Abstract
Endocytosis – the uptake of extracellular ligands, soluble molecules, protein and lipids from the extracellular surface – is a vital process, comprising multiple mechanisms, including phagocytosis, macropinocytosis, clathrin-dependent and clathrin-independent uptake such as caveolae-mediated and non-caveolar raft-dependent endocytosis. The best-studied endocytotic pathway for internalizing both bulk membrane and specific proteins is the clathrin-mediated endocytosis. Although many papers were published about the caveolar endocytosis, it is still not known whether it represents an alternative pathway with distinct cellular compartments to avoid lysosomal degradation or ligands taken up by caveolae can also be targeted to late endosomes/lysosomes. In this paper, we summarize data available about caveolar endocytosis. We are especially focussing on the intracellular route of caveolae and providing data supporting that caveolar endocytosis can join to the classical endocytotic pathway.
Keywords: alternative endocytosis, caveolae, caveosomes, endosomes/lysosomes, kinases and phosphatases
Introduction
Cavolae on the plasma membrane
-
Internalization of caveolae
Are caveolae stable, immobile invaginations at the plasma membrane?
Caveolar budding and pinching off from the plasma membrane
Intracellular route of caveolae
Conclusion
Introduction
Caveolae were described first in 1953 by Palade [1] as flask-or omega-shaped plasma membrane invaginations, with a diameter of 50–100 nm. These characteristic membrane invaginations are abundantly present in many but not all eukaryotic cell membrane [2]. Biochemical studies revealed that caveolae are detergent resistant, highly hydrophobic membrane domains composed of mainly cholesterol and sphingolipids. These detergent-resistant liquid-ordered membrane domains are currently referred to lipid rafts [3–6].
The main structural proteins of caveolae are members of the caveolin gene family, caveolin-1, caveolin-2 and caveolin-3. Morphologically defined caveolae can be formed by the expression of caveolin-1, which is a small integral membrane protein, whose 34 hydrophobic amino acids inserted into the inner leaflet of the membrane bilayer in a special hairpin-like form and never reaches the outside of the cell. The rest of the protein is cytosolic; the N-terminal region has a special amino acid sequence functioning as scaffolding domain and has been suggested to be important for binding caveolin to cholesterol and sphingolipid-rich membrane domains [7, 8]. This domain is also implicated in binding to signalling molecules [2]. The C terminus aligns along the inner leaflet of the bilayer by multiple palmitoylations [9]. Individual caveolae were estimated to contain 144 molecules of caveolin [10].
Caveolae have been implicated in numerous functions, including cell signalling, lipid regulation, vesicular transport via their endo-cytosis, and they have been suggested to play a role in a variety of diseases, including cancer, diabetes and virus infection [11]. In this paper, we focus on the function of caveolae in endocytosis.
Cavolae on the plasma membrane
Expression of caveolin-1 has been described to be necessary and sufficient for the formation of morphologically defined caveolae [12, 13]. The function of caveolin-2 has not yet been defined in details; it was thought to have accessory function in caveolae formation. Recent result suggests that in addition to caveolin-1, caveolin-2 is necessary for formation of deep plasma membrane-attached caveolae [14].
At the level of endoplasmic reticulum, caveolin-2 interacts with caveolin-1 to form high molecular mass hetero-oligomeric complex. The current model of caveolae biogenesis suggests that cavolae form in the Golgi complex by binding these caveolin-1/caveolin-2 heterooligomers to cholesterol. Interaction of caveolin-1 with caveolin-2 renders caveolin-2 detergent insoluble, and targets Golgi localized caveolin-2 to the plasma membrane. Exit from the Golgi complex is accelerated by cholesterol [15] and inhibited by glycosphingolipid depletion [16]. The small ‘caveolae precursors’ (also called ‘exocytic caveolar carriers’[17]) are trafficking to the plasma membrane [18]. When the small caveolar carriers are inserted to the plasma membrane, caveolae are becoming relatively immobile structures.
Based on ultrastructural studies, it seems likely that caveolae formation requires proteins other than caveolins. Recently, PTRF-cavin (p-cavin), a protein initially identified as polimerase I, a transcript release factor was found to be also required for caveolae formation in mammalian and zebrafish cells [19–22]. PTRF-cavin is a soluble cytosolic protein recruited to the membrane to generate caveolae, and it most likely operates as a coat protein for caveolae. Binding of PTRF-cavin to membrane domain containing oligomerized caveolins, cholesterol and phosphatidylserine stabilizes the membrane curvature to produce the classical flask shape of cavolae. Biochemical and morphological data [21, 22] suggest that p-cavin associates with mature caveolae at the plasma membrane but does not associate with non-caveolar caveolin present in the Golgi complex.
Caveolae are anchored to the plasma membrane by cytoskeletal components [23]. An actin cross-linking protein, filamin, is one of the proteins identified as a ligand for caveolin-1 [24]. However, the molecular mechanism for binding caveolae to the cytoskeleton has not been established. Liu and Pilch [22] provide experimental data suggesting that PTRF-cavin may serve as direct connection between caveolar components and the cytoskeleton.
Cholesterol itself is essential for caveolae formation and caveolin transcription as well [5, 25]. Cells treated with agents that remove cholesterol (filipin, methyl-β-cyclodextrin or nystatin) lose caveolin and caveolae, resulting in flattened plasma membranes [26].
Internalization of caveolae
Are caveolae stable, immobile invaginations at the plasma membrane?
