Abstract
Sjögren’s syndrome (SS) patients have low salivary dehydroepiandrosterone (DHEA) and androgen biomarker levels, but high salivary oestrogen levels. The hypothesis was that the healthy glands contain DHEA-sulphate processing intracrine machinery; the local androgen/oestrogen imbalance suggests that this is disarranged in SS. Indirect immunofluorescence and quantitative real-time PCR (qRT-PCR) of steroid sulphatase, sulfotransferase, 3β- and 17β-hydroxysteroid dehydrogenases (3β- and 17β-HSD), 5α-reductase and aromatase were performed for labial salivary glands of healthy controls and persons with SS. In control acini steroid sulphatase and sulfotransferase immunoreactivities were located in the basolateral cell parts. 3β- and 17β-HSD formed strong, interrupted bands along the basal cell parts. 5α-reductase was mainly located in acinar cell nuclei and aromatase in the apical cell membrane. All enzymes were more widespread in ducts. In SS, steroid sulphatase was weak and deranged, 3β- and 17β-HSD had lost their strict basal acinar cell localization and 5α-reductase was mainly found in the cytoplasm of the acinar cells, whereas aromatase showed similar staining in SS and controls. qRT-PCR of labial salivary glands disclosed all corresponding enzyme mRNAs with the levels of 3β-HSD in SS being the lowest. Healthy tubuloacinar epithelial cells contain complete intracrine machineries for DHEA(-sulphate) pro-hormone processing. These enzymes have in healthy acini an organized architecture, which corresponds with DHEA uptake from the circulation, nuclear site of production of the active dihydrotestosterone (DHT) end product and production of oestrogens into saliva for export to ductal and oral epithelial cells. SS is characterized by low 3β-HSD levels, which together with impaired subcellular compartmentalization of HSDs and 5α-reductase may explain the low local DHT and androgen biomarker levels in SS.
Keywords: Sjögren’s syndrome, salivary glands, dehydroepiandrosterone, dihydrotestosterone, intracrinology
Introduction
Sjögren’s syndrome (SS) is an autoimmune disease characterized by focal lymphoplasmacytoid adenitis and salivary and lachrymal gland atrophy associated with production of SS auto-antibodies, xerostomia and keraconjunctivitis sicca. Its prevalence is 0.1–0.6%[1]. This common autoimmune disease affects primarily women, with the female : male ratio being 9 : 1, and often at the age of 40–50 years [2]. It does not seem to be a coincidence that many women develop SS at this age.
During menopause, the declining ovarian activity leads to lowered oestrogen levels [3]. However, in SS the oestrogen levels in saliva are abnormally high compared to their healthy peers [4]. Oestrogens and, in particular, an augmented oestrogen to androgen ratio, are generally considered as enhancers of cell proliferation and humoural immune responses [5–7]. At the same age women also develop adrenopause and, moreover, patients with SS a premature and/or abnormally deep adrenopause so that their systemic dehydroepiandrosterone (DHEA)-sulphate levels are 60%[8] and their salivary DHEA levels only 50% of those of the age- and gender-matched controls. The local intracrine conversion of DHEA to dihydrotestosterone (DHT) does not suffice to compensate these low local androgen precursor sex steroid (DHEA-sulphate and DHEA) levels because the salivary DHT values are also low in persons with SS (51% of the controls; [4]) and the DHT-regulated androgen biomarker, cysteine-rich secretory protein-3, is very low in salivary glands and in the saliva of patients with primary SS [9]. Thus, the excess local oestrogen and androgen deficiency, which have been suggested to predispose towards lymphocyte infiltration and acinar cell atrophy [5–7, 10, 11], hallmarks of the disease target tissue [12, 13], are already relatively firmly established.
Primates are unique in having adrenals with a reticular zone that secretes large quantities of the precursor sex steroids DHEA and its sulphate. Precursor sex steroids can be tailor-made to various active sex steroids according to the local tissue needs. The role of DHEA metabolism in different models of autoimmune diseases cannot therefore be easily studied in lower species, such as rodents. Intracrine DHEA-sulphate metabolism into androgens and/or oestrogens, as known in female breast and uterus or male prostate, is shown in Fig. 1[14], but has not been studied in salivary glands except for two single enzymes [15, 16]. This Fig. 1 also lays the groundwork to our two-part working hypothesis. Based on the local oestrogen/androgen imbalance in SS and the differences in the serum and salivary sex steroid profiles in healthy controls and patients [4], it was suggested that (i) there is a local DHEA processing intracrine machinery in healthy human salivary glands and (ii) that this is deranged in SS.
