Abstract
Proteins of the annexin family bind to phospholipids in a Ca2+ dependent manner. The exposure of phosphatidylserine (PS) by apoptotic as well as necrotic cells is one major eat-me-signal for macrophages. Annexin A5 (Anx A5) preferentially binds to PS. The availability of Anx A5 knock out (KO) mice allowed us to investigate for the first time if endogenous Anx A5 modulates the immune response towards allogeneic cells. Furthermore, the effect of Anx A5 gene deletion on the phagocytic process as well as on the inflammatory reaction of macrophages was explored. We found that Anx A5 KO mice have a strongly reduced allogeneic cellular immune reaction against primary as well as secondary necrotic cells. In vivo phagocytosis experiments revealed that macrophages of Anx A5 KO mice displayed an increased uptake of necrotic cells. Additionally, an increased secretion of the anti-inflammatory cytokine IL-10 of isolated macrophages of Anx A5 KO mice after contact with necrotic cells was observed. Furthermore, the promoter activity of the Anx A5 gene was enhanced after stimulation of macrophages. The tumour size of an allogeneic tumour regressed faster when endogenous Anx A5 was present. These data demonstrate that endogenous Anx A5 influences the phagocytosis of necrotic cells, modulates the immune response towards allogeneic cells and acts as an inflammatory protein.
Keywords: annexin A5, macrophages, primary necrosis, secondary necrosis, allogeneic cells, phagocytosis, inflammation, immune modulation
Introduction
Annexins bind to phospholipids in a Ca2+ dependent manner. Phospholipids like phosphatidylserine (PS) and lyso-phosphatidylcholine (LPC) are involved in the clearance of apoptotic and necrotic cells. The exposure of PS by dying and dead cells is one major eat-me-signal for phagocytes [1]. Macrophages produce the milk fat globule-EGF-factor 8 (MFG-E8), which binds to apoptotic cells by recognizing aminophospholipids such as PS [2]. The receptors responsible for the engulfment of PS exposing apoptotic cells were identified recently as being Tim4 and Tim1 [3]. The secretion of LPC by apoptotic cells leads to the attraction of phagocytes and is therefore considered as an important soluble find-me-signal [4]. Recently, it has been shown that annexin A1 (Anx A1) and its peptide derivatives are released by apoptotic cells and also very efficiently promote the phagocytosis of apoptotic cells [5]. The latter is an important physiologic homeostatic mechanism that is associated with non-inflammatory or anti-inflammatory sequalae (reviewed in [6]). Anx A1 is a mediator of the anti-inflammatory actions of glucocorticoids (reviewed in [7]), which further promote non-inflammatory or even anti-inflammatory phagocytosis of apoptotic cells [8]. In contrast, exogenous Anx A5, which preferentially binds to PS, skewed the cytokine secretion of macrophages after contact with apoptotic cells towards pro-inflammatory cytokines in in vitro assays [9]. The same effects were observed for secondary necrotic cells (suppl. Fig. 3). Furthermore, the addition of Anx A5 to apoptotic cells significantly increased their in vivo immunogenicity [10]. Importantly, Anx A5-coupled apoptotic tumour cells induced the regression of growing tumours [11]. In an infection model, Anx A5 bound to PS of apoptotic Leishmania promastigotes also enhanced the release of the inflammatory cytokine TNF- by granulocytes [12]. Anx A5 inhibits apoptotic and necrotic cell uptake by macrophages most likely through interference with the availability of PS for recognition [13, 14]. Anx A5, in contrast to Anx A1, inhibits the phagocytosis of apoptotic cells by internalization of PS exposing membrane areas [15].
Figure 3.