Caveolae and caveolin-containing membrane domains on the plasma membrane have various curvatures and shapes (Ω, elongated flask, curved membrane invaginations with wide opening and narrow neck). This morphology strongly suggests that caveolae could be involved in endocytosis operating parallelly to clathrin-mediated endocytosis. In spite of the morphological evidence, it has been debated for a long time that caveolae can really pinch off from the plasma membrane. Studying the dynamic properties of caveolae by green-fluorescent-protein (GFP)-tagged caveolin-1 fusion protein, it revealed that the exchange of caveolin-1-GFP between plasma membrane and intracellular pool is surprisingly slow; indicating that plasma membrane caveolae are immobile structures [27]. Uptake of albumin receptor (gp60) localized in caveolae in endothelial cells is inhibited by over-expression of caveolin-1 [28], suggesting that caveolin-1 itself can be responsible for stabilizing caveolae on the plasma membrane.
Increasing number of evidence show, however, that although caveolae are not normally involved in endocytosis, interaction of caveolae or caveolin with specific ligands can trigger the rapid internalization of caveolae. Several ligands like folic acid [29, 30], albumin [31], autocrine motility factor [32], alkaline phosphatase [33], lactosyl ceramide [34] and pathogens as ganglioside-bound cholera toxin [33], SV40 virus [2, 35], polyoma virus [36], echovirus1 [37], HIV virus [38], respiratory syncytial virus [39], certain FimH-expressing bacteria [40] are known to be internalized by caveolae. Binding of various ligands to caveolin/caveolae, cross-linking caveolar components, receptors accumulating in caveolae promote downstream of signalling events resulting in caveolar internalization [41].
Based on morphological and biochemical data recently, it is generally accepted that caveola-mediated endocytosis functions as a true uptake mechanism parallel to the clathrin-mediated pathway. Being ligand-triggered, caveolar endocytosis provides a highly regulated way for uptake of specified substances.
Caveolar budding and pinching off from the plasma membrane
Caveolar budding is regulated by kinases and phosphatases. Caveolin was first described as a substrate for Src-family tyrosine kinase [42, 43]. Later a special interaction between Src and caveolin-1 was found: the scaffolding domain of caveolin-1 binds Src [44–46]. Palmytoilation of caveolin-1 at cystein 156 also contributes to the coupling of Src to caveolin-1 [44, 45]. Caveolin-1 was described to be phosphorylated [41] on tyrosine residue 14 by Src family kinase [33, 43 and 46]. Binding of albumin to gp60, (receptor for albumin localized in caveolae) induces tyrosine phosphorylation of both gp60 and caveolin-1 [47]. Tyrosine kinase inhibitors (herbimycid A and genistein) prevented the gp60-activated vesicle formation and albumin endocytosis [47]. These data suggest that caveolin-1 phosphorylation results in caveolar internalization.
Lee et al.[48] showed that the other caveolin isoform, caveolin-2 also undergoes Src-induced phosphorylation on tyrosine 19. Phosphocaveolin-2 (TyrP19) was strictly co-localized with phosphocaveolin-1 (TyrP14) indicating that the simultaneous phosphorylation of caveolin-1 and caveolin-2 might equally be important in regulation of caveolae pinching off from the plasma membrane. Similarly, phosphorylation of caveolin-2–induced internalization of cavolae in peritoneal macrophages [49].
As expected, phosphatases play an important role in caveolar internalization. Vanadate, a tyrosine phosphatase inhibitor stimulates caveolar endocytosis by causing hyperphosphorylation of tyrosine residues of caveolin-1 [50]. Treatment with a serine/threonine phosphatase inhibitor (ocadaic acid) causes massive mobilization of caveolae [33, 34, 49–52]. Ocadaic acid is a polyether metabolite, isolated from the black marine sponge Halichondria ocadai and inhibits protein phosphatases, especially PP1 and PP2A [53]. PP1 and PP2A are two major classes of serine/threonine protein phosphatases that dephosphorylate a broad spectrum of protein kinases [54]. The exact mechanism by which serine/threonine phosphatase (PP1 and PP2A) inhibitors can stimulate caveolar endocytosis is not known. Because PP2A is a specific inhibitor of Src kinase, one possible explanation for ocadaic acid effect to stimulate caveolar internalization is that these sequential inhibitions activate Src kinase that can phosphorylate caveolin-1 and/or -2 (Fig. 1).
Figure 1.
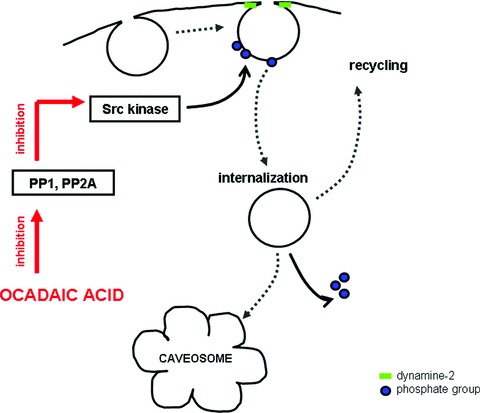
The possible mechanism by which ocadaic acid can cause tyrosine phosphorylation of caveolin and results in the internalization of caveolae. Ocadaic acid is a specific serine/threonine phosphatase (PP1 and PP2A) inhibitor. Src kinase is specifically inhibited by PP1 and PP2A. The sequential inhibitions activate Src kinase that can phosphorylate tyrosine residues of caveolin-1 and/or -2. Dephosphorylation of caveolin supposed to be necessary for the recycling of caveolae to the cell surface.