Figure 1.
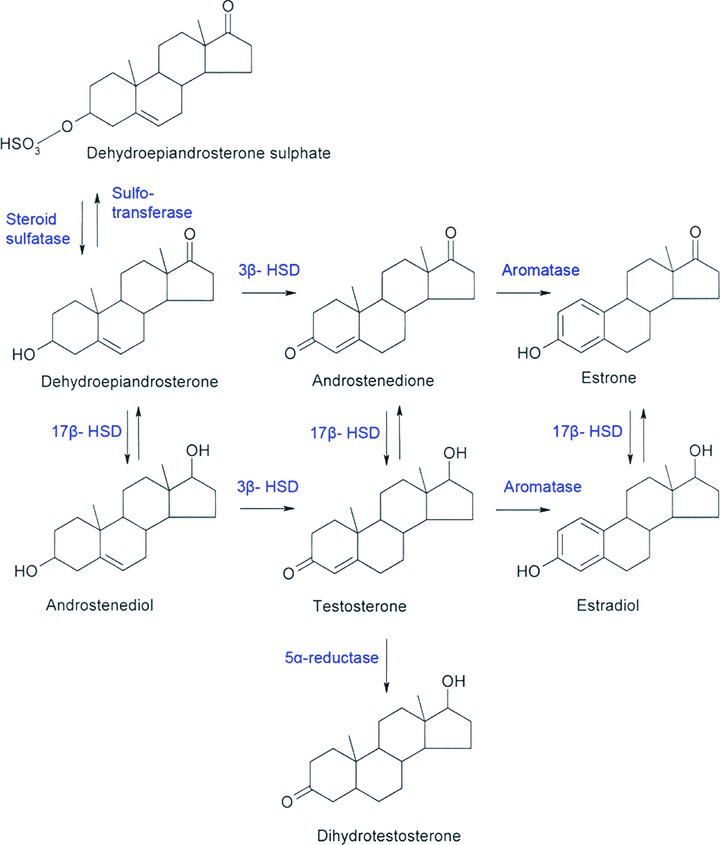
Intracrine metabolism of the dehydroepiandrosterone sulphate (DHEA-S) pro-hormone to various sex steroids. DHEA-sulphate can be subjected to desulphation by steroid sulphatase, but the desulphated DHEA can again be resulphated by sulfotransferase. DHEA is further metabolized by the following key enzymes as shown in the figure: 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD), 17β-HSD, 5α-reductase and/or aromatase.
Material and methods
Patients and samples
Five to eight labial salivary glands from four patients with primary SS and four healthy controls were processed for immunohistochemistry. Patients with primary SS included in this study fulfilled the American-European Consensus Criteria for SS [17]. Labial salivary glands were individually removed as part of a routine histopathological diagnostic procedure at the Department of Rheumatology, Medical Centre of Ljubljana in Slovenia. They were snap frozen in liquid nitrogen and embedded in and optimal compound for tissue embedding (O.C.T.) compound (Tissue-Tek, Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands).
Immunohistochemistry
Tissue samples were cut to 5-μm-thick cryostat sections and fixed in 4% paraformaldehyde for 5 min. Unspecific binding sites were blocked with 1 : 10 diluted normal donkey serum (Jackson ImmunoResearch Europe Ltd., Suffolk, UK) for 1 hr at room temperature. Tissue sections were then incubated (1) in enzyme specific affinity purified primary antibodies diluted in 10 mM phosphate buffered, 140 mM saline [phosphate buffered saline (PBS)], pH 7.2, containing 40% glycerol and 0.02% sodium azide, using 20 μg/ml goat anti-human steroid sulphatase E.C.3.1.6.2 IgG (Santa Cruz Biotechnology, Heidelberg, Germany), goat anti-human sulfotransferase E.C.2.8.2.15 IgG (Santa Cruz Biotechnology), 20 μg/ml goat anti-human 3β-hydroxysteroid dehydrogenase E.C.1.1.1.145 (3β-HSD) IgG (Santa Cruz Biotechnology), 20 μg/ml goat anti-human 17β-HSD E.C.1.1.1.63 IgG (Santa Cruz Biotechnology), 20 μg/ml goat anti-human aromatase E.C.1.14.14.1 IgG (Santa Cruz Biotechnology) and 20 μg/ml goat anti-human 5α-reductase E.C.1.3.99.5 IgG (Santa Cruz Biotechnology), (2) washed in 0.1% Triton-X in PBS, pH 7.5 and then PBS alone, (3) 10 μg/ml donkey anti-goat IgG (Alexa Fluor, Molecular Probes, Eugene, OR, USA) for 1 hr at room temperature in dark, (4) washed as above, (5) sometimes counterstained with 0.014 mmol DAPI (Sigma-Aldrich) nuclear stain in dH2O for 5 min. at room temperature in the dark, (6) washed as above and 7) mounted in fluorescent mounting medium Vectashield (Vector Laboratories, Burlingame, CA, USA).
