254–5. doi: 10.1016/j.ijgo.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Duquette RA, Shmygol A, Vaillant C, et al. Vimentin-positive, c-kit-negative interstitial cells in human and rat uterus: a role in pacemaking. Biol Reprod. 2004;72:276–83. doi: 10.1095/biolreprod.104.033506. [DOI] [PubMed] [Google Scholar]
- 63.Cretoiu D, Ciontea SM, Popescu LM, et al. Interstitial Cajal-like cells (ICLC) as steroid hormone sensors in human myometrium: immunocytochemical approach. J Cell Mol Med. 2006;10:789–95. doi: 10.1111/j.1582-4934.2006.tb00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutchings G, Gevaert T, Deprest J, et al. Effect of prolonged c-kit receptor inhibition by imatinib mesylate on the uterine contractility of pregnant rabbits. Gynecol Obstet Invest. 2008;65:108–11. doi: 10.1159/000109080. [DOI] [PubMed] [Google Scholar]
- 65.Mei F, Zhu J, Guo S, et al. An age-dependent proliferation is involved in the postnatal development of interstitial cells of Cajal in the small intestine of mice. Histochem Cell Biol. 2009;131:43–53. doi: 10.1007/s00418-008-0515-7. [DOI] [PubMed] [Google Scholar]
- 66.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laguens R, Lagrutta J. Fine structure of human uterine muscle in pregnancy. Am J Obstet Gynecol. 1964;89:1040–7. doi: 10.1016/0002-9378(64)90296-0. [DOI] [PubMed] [Google Scholar]
- 68.Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med. 2008;12:1944–55. doi: 10.1111/j.1582-4934.2008.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streutker CJ, Huizinga JD, Driman DK, et al. Interstitial cells of Cajal in health and disease. Part II: ICC and gastrointestinal stromal tumours. Histopathology. 2007;50:190–202. doi: 10.1111/j.1365-2559.2006.02497.x. [DOI] [PubMed] [Google Scholar]
- 70.Junquera C, MartÌnez-Ciriano C, Castiella T, et al. Immunohistochemical and ultrastructural characteristics of interstitial cells of Cajal in the rabbit duodenum. Presence of a single cilium. J Cell Mol Med. 2007;11:776–87. doi: 10.1111/j.1582-4934.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinescu ME, Popescu LM, Gherghiceanu M, et al. Interstitial Cajal-like cells in rat mesentery: an ultrastructural and immunohistochemical approach. J Cell Mol Med. 2008;12:260–70. doi: 10.1111/j.1582-4934.2008.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ward SM, Harney SC, Bayguinoy JR, et al. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol (Lond) 1997;505:241–58. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torihashi S, Nishi K, Tokutomi Y, et al. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–8. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 74.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–53. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 75.Hutchings G, Deprest J, Nilius B, et al. The effect of imatinib mesylate on the contractility of isolated rabbit myometrial strips. Gynecol Obstet Invest. 2006;62:79–83. doi: 10.1159/000092530. [DOI] [PubMed] [Google Scholar]
- 76.Allix S, Reyes-Gomez E, Aubin-Houzelstein G, et al. Uterine contractions depend on KIT-positive interstitial cells in the mouse: genetic and pharmacological evidence. Biol Reprod. 2008;79:510–7. doi: 10.1095/biolreprod.107.066373. [DOI] [PubMed] [Google Scholar]
- 77.Kubota Y, Kajioka S, Biers SM, et al. Investigation of the effect of the c-kit inhibitor Glivec on isolated guinea-pig detrusor preparations. Auton Neurosci. 2004;115:64–73. doi: 10.1016/j.autneu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Popescu LM, Vidulescu C, Curici A, et al. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–81. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 79.Shimojima N, Nakaki T, Morikawa Y, et al. Imatinib blocks spontaneous mechanical activities in the adult mouse small intestine: possible inhibition of c-kit signaling. Pharmacology. 2005;74:95–9. doi: 10.1159/000084021. [DOI] [PubMed] [Google Scholar]
- 80.Van Helden DF, Imtiaz MS. Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus. J Physiol. 2003;548:271–96. doi: 10.1113/jphysiol.2002.033720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shmygol A, Blanks A, Bru-Mercer G, et al. Tissue-level Ca2+ signalling in human myometrium: a possible role for ICCs. University College London 2006. Proc Physiol Soc. 3:C114. [Google Scholar]
- 82.Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol. 2004;16:507–12. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 83.Leybaert L, Braet K, Vandamme W, et al. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10:251–7. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- 84.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147:S172–81. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hutchings G, Gevaert T, Deprest J, et al. The effect of extracellular adenosine triphosphate on the spontaneous contractility of human myometrial strips. Eur J Obstet Gynecol Reprod Biol. 2009;143:79–83. doi: 10.1016/j.ejogrb.2008.12.004. [DOI] [PubMed] [Google Scholar]