Sowa et al.[14] mapped two serine phosphorylation sites in the Nterminal region (23, 36) of caveolin-2. They showed that phosphorylation of each site contributes to caveolin-1-dependent caveolae formation. Mutation of serine 36 to alanine markedly reduced the number of plasma membrane–attached caveolae. Phosphorylation of serine 23 had marginal influence on the formation of caveolae on the plasma membrane but enhanced the number of uncoated vesicles in the cytoplasm. When both 23 and 36 serine residues were replaced by alanine, the surface-attached caveolae were eliminated but appearance of non-coated vesicles in the cytoplasm was markedly increased. These results suggest that dephosphorylation of serine residues of caveolin-2 might also be important to regulate caveolae pinching off from the plasma membrane.
The GTP-binding protein dynamin is known to play an important role in pinching off of clathrin-coated vesicles [55]. It was a surprising finding when dynamin was found to recruit also to caveolar membranes [56, 57]. Subsequent to the hydrolysis of GTP, dynamin triggers fission of caveolae by constricting its neck. Dynamin, however, is not a permanent component of caveolae; association of dynamin with caveolae appears to be a transient phenomenon [2]. Intersectin-2 has been reported to localize also at the neck of caveolae controlling caveolar endocytosis by regulating the GTP-ase activity of dynamin [58]. Recently, the endothe-lial NO synthase (eNOS)-trafficking inducer, NOSTRIN, was found to function as an adaptor recruiting dynamin-2 molecules required for vesicle membrane fission [59, 60].
The cytoskeleton has also important function in regulating the steady-state distribution of caveolae [23]. Although the cortical actin cytoskeleton appears to confine caveolae/caveolin-1 to the cell surface acting as simple physical barriers to the detachment of caveolae, microtubules serve as tracks for the transport of caveolae in the cytoplasm. Thus, the internalization of caveolae depends on the integrity and/or reorganization of the cytoskeleton [33], and local disassembly of cortical actin network is essential to initiate inward transport of caveolae along microtubules. Reorganization of the actin cytoskeleton was found to be crucial for SV40 virus entry. Accumulation of virus in caveolae initiates a signalling cascade that leads to tyrosine phosphorylation and depolymerization of cortical actin cytoskeleton. Actin monomers are recruited to the virus-loaded caveolae and actin patches are formed. After internalization of caveolae, the cortical cytoskeleton returns to its normal pattern [2]. Ocadaic acid can also cause reorganization of the actin cytoskeleton, leading to caveolar internalization [27].
There are data suggesting that integrins, or rather integrin-mediated adhesion, also regulate the caveolar endocytosis [61, 62]. Integrins connect the extracellular matrix to the actin cytoskeleton at special structures called focal adhesions through a protein complex that includes vinculin, pallixin, tallin and α-actinin [63]. Caveolin-1 – especially its tyrosin-phosphorylated (pY14cav-1) form – was found to strongly colocalize with vinculin at focal adhesion [61]. Although caveolin phosphorylation induces internalization, integrins inhibit internalization by retaining phospho-caveolin in focal adhesion. Cell detachment triggers a shift in phospho-caveolin localization to caveolae where it induces internalization of this membrane domain [61, 62].
Summarizing, phosphorylation of caveolin(s), recruitment and activation of dynamin at the caveolar neck, reorganization of the cytoskeleton are essential for caveola internalization. Once caveolae pinched off from the plasma membrane, they may associate with motor molecules that propel them along the microtubules [23].
Intracellular route of caveolae
Less extensively documented are the trafficking and the intracellular fate of caveolae. Caveolae were shown to be equipped with several components of fusion machinery [64]. NSF, SNAP, VAMP were described to concentrate in caveolae and possibly associate with caveolin-1 [65, 66]. Thus, internalized caveolae co-opt the same mechanism used in trafficking of other vesicles [65–67]; therefore, they can dock and fuse with cytoplasmic organelles [33, 67].
The basic question is whether the internalized caveolae can fuse with endosomes and follow the classical endocytotic degradative pathway or the alternative endocytosis involves alternative cellular compartments. Stimulated caveolar internalization is always accompanied by the appearance of grape-like multi-caveolar complexes (Fig. 2A). Studying the entry of SV40 viruses taken up by caveolae, the virus particles were detected in these multi-caveolar complexes of neutral pH, distinct from classical endocytotic compartments [35]. These multi-caveolar complexes never fused with lysosomes; thus, viruses could escape lysosomal degradation. Since these structures were labelled with caveolin-1, they were named caveosomes [35, 68]. Until now, only a few electron microscopical pictures were published about the morphology of these organelles [35, 69].
Figure 2.