Non-immune, normal goat IgG was used instead of and at the same concentration as the primary specific antibodies for staining controls.
Doublestaining
For immunofluorescence doublestaining the sections were incubated in 1:50 diluted primary affinity purified rabbit anti-human lactoferrin immunoglobulin fraction (DAKO, Glostrup, Denmark) and 0.1 μg/ml monoclonal mouse anti-human anti-smooth muscle actin (Chemicon International, Temecula, CA, USA) as recommended by the manufacturers, followed by incubation in 10 μg/ml donkey anti-rabbit IgG (Alexa Fluor) and 10 μg/ml donkey antimouse IgG (Alexa Fluor), respectively.
Microscopy and photographing
Stained sections were examined under an Olympus AX70 (Olympus Optical, Hamburg, Germany) microscope coupled with a charge-coupled device (CCD) camera (Olympus DP71).
Tissue culture
For tissue culture studies labial salivary glands were obtained from four healthy controls who were treated for mucocele and three patients with SS. Glands were minced into pieces (∼2 mm3) and cultured overnight in Dulbecco’s modified minimal essential medium (DMEM)-Ham’s F-12 medium (Gibco BRL) containing 10% foetal calf serum (FCS), L-glutamine, 10× antibiotics and Fungizone (2.5 μg/ml; Gibco BRL, Grand Island, NY, USA). The next day, the media were changed to basal DMEM-Ham’s F-12 media with 10% serum-stripped FCS, L-glutamine, 1X antibiotics and 2.5 μg/ml Fungizone. Tissues were cultured for 72 hrs.
RNA extraction and cDNA synthesis
Tissue RNA was isolated with High Pure RNA Tissue kit (Roche, Basel, Switzerland), followed by cDNA synthesis done with the SuperScript First Strand cDNA Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
qRT-PCR reaction was done with a LightCycler(tm) PCR instrument (Roche). The primers used for the detection of the steroidogenic enzymes for steroid sulphatase 5′-GGAAGGCCTTTTTCTTCACC-3′ and 5′-AGGGTCTGGGTGTGTCTGTC-3′, for sulfotransferase 2B1 5′-CCTG-GTGTGAGACCATTGTG-3′ and 5′-GAGCCCTGTAAGTCCTGCTG-3′, for 3β-HSD-1 5′-CCTTCAACCGCCACATAGTC-3′ and 5′-GCTTGTGCC-CTTGTCACTTT-3′, for 17β-HSD-5 5′-TGCGTGAGATTCTCCAGATG-3′ and 5′-AATGGCTTGGGAGAAGGTTT-3′, for 5α-reductase-1 5′-ATGTTC-CTCGTCCACTACGG-3′ and 5′-AGCCACACCACTCCATGATT-3′ and for aromatase 5′-GTGGACGTGTTGACCCTTCT-3′ and 5′-CAACTCAGTG-GCAAAGTCCA-3′. 3β-HSD-1 and 17β-HSD-5 form the major isotypes of the corresponding HSD enzymes catalysing conversion of the intermediate metabolites of DHEA towards the active sex steroids. 5α-reductase-1 is the isotype of 5α-reductase found in the human labial salivary glands.
Results
Expression of the steroidogenic enzyme mRNAs in labial salivary glands
All six steroidogenic enzymes studied were expressed at the mRNA level in labial salivary glands both in healthy controls and persons with SS (Fig. 2).
Figure 2.
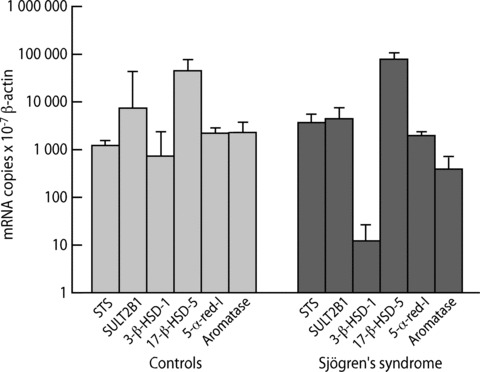
Expression of mRNA of steroidogenic enzymes in labial salivary glands in healthy controls (n= 4) and in patients with Sjögren’s syndrome (SS, n= 3). STS = steroid sulphatase; SULT2B1 = steroid sulfo-transferase; 3β-HSD-1 = 3β-hydroxysteroid dehydrogenase, isotype 1; 17β-HSD-5 = 17β-hydroxysteroid dehydrogenase; 5-α-red-I = 5α–reductase, isotype 1. Values represent means ± S.E. of the means.
Healthy controls
The enzyme catalysing desulphation of DHEA-sulphate, steroid sulphatase, stained strongly mainly the basal parts of the acinar cells. This sulphatase staining covered most segments of the acinar profiles, but did not always totally encircle them (Fig. 3A). In ductal epithelial cells steroid sulphatase was found to varying degree in the whole cytoplasm of most, but not all ductal cells (insert in Fig. 3A). Sulfotransferase, catalysing resulphation of DHEA, displayed granular cytoplasmic staining pattern in the acinar cells, with a preferential localization in the basal aspects of the cells. In the ductal epithelial cells sulfo-transferase reached considerable levels of expression in the cell cytoplasm (Fig. 3C).
Figure 3.
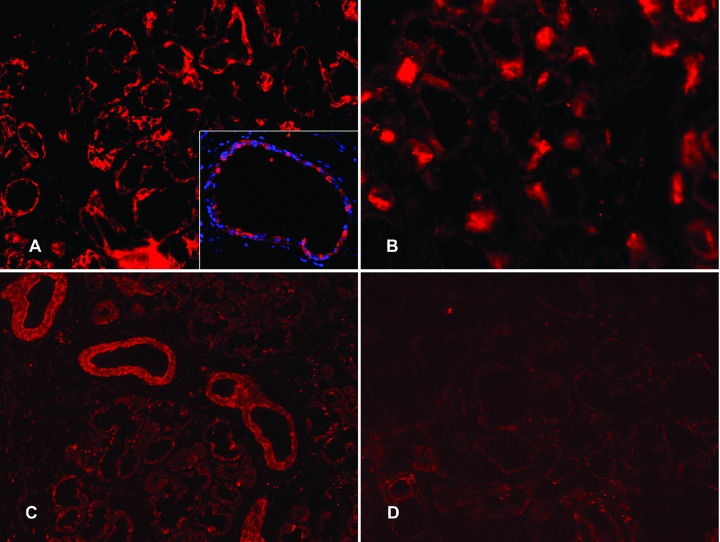
Expression of steroid sulphatase and sulfotransferase in labial salivary glands. (A) In healthy labial salivary glands, steroid sulphatase staining (red colour) was strong and confined to the basal parts of the acinar cells. Insert in (A): In salivary ducts steroid sulphatase (red colour) was found to varying degree in the whole cytoplasm of most, but not all ductal epithelial cells (blue nuclear DAPI counterstain). (B) In labial salivary glands of patients with SS, steroid sulphatase was staining in specific parts of the acini like the healthy controls but was overall decreased (compared to A). (C) In healthy salivary glands sulfotransferase staining (red colour) was mostly seen in salivary ducts, while acini stained weakly with somewhat granular staining pattern, mostly seen in the basal parts of the acinar cells. (D) In SS salivary glands sulfotransferase showed a decreased expression in the acini (compared to C). Objective magnification ×200 (in A, B, C and D) or ×400 (in insert).
Both 3β- and 17β-HSDs were located in the immediate vicinity of the basal plasma membrane of the acinar cells (Fig. 4A and C, respectively). Staining intensity was very strong and staining did not seem to extend far to the cytoplasm of the acinar cells. These narrow HSD bands were always interrupted. Double staining experiments disclosed that they embraced the mucous segments of the acini, but not the lactoferrin-containing seromucous demilunes ([18]; Fig. 5A). In spite of this staining pattern, double labelling experiments of HSDs with smooth muscle α-actin for myoepithelial cells disclosed that these enzymes were truly located in the acinar cells (Fig. 5B) although occasionally also seen in some parts of the myoepithelial cells (data not shown). In salivary ducts these two HSDs were more diffusely located (data now shown) although they were occasionally located only in the immediate vicinity of the apical (as opposed to the basal location in acini) plasma membrane as a relatively narrow immunoreactive line (insert in Fig. 4C).