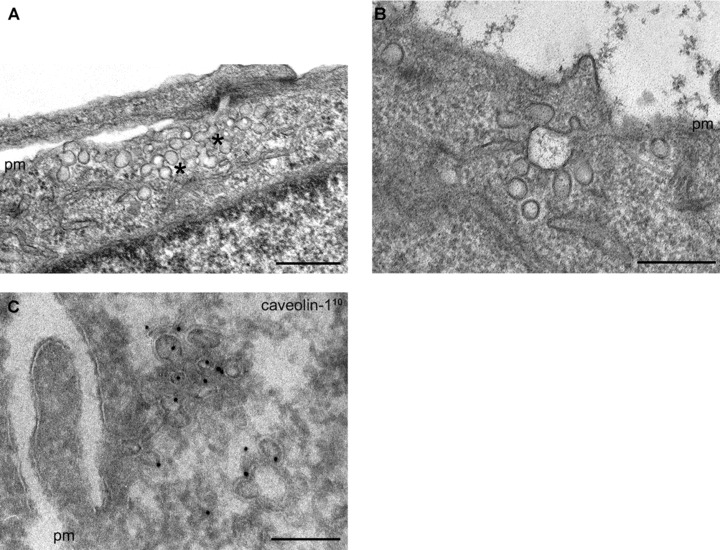
(A) Stimulated internalization of caveolae results in the appearance of caveola-clusters (stars). (B) and (C) Some of these clusters are in connection with the cell surface through narrow tubular invagination of the plasma membrane. (C) Using anti-caveolin-1 antibody on ultra-thin–frozen sections, these multi-caveolar clusters were always found to be caveolin-positive. (The first antibody was visualized by colloidal gold-conjugated protein-A. The gold particles represent caveolin-1 and the diameter of the gold particles is shown in index.) pm: plasma membrane. Bars: 200 nm.
According to recent knowledge, caveosomes are multi-caveolar structures of heterogeneous morphology; they are supposed to be early endosome-equivalent intermediate organelles in caveolar endocytosis. The further fate of caveosomes is not entirely known. Viruses were described to be sorted from caveosomes into vesicular and tubular structures that travel along microtubules to the smooth endoplasmic reticulum [35, 37].
There are data, however, showing that ligands internalized by cavolae can be driven to the classical endocytotic organelles. Cholera toxin entering the cells by caveolar endocytosis passes through early endosomes and accumulates in the Golgi complex [70]. It was also shown that under normal conditions, caveolae carrying SV40 virus particles can transiently interact with early endosomes [71, 72]. The existence of two caveolar trafficking routes involving caveosomes and early endosomes raises the questions whether caveosomes are independent structures or the downstream caveosomes interact with the classical endocytotic compartments. When we studied caveolar internalization in HepG2 cells, we found that many of these caveosome-like multi-caveolar complexes were connected with the cell surface by a narrow tubular plasma membrane invaginations (Fig. 2B and C), but some of them seemed to be independent structures in the cytoplasm. When Ruthenium red (Ru red) – an electron dense dye – was used to label the cell surface, many of these structures were Ru red–positive (Fig. 3A–C) indicating that they were still connected with the cell surface. These results support the idea that a significant portion of these multi-caveolar complexes described as caveosomes are not independent structures.
Figure 3.
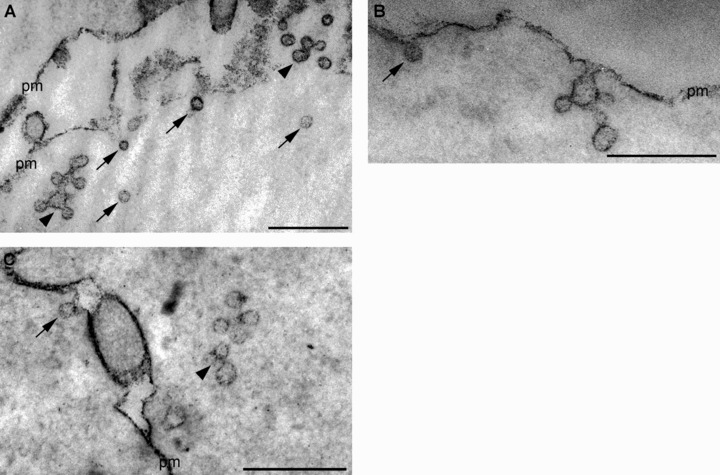
Using Ruthenium red (Ru red) to label the cell surface, single vesicles (arrows) as well as caveolar clusters (arrowheads) are found to be Ru red-positive. This finding indicates that they are still in connection with the cell surface. Note that many of the vesicles deeper in the cytoplasm are also labelled with Ru red showing that they are still connected with the cell surface. The caveolar clusters (arrowheads) are often described as caveosomes. The Ru red staining clearly shows that many of them are not independent structures. pm: plasma membrane. Bars: 400 nm.
The sub-cellular distribution of caveolin could provide insights into the endocytotic pathways. Caveolin-1 in many cells is evident on the cell surface and within the Golgi complex, and only partial colocalization can be detected with endosomal markers such as EEA1, a marker of the early sorting endosome [71] or CD63, late endosomal marker [73]. When caveolar endocytosis was provoked by albumin, the number of CD63 and caveolin-1 double-labelled multi-vesicular bodies or late endosomes significantly increased [73]. Studying the long-term internalization of albumin in HepG2 cells, albumin was found to accumulate in large, caveolin-1-positive caveosome-like caveolar clusters. At the same time, the number of caveolin-1 and CD63 containing multi-vesicular bodies significantly increased (Fig. 4) indicating that caveolae-mediated endocytosis of albumin resulted in an increased caveolar trafficking along the classical endosomal degradative pathway [73].
Figure 4.
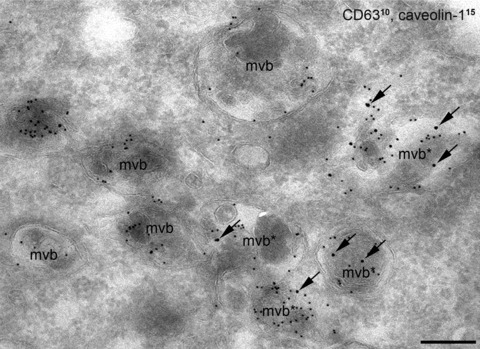
Long-term internalization of albumin in HepG2 cells. Caveolin-1 and CD63 (late endosomal marker) double labelling on ultrathin–frozen section. Numerous caveolin-1 and CD63 containing multi-vesicular bodies (mvb) are present in the cytoplasm indicating that caveola-mediated endocytosis of albumin resulted in an increased trafficking of caveolae to the classical degradative pathway. CD63 was detected with smaller (d : 10 nm), whereas caveolin-1 was labelled with the larger (d : 15 nm) gold particles. (Arrows show caveolin-1 labelled with larger gold particles). Bars: 200 nm.
Analysing the involvement of several small GTPases in the trafficking of caveolae, Pelkmans et al.[71] found that caveolae could move to early endosomes in a Rab5-dependent manner. Although caveolae dock to and fuse with endosomal membrane, their membrane domains do not mix with each other. After some time, the caveolin-1 sub-domains can pinch off again from early endo-somes as membrane vesicles. If caveolae transiently fuse with early endosomes, one can suppose that they should be able to pick up some fluid from that compartment and potentially carry it to caveosomes, suggesting that the traffic is bidirectional. Experiments using a fluid-phase tracer Lucifer yellow (LY) showed that after long-time incubation, small but significant amount of LY accumulated in caveosomes [72]. Human polyoma virus (JCV) that is known to enter the cell by clathrin-dependent endocytosis and transported immediately to early endosomes can also be sorted to a caveolin-1-positive endosomal compartment. This transport is also dependent on Rab5-GTP-ase [74]. These experimental data clearly show that there is communication between caveola-mediated and classical endocytotic pathways and suggest that the communication between caveosomes and early endosomes is bidirectional.
The morphological entities by which this communication can occur are not known. Caveolin-1 containing sub-domains pinching off from the early endosomes and/or caveosomes as vesicles can function as mediators between the two pathways. Since dynamin was found to accumulate along the surface-connecting tubular part of the caveolar clusters [75], it cannot be excluded, however, that caveolar clusters ‘en mass’ can fuse with endosomes.
Kinases and phosphatases also have regulatory role in fusion of caveolar carriers with endosomal compartments/or caveosomes. Sorting, distribution, transfer to final destination of the cargo strongly depend on kinases and phosphatases. Using high-throughput RNA interference and automated image analysis, Pelkmans et al.[76] showed that a high number (about 43) of kinases are required for caveolar entry of SV40 virus. By this genomewide screen, they could show specific role of kinases at different stages of the caveolar assembly and transport. Large group of these kinases was found to function in various signalling system indicating that endocytotic transport and signal transduction are tightly coupled.
Not only phosphorylation but also dephosphorylation of membrane-associated receptors or proteins can be critical to determine the sorting. The regulatory role of phosphatases, however, is less known. There are data indicating that PP2A – a serine/threonine phosphatase – plays an important role in endosomal sorting and movement of endocytotic compartments along the microtubules [77–79]. By dephosphorylation of proteins present in the endosomal membrane, PP2A can regulate maturation of endosomes, fusion of endosomes with lysosomes [78, 80, 81]. If PP2A is inhibited, the classical endocytotic sorting is blocked and the cargo can stuck in one of the intermediate compartments. The small T-antigen of SV40 virus is known to bind and inhibit PP2A [82], by which it can interfere the maturation of virus and caveolin-containing endosomes (caveosomes) and endo-some/lysosome fusion. This might explain why SV40 virus is retained in caveosomes resulting in an escape from lysosomal degradation. Caveolin-1 itself is known to interact with and inhibit PP2A [83] that can result in accumulation of caveolae in caveo-somes. It seems likely that the interaction of the cargo with caveolar components, caveolin itself or any of the regulatory kinases and/or phosphatases can be an important determinant for the final destination of the cargo.
It has been suggested that cholesterol itself plays an important role in intracellular transport [25]. Normally, cholesterol is present in the plasma membrane and early endosomes and is sorted away from late endosomes and lysosomes [84]. Recent studies have revealed that cholesterol is not a passive component of endosomal membranes but rather directly involved in the sorting and transport of endocytotic vesicles [85].
Conclusions
Although caveolae are immobile lipid domains of the plasma membrane, under special conditions (like binding-specific ligands to their receptors) they can pinch off from the plasma membrane. Nowadays, it is generally accepted that caveola-mediated endocytosis functions as a true uptake mechanism parallel to the clathrin-mediated pathway. Being ligand-triggered, caveolar endocytosis provides a more selective and highly regulated way for uptake of specified substances. Caveolar endocytosis is regulated by kinases and phosphatases. Tyrosin phosphorylation of caveolin-1 (and maybe caveolin-2) can initiate budding and internalization of caveolae. The GTP-binding protein dynamin that is temporally associated to caveolae triggers fission of caveolae by constricting its neck subsequent to the hydrolysis of GTP. The internalization of caveolae depends on the integrity and/or reorganization of the cytoskeleton: local disassembly of cortical actin network is essential to initiate inward transport of caveolae [33]. Once caveolae pinch off from the plasma membrane, they may associate with motor molecules that propel them along the microtubules [23]. Although the cortical actin cytoskeleton appears to confine caveo-lae/caveolin-1 at the cell surface acting as simple physical barriers to the detachment of caveolae, microtubules serve as tracks for the transport of caveolae in the cytoplasm.