Figure 4.
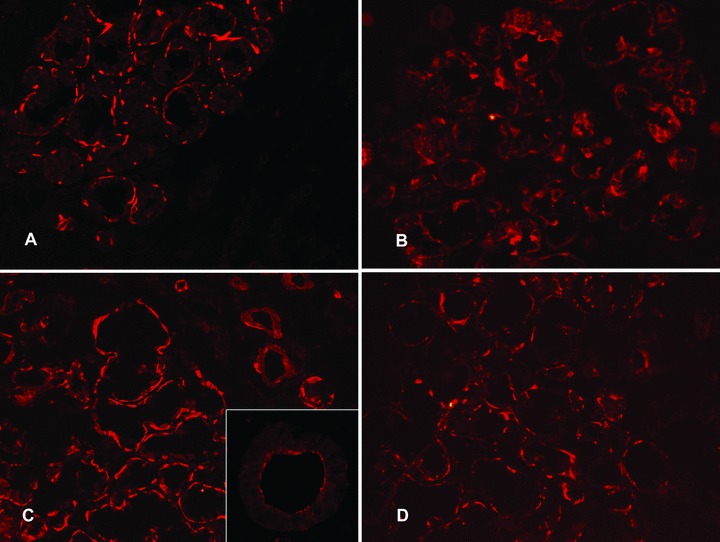
Expression of 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase in labial salivary glands. In healthy controls, 3β-hydroxysteroid dehydrogenase (A) and 17β-hydroxysteroid dehydrogenase (C) stained the basal parts of the acinar cells leading to a narrow interrupted band-like staining. Insert in (C): In contrast, ductal staining was often characterized by a narrow band of apical staining. In labial salivary glands from patients with SS, 3β-HSD staining was more diffuse (B, compared to A) and the same pattern was seen in 17β-HSD staining (D, compared to C). Objective magnification ×200 (in A, B, C and D) or ×400 (in the insert).
Figure 5.
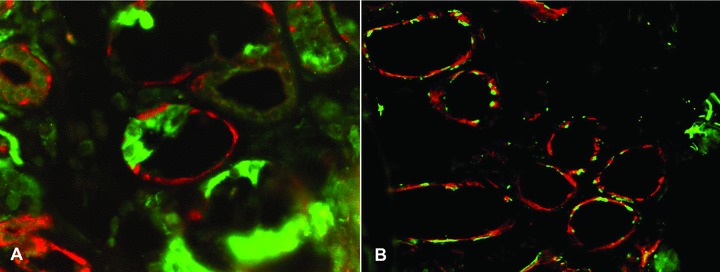
Hydroxysteroid dehydrogenases (HSD) were in the healthy labial salivary glands restricted to the mucous segments of the acini. (A) 17β-HSD (red colour) was stained together with lactoferrin (green colour), which is restricted to the serous demilunes. It was found that 17β-HSD only embraced the mucous segments of the acini, but did not embrace the seromucous half moons. (B) To exclude exclusive staining of myoepithelial cells, HSDs (17β-HSD in the panel, red colour) and myoepithelial cell marker smooth muscle actin showed very little overlap (which would produce yellow colour). Objective magnification ×200.
5α-reductase was mainly located in the nuclei of the acinar cells so that cytoplasmic staining was relatively weak. This nuclear localization was clearly seen in nuclear DAPI counterstained sections (Fig. 6A), whereas its localization was more diffuse in the ductal cells (arrowhead). Aromatase showed a very intimate association with the apical (and apolateral) acinar cell plasma membrane; it was not found on the basal plasma membrane domains of the acinar cells (Fig. 6C). In contrast, in ductal cells it was located on both the apical and basal plasma membrane domains (insert in Fig. 6C). Aromatase was not found in the cell cytoplasm or in the nuclei of any of the acinar or ductal cells.
Figure 6.
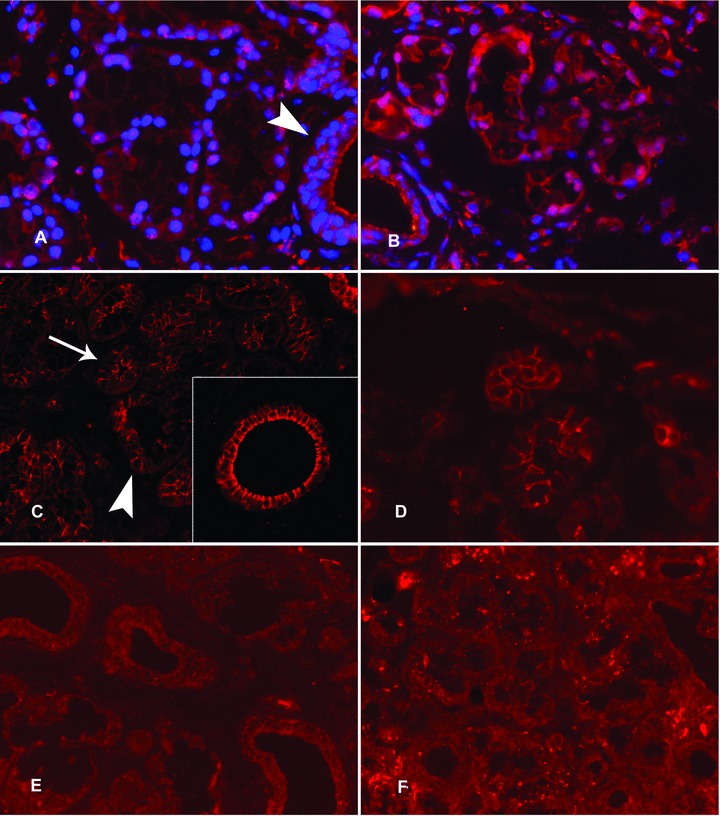
Expression of 5α-reductase and aromatase in labial salivary glands. In healthy labial salivary glands, 5α-reductase staining was strong in the nuclei of the acinar cells, whereas it had a more diffuse localization in the ductal epithelial cells (A, arrow head). This partial nuclear localization of 5α-reductase is very evident with nuclear DAPI counterstain (A). In contrast, aromatase immunoreactivity was polarized in the acinar cells so that it was expressed strongly on apical and lateral cell membrane domains (C, arrowhead) but was barely seen in the basal cell membrane (C, arrow) (C). Insert in (C): In salivary ducts aromatase was often seen also on the basal aspects of the ductal epithelial cells. In labial salivary glands of patients with SS, 5α-reductase staining was mostly cytoplasmic (B, compared to A). For the expression of aromatase in SS salivary glands, a similar expression was found as in healthy controls (D, compared to C). (E) and (F): The negative staining controls with normal, non-immune goat IgG, used instead of and at the same concentration as the specific primary antibodies, confirm the specificity of the stainings. Objective magnification ×200 in all panels and ×400 in the insert.
Sjögren’s syndrome
Steroid sulphatase staining was in SS very weak in acinar cells compared to the acini of the healthy controls and it did not so closely follow the basal aspects of the acini as it did in controls (Fig. 3B; compare with Fig. 3A). In SS, sulfotransferase, although clearly present in the ductal epithelial cells, was very weak or even absent in the acinar cells (Fig. 3D; compare with Fig. 3C).
In SS, both 3β- and 17β-HSD staining extended much further into the cell cytoplasm than in the corresponding healthy control glands so that both HSDs had more diffuse staining patterns (Fig. 4B and D, respectively; compare with Fig. 4A and C).
In SS, 5α-reductase was not so strictly localized to the nuclei but was to a large extent localized in the cytoplasm of the acinar cells (Fig. 6B; compare with Fig. 6A), whereas aromatase stained similarly in SS as in the healthy controls (Fig. 6D; compare with Fig. 6C).
Staining controls
Negative staining controls with normal goat IgG were negative for healthy controls (Fig. 6E) and for patients with SS (Fig. 6F) and confirmed the specificity of the staining.