After internalization, grape-like multi-caveolar complexes called caveosomes are appearing in the cytoplasm [2, 35, 68]. It is still debated whether these caveolar clusters are independent entities because many of them are connected to the cell surface by very narrow tubular plasma membrane invaginations. Viruses are accumulated in these multi-caveolar complexes of neutral pH, distinct from classical endocytotic compartments [35]. They never fuse with lysosomes; thus, viruses can escape lysosomal degradation. Other ligands using caveolae to enter the cells, however, are present in early endosomes following the classical endocytotic route. Long-term incubation with albumin also results in the appearance of caveolin-1 in late endosomes/multi-vesicular bodies indicating that caveolae or caveosomes communicate with the classical endocytotic compartments. These data strongly suggest that although the first step of the uptake is different, but the intermediate organelles (caveosomes/early endosomes) can communicate with each other; thus, ligand internalized by caveolae can be driven to the classical endocytotic pathway.
The route followed by different ligands is regulated by small GTPases (Rab molecules), kinases and phosphatases. (Fig. 5 summarizes the possible route for caveolae after internalization.) It seems likely that the interaction of the cargo with caveolar components, caveolin itself or any of the regulatory kinases and phosphatases can have determining influence on the final destination of the cargo.
Figure 5.
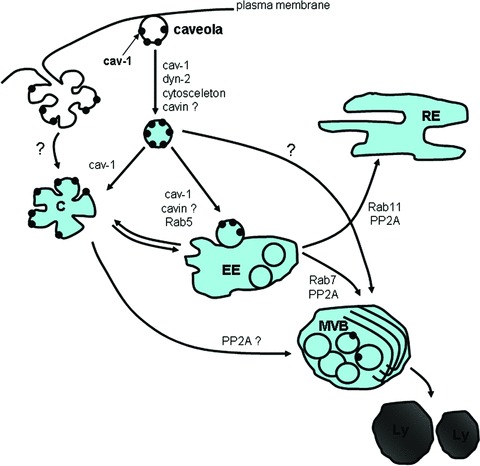
Intracellular trafficking of caveolae. Phosphorylation of caveolin(s), association of dynamin with the neck of caveolae, reorganization of the cytoskeleton result in pinching off of caveolae from the plasma membrane. Stimulated caveola internalization can result in formation of caveolar clusters as well. Internalized caveolae can fuse with early endosomes (EE) in a Rab5-dependent manner. Pinched-off single caveolae might fuse with pre-existing caveosomes (C) or caveolar clusters ‘en mass’ detaching from the plasma membrane can form caveosomes. The caveo-some–endosome pathways seem to be bidirectional. Further sorting from early endosomes can occur to recycling endosomes (RE) or multi-vesicular bodies (MVB)/lysosomes (Ly). The sorting depends on Rabs and PP2A as well.
Since numerous signalling molecules were identified to accumulate in caveolae, they can also be called ‘signaling organelles’[86]. Large group of kinases identified in endocytosis function in various signalling pathways strongly support the idea that endocytotic transport and signal transduction are tightly coupled and the caveolar internalization should play an important role to regulate signal transduction as well.
It has to be point out that there are caveolae that are immobile; they never pinch off from the plasma membrane. Smooth muscle cells have huge amount of caveolae on their plasma membrane by which the surface area is enlarged over 80%. These caveolae, however, are never taking part in endocytosis, calcium handling seems to be one of the most important role of them [87, 88].
Acknowledgments
The authors are grateful to Margit Kutasi and Katalin Löcsey for their valuable technical assistance. We gratefully acknowledge Prof. Pál Röhlich for critical reading of the manuscript and Dr. Elisabeth Fromm for the language correction of the manuscript.