Discussion
Present results demonstrate for the first time that the epithelial cells of the healthy human labial salivary glands house a complete and well-organized intracrine machinery required for the conversion of DHEA(-sulphate) pro-hormone to its most active sex steroid metabolites, DHT and 17β-estradiol [15, 16]. This cellular architecture seems to reflect the preferential site of action (Fig. 7) [19]. Steroid sulphatase, which converts DHEA-sulphate (storage form) to DHEA (pro-hormone form), and sulfotransferase working in opposite direction, were preferentially localized to the basal aspects of the acinar cells (Fig. 7) as in gastric glands [20]. Also the next enzymes in the intracrine processing chain, 3β- and 17β-HSDs, were in acinar cells restricted to their basal aspects. This would bring the initial and intermediate conversion steps close to the site of the endocrine delivery of DHEA-sulphate and DHEA from the circulation. Nuclear localization of 5α-reductase type 1, the isotype found in salivary glands [4], is probably a reflection of the intracrine mode of action of locally produced DHT [21]. Localization of aromatase in the apical acinar cell membrane may reflect production of 17β-oestradiol for export and its paracrine mode of action on salivary gland and oral epithelial cells [22]. This would also prevent competition with DHT in the control of transcription of genes in the acinar cell nuclei [23].
Figure 7.
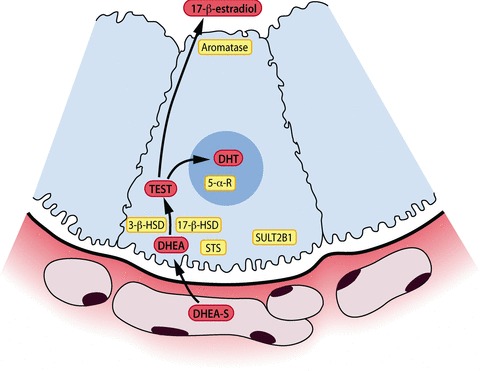
The architecture of the steroidogenic enzymes in a healthy salivary gland acinar cell. Sex steroids are represented in yellow background and follow a route from the basal part towards apical parts of the acinar cell. STS: steroid sulphatase, SULT: steroid sulfo-transferase, 3β-HSD: 3β-hydroxysteroid dehydrogenase, 17β-HSD: 17β-hydroxys-teroid dehydrogenase. DHEA-sulphate = dehydroepiandrosterone sulphate, TEST = testosterone, DHT = dihydrotestosterone.
In SS salivary glands the architecture of the intracrine enzymes producing active androgens was deranged. Steroid sulphatase, 3β- and 17βHSDs had in SS lost their close spatial relationship with the basal parts of the acinar cells. The key enzyme 5α-reductase, converting testosterone to DHT, was in SS quite strongly expressed in the cell cytoplasm instead of its nuclear localization in as in healthy controls. Finally, based on the qRT-PCR data, 3β-HSD is very low in SS. Current work supports our earlier results about the disturbed sex steroid levels in saliva of SS patients [4, 9]. First, the enzymatic machinery processing DHEA to DHT is topologically deranged in SS salivary glands, which together with the low 3β-HSD could explain the diminished local concentrations of DHT and androgen-regulated biomarker CRISP-3. Aromatase, however, seems to be similarly expressed in healthy and SS salivary glands; this observation is convergent with our results of slightly increased concentrations of 17-β-estradiol in saliva of patients with SS [4]. Diminished usage of DHEA to DHT synthesis might provide more intracellular testosterone for conversion to 17β-estradiol.
In conclusion, the work shows that a DHEA processing enzymatic machinery is expressed in healthy salivary gland epithelial cells and that this machinery is deranged in SS. This dysfunction, together with low serum levels of DHEA-sulphate, may explain the local androgen deficiency seen in salivary glands of patients with SS. Women may be particularly vulnerable to such dysregulation of the acinar cell intracrinology as their local DHT production is totally dependent on DHEA input into this machinery. Men, instead, can to a large extent bypass this need for local sex steroid tailor-making by feeding in testosterone, which is only one step away of becoming DHT. Local DHEA processing may deserve more attention when reasons for the female dominance, age of onset and exocrine gland targeting typical for SS are analysed in depth [24].
Acknowledgments
This article is dedicated to my late friend, Professor Michael Humphreys-Beher, for numerous rewarding in-depth discussions all over the world, including several times in Finland, on the eventual role of the acinar cell changes in the induction phase of SS. This research was funded by Finska Läkaresällskapet, evo grants, Invalid Foundation, the Finnish Dental Society Apollonia, CIMO, Orion-Farmos Research Foundation and Helsinki University. During her fellowship, M.S. was supported by ErasmusMC-Scholarship from Erasmus University and by the Erasmus-Scholarship from the European Union.
References
- 1.Bowman SJ, Ibrahim GH, Holmes G, et al. Estimating the prevalence among Caucasian women of primary Sjögren’s syndrome in two general practices in Birmingham, UK. Scand J Rheumatol. 2004;33:39–43. doi: 10.1080/03009740310004676. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjögren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjögren’s Syndrome. Ann Rheum Dis. 1996;55:116–21. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 4.Porola P, Virkki L, Przybyla BD, et al. Androgen deficiency and defective intracrine processing of DHEA in the salivary glands in Sjögren’s syndrome. J Rheumatol. 2008;35:2229–35. doi: 10.3899/jrheum.080220. [DOI] [PubMed] [Google Scholar]
- 5.Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34:241–51. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- 6.Cutolo M, Straub RH, Bijlsma JW. Neuroendocrine-immune interactions in synovitis. Nat Clin Pract Rheumatol. 2007;3:627–34. doi: 10.1038/ncprheum0601. [DOI] [PubMed] [Google Scholar]
- 7.Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: friend or foe. Rheumatology. 2008;43:2–5. doi: 10.1093/rheumatology/ken150. [DOI] [PubMed] [Google Scholar]
- 8.Valtysdottir ST, Wide L, Hällgren R. Low serum dehydroepiandrosterone sulfate in women with primary Sjögren’s syndrome as an isolated sign of impared HPA axis function. J Rheumatol. 2001;28:1259–65. [PubMed] [Google Scholar]
- 9.Laine M, Porola P, Udby L, et al. Low salivary dehydroepiandosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjögren’s syndrome. Arthritis Rheum. 2007;56:2575–84. doi: 10.1002/art.22828. [DOI] [PubMed] [Google Scholar]
- 10.Konttinen YT, Porola P, Konttinen L, et al. Immunohistopathology of Sjögren’s syndrome. Autoimmun Rev. 2006;6:16–20. doi: 10.1016/j.autrev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Porola P, Laine M, Virkki L, et al. The influence of sex steroids on Sjögren’s syndrome. Ann N Y Acad Sci. 2007;1108:426–32. doi: 10.1196/annals.1422.045. [DOI] [PubMed] [Google Scholar]
- 12.Ryu OH, Atkinson JC, Hoehn GT, et al. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology. 2006;45:1077–86. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- 13.Hu S, Wang J, Meijer J, et al. Salivary proteomic and genomic biomarkers for primary Sjögren’s syndrome. Arthritis Rheum. 2007;56:3588–600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:113–8. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 15.Onodera K, Sasano H, Ichinohasama R, Ooya K. Immunolocalization of aromatase in human minor salivary glands of the lower lip with primary Sjögren’s syndrome. Pathol Int. 1998;48:786–90. doi: 10.1111/j.1440-1827.1998.tb03838.x. [DOI] [PubMed] [Google Scholar]
- 16.Lam K, Zhang L, Yamada KM, Lafrenie RM. Adhesion of epithelial cells to fibronectin or collagen I induces alterations in gene expression via a protein kinase C-dependent mechanism. J Cell Physiol. 2001;189:79–90. doi: 10.1002/jcp.1142. [DOI] [PubMed] [Google Scholar]
- 17.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konttinen YT, Kulomaa M, Malmström M, et al. Lactoferrin in Sjögren’s syndrome. Arthritis Rheum. 1984;27:462–467. doi: 10.1002/art.1780270416. [DOI] [PubMed] [Google Scholar]
- 19.He D, Meloche CA, Dumas NA, et al. Different subcellular localization of sulphotransferase 2B1b in human placenta and prostate. Biochem J. 2004;379:533–40. doi: 10.1042/BJ20031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tashiro A, Sasano H, Nishikawa T, et al. Expression and activity of dehydroepiandrosterone sulfotransferase in human gastric mucosa. J Steroid Biochem Mol Biol. 2000;72:149–54. doi: 10.1016/s0960-0760(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Dalrymple SL, Becker RE, et al. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12:4072–9. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 22.Välimaa H, Savolainen S, Soukka T, et al. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180:55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- 23.Cutolo M, Seriolo B, Villaggio B, et al. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:131–42. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 24.Konttinen YT, Käsnä-Ronkainen L. Sjögren’s syndrome: viewpoint on pathogeneis. One of the reasons I was never asked to write a textbook chapter on it. Scand J Rheumatol. 2002;116:15–22. [PubMed] [Google Scholar]