References
- 1.Palade GE. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 2.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–20. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 3.Smart EJ, Graf GA, Mc Nives MA, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargiacomo M, Sudol M, Tang A, Lisanti MP. Signal transducing molecules and GP1-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata M, Peranen J, Schreiner R, et al. VIP 21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harder T, Simons K. Caveolae, DIGs and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–42. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 7.Tang Z, Scherer PE, Okamoto T, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–61. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 8.Machleidt T, Li WP, Liu P, Anderson RG. Multiple domains in caveolin-1 control its intracellular traffic. J Cell Biol. 2000;148:17–28. doi: 10.1083/jcb.148.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietzen DJ, Hastings WR, Lublin DM. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270:6838–42. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- 10.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12:796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655–9. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipardi E, Mora R, Colomer V, et al. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidilinositol (GPI)-anchored protein sin epithelial cells. J Cell Biol. 1998;140:617–26. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowa G, Pypaert M, Fulton D, Sessa WC. The phosphorylation of caveolin-2 on serines 23 and 36 modulates caveolin-1-dependent caveolae formation. Proc Natl Acad Sci USA. 2003;100:6511–6. doi: 10.1073/pnas.1031672100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pol A, Martin S, Fernandez MA, et al. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng ZJ, Singh RD, Sharma DK, et al. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Cell Biol. 2006;17:3197–210. doi: 10.1091/mbc.E05-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parton RG, Simons K. The multiple faces of caveolae. Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 18.Tagawa A, Mezzacasa A, Hayer A, et al. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170:769–79. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinten J, Voldstedlund M, Clausen H, et al. A 60-kDa protein abundant in adipocyte caveolae. Cell Tissue Res. 2001;305:99–106. doi: 10.1007/s004410100389. [DOI] [PubMed] [Google Scholar]
- 20.Vinten J, Johnsen AH, Roepstorff P, et al. Identi fication of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta. 2005;1717:34–40. doi: 10.1016/j.bbamem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Hill MM, Bastiani M, Luettrforst R, et al. PTRF-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–24. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–22. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 23.Mundy DI, Machleidt T, Ying YS, et al. Dual control of caveolae membrane traffic by microtubules and actin cytoskeleton. J Cell Sci. 2002;115:4327–39. doi: 10.1242/jcs.00117. [DOI] [PubMed] [Google Scholar]
- 24.Stahlhut M, Van Deurs B. Identification of filamin as a novel ligand for caveolin-1: evidence for organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol Biol Cell. 2000;11:325–37. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–54. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- 26.Rothberg KG, Heuser JE, Donzell WC, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 27.Van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13:92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 28.Minshall RD, Tiruppathi C, Vogel SM, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–70. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–1. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- 30.Rothberg RG, Ying YS, Kolhouse JF, et al. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990;110:637–49. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benlimame N, Le PU, Nabi IR. Localization of autocrine motility factor receptor to caveolae and clathrin-inde-pendent internalization of its ligand to smooth endoplasmic reticulum. Mol Biol Cell. 1998;9:1773–86. doi: 10.1091/mbc.9.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puri V, Watanabe R, Singh RD, et al. Clathrin-dependent and independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol. 2001;154:535–47. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step reticular transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 36.Richterova Z, Liebe D, Horak M, et al. Caveolae are involved in the trafficking of mouse polyoma virus virion and artificial VP1 pseudocapsids toward cell nuclei. J Virol. 2001;75:10880–91. doi: 10.1128/JVI.75.22.10880-10891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pietiäinen V, Marjomäki V, Upla P, et al. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin and signaling events. Mol Biol Cell. 2004;15:4911–25. doi: 10.1091/mbc.E04-01-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell SM, Crowe SM, Mark J. Lipid rafts and HIV-1 from viral entry to assembly of progeny virion. J Clin Virol. 2001;22:217–27. doi: 10.1016/s1386-6532(01)00193-7. [DOI] [PubMed] [Google Scholar]
- 39.Weiling D, Hope JC, Chaplin P, et al. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–8. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 40.Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–8. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- 41.Pelkmans L, Purterer D, Helenius A. SV 40 induced internalization of caveolae involves local actin polymerization and dynamin-recruitment. Science. 2002;296:535–39. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 42.Glenney JR., Jr Tyrosine phosphorylation of a 22 kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–6. [PubMed] [Google Scholar]
- 43.Lee H, Volonte D, Galbiati F, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a cSrc/cav1/Grb7 signaling casette. Mol Endocrinol. 2000;14:1750–75. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 44.Galbiati F, Volonte D, Meani D, et al. The dually acylated NH2-terminal domain of Gi1a is sufficient to target a green fluorescent protein reporter to caveolin-enriched plasma membrane domains. Palmitoylation of caveolin-1 is required for the recognition of dually acylated G-proteinα subunits in vivo. J Biol Chem. 1999;274:5843–50. doi: 10.1074/jbc.274.9.5843. [DOI] [PubMed] [Google Scholar]
- 45.Lee H, Woodman SE, Engelman JA, et al. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the cSrc tyrosine kinase. Targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolin-1 (Tyr 14) J Biol Chem. 2001;276:35150–8. doi: 10.1074/jbc.M104530200. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tyrosine kinases. The α-isoform of caveolin is selectively phosphorylated by vSrc in vivo. J Biol Chem. 1996;271:3863–8. [PubMed] [Google Scholar]
- 47.Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by a tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Park DS, Wang XB, et al. Srcinduced phosphorylation of caveolin-2 on tyrosin 19. J Biol Chem. 2002;277:34556–67. doi: 10.1074/jbc.M204367200. [DOI] [PubMed] [Google Scholar]
- 49.Kiss AL, Botos E, Turi A, Miillner N. Ocadaic acid treatment causes phosphorylation of caveolin-2 and induces internalization of caveolae in rat peritoneal macrophages. Micron. 2004;35:707–15. doi: 10.1016/j.micron.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Botos E, Turi A, Miillner N, et al. Regulatory role of kinases and phosphatases on the internalization of caveolae in HepG2 cells. Micron. 2007;38:313–20. doi: 10.1016/j.micron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Thomsen P, Roepstooff K, Stahlhut M, Van Deurs B. Caveolae are highly immobile plasma membrane microdomains which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–50. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma DK, Choudhury A, Singh RD, et al. Glycosphingolipids internalized via caveola-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem. 2003;278:7564–72. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez JJ, Candenas ML, Suoto ML, et al. Ocadaic acid, useful tool for studying cellular processes. Curr Med Chem. 2002;9:229–62. doi: 10.2174/0929867023371247. [DOI] [PubMed] [Google Scholar]
- 54.Cohen PT. Protein phosphatase I – targated in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 55.De Camilli P, Takei K, McPherson PS. The function of dynamin in endocytosis. Curr Opin Neurobiol. 1995;5:559–65. doi: 10.1016/0959-4388(95)80059-x. [DOI] [PubMed] [Google Scholar]
- 56.Oh P, Mc Intosh D, Schnitzer D. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fusion from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–44. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henley JR, Kroueger EW, Oswald BJ, Mc Niven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:86–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain HK, Jenna S, Glogauer M, et al. Endocytic protein intersectin-1 regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–32. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 59.Schilling K, Opitz N, Weisenthal A, et al. Translocation of endothelial nitric oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–80. doi: 10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tissue Res. 2009;335:27–40. doi: 10.1007/s00441-008-0688-3. [DOI] [PubMed] [Google Scholar]
- 61.Del Pozo MA, Balasubramanian N, Alderson NB, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salanueva IJ, Cerezo A, Guadamillas MC, DelPozo MA. Integrin regulation of caveolin function. J Cell Mol Med. 2007;11:969–80. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–9. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 64.Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol. 1995;268:H48–55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- 65.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking and fusion including VAMP, NSF, SNAP, annexin and GTPase. J Biol Chem. 1995;270:14399–404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 66.Predescu SA, Predescu DN, Palade GE. Endothelial transcytotic machinery involves supramolecular protein-lipid complexes. Mol Biol Cell. 2001;12:1019–33. doi: 10.1091/mbc.12.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Razani B, Woodman SE, Lisanti M. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 68.Nicols BJ. A distinct class of endosome mediates clathrin independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–8. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 69.Peter P, Mironov AJ, Vey M, et al. Trafficking of prion proteins through a caveolae-mediated endosomal pathway. J Cell Biol. 2003;162:703–17. doi: 10.1083/jcb.200304140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richards AA, Stang E, Peppehok R, Parton RG. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol Biol Cell. 2002;13:1750–64. doi: 10.1091/mbc.01-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parton RG, Richard AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–38. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 72.Pelkmans L, Bürli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–80. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Botos E, Klumperman J, Oorschot V, et al. Caveolin-1 is transported to multi-vesicular bodies after albumin-induced endocytosis of caveolae in HepG2 cells. J Cell Mol Med. 2008;12:1–9. doi: 10.1111/j.1582-4934.2007.00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Querbes W, O’ Hara BA, Williams G, Atwood WJ. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of non-caveolae ligands. J Virol. 2006;80:9402–13. doi: 10.1128/JVI.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiss AL, Botos E. Ocadaic acid retains caveolae in multicaveolar clusters. Pathol Oncol Res. 2008 doi: 10.1007/s12253-008-9139-4. ; doi: DOI: 10.1007/s12253--00809139-4. [DOI] [PubMed] [Google Scholar]
- 76.Pelkmans L, Fava E, Grabner H, et al. Genome-wilde analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;3571:1–9. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 77.Price NE, Mumby MC. Brain protein serine/threonine phosphatases. Curr Opin Neurobiol. 1999;9:336–42. doi: 10.1016/s0959-4388(99)80049-x. [DOI] [PubMed] [Google Scholar]
- 78.Molloy SS, Thomas L, Kamibayashi C, et al. Regulation of endosome sorting by a specific PP2A isoform. J Cell Biol. 1998;142:1399–411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sontag E, Nunbhahdi-Craig V, Lee G, et al. Molecular interactions among protein phosphatase 2A, tau and microtubules. J Biol Chem. 1999;274:25490–8. doi: 10.1074/jbc.274.36.25490. [DOI] [PubMed] [Google Scholar]
- 80.Schapiro F, Soe TT, Mallet G, Maxfield F. Role of cytoplasmic domain serines in intracellular trafficking of furin. Mol Biol Cell. 2004;15:1882–94. doi: 10.1091/mbc.E03-09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varlamov O, Kalinina E, Che F-Y, Fricker LD. Protein phosphatase 2A binds to the cytoplasmic tail of car-boxypeptidase D and regulates post-trans-Golgi network trafficking. J Cell Sci. 2000;114:311–22. doi: 10.1242/jcs.114.2.311. [DOI] [PubMed] [Google Scholar]
- 82.Rundell K, Parakati R. The role of the SV40ST antigen in cell growth promotion and transformation. Semin Cancer Biol. 2001;11:5–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]
- 83.Li L, Ren CH, Tahir SA, et al. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interaction with and inhibition of serine/threonine protein phosphatases PP1 and PP2. Mol Cell Biol. 2003;23:9389–404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maxfield FR, Wustner D. Intracellular cholesterol transport. J Clin Invest. 2002;110:891–8. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen H, Yang J, Low PS, Cheng J-X. Cholesterol level regulates endosome mobility via Rab proteins. Biophys J. 2008;94:1508–20. doi: 10.1529/biophysj.106.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okamoto T, Schlegel A, Scherer PE, Lisanti M. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–22. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 87.Gherghiceanu M, Popescu LM. Caveolar nanospaces in smooth muscle cells. J Cell Mol Med. 2006;10:519–28. doi: 10.1111/j.1582-4934.2006.tb00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Popescu LM, Gherghiceau M, Mandache E, Cretoiu D. Caveolae in smoothe muscles: nanocontacts. J Cell Mol Med. 2006;10:960–90. doi: 10.1111/j.1582-4934.2006.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