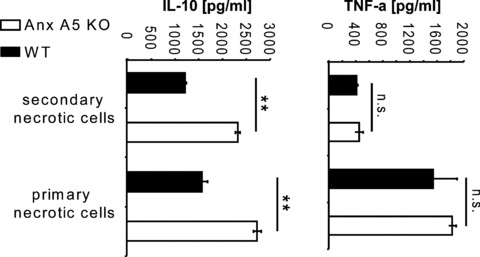
Cytokine secretion of activated macrophages of WT and Anx A5 KO mice after stimulation with necrotic cells. The secretion of murine TNF-α and IL-10 by LPS activated peritoneal macrophages after contact in cell culture with mechanical stress-induced primary necrotic Sp2O cells or UV-B light induced secondary necrotic cells, respectively, was quantified by ELISA. Note: macrophages of Anx A5 KO mice showed a significantly enhanced secretion of IL-10. Values are the mean +/– SD of three assays. KO: knock out; WT: wild type; **P < 0.01
From the immunological point of view, apoptotic cells are non- or even anti-inflammatory [16] while primary as well as secondary necrotic cells stimulate the immune system [17]. During apoptosis the re-transport of PS from the outer to the inner leaflet of the plasma membrane declines. Besides the massive disturbance of plasma membrane morphology, the plasma membrane of apoptotic cells is known to remain ion selective for a long time. If apoptotic cells are not swiftly cleared, they change their membrane composition [18] and turn to secondary necrosis. Secondary necrotic cells have undergone apoptosis for a while and then loose their membrane integrity. Primary necrotic cells result from viable ones, which have immediately lost their membrane integrity due to strong external impacts. Cytotoxic components can then get in contact with immune competent cells and trigger an immune response [19]. Danger signals like HMGB1 [20], uric acid [21] or ATP [22] are all released by primary necrotic or damaged cells. These signals can get lost upon washing of cells that are prepared for cell-based assays or animal injections. We observed in previous studies that necrotic cells produced by heat-treatment (30 min., 56°C) had the same anti-inflammatory effects on activated macrophages as apoptotic ones [14]. Furthermore, there was no significant difference between unwashed and washed necrotic cells, if necrosis was induced by heat treatment and an anti-inflammation dominated response of the macrophages was detected in all cases. In contrast, unwashed mechanical stress-induced necrotic cells induced a decreased secretion by activated macrophages of the anti-inflammatory cytokine IL-10 in comparison to heat-induced necrotic cells [23]. This again highlights the importance of labile and short-lived danger molecules as additional signal for the induction of adaptive immune responses [24], which are only preserved in the case of mechanical stress-induced necrosis.
To elucidate the role of Anx A5 under normal physiological conditions we generated Anx A5 knock out (KO) mice [25]. Surprisingly, initial studies did not detect an altered phenotype of these mice [26]. By performing analyses with respect to the clearance of dying cells we could also detect no significant differences between WT and Anx A5 KO mice in the immune response towards apoptotic cells [23]. The endogenous levels of Anx A5 are low (<10 ng/ml in serum; [27]) and the expected immunomudulatory potential of this protein, therefore, is weak. If higher levels of Anx A5 are present (e.g. by the addition of exogenous Anx A5), we have already shown that the immune response even towards syngeneic cells is enhanced in the presence of Anx A5 [11].
Here we examine how endogenous Anx A5 modulates the immune reaction against allogeneic cells. The latter lead to an activation of the immune system since non-self antigens are recognized. We induced primary necrosis by mechanical stress and injected the necrotic cells within 0.5 hr after starting the necrotizing procedure into mice to preserve heat-labile short-lived danger signals. We also included secondary necrotic cells in our immunization protocol, because they are known to have a high inflammatory potential. Thus, primary as well as secondary necrotic cells are well suited to prime cellular immunity. Furthermore, we investigated the effect of Anx A5 gene deletion on the phagocytic process as well as on the inflammatory reaction of macrophages.
Materials and methods
Animals
The experiments were performed with 10–12-week-old Anx A5-deficient and wild type (WT) mice displaying mixed genetic background of C57/BL6 129/SvJ. The Anx A5 deficient mouse strain was generated by homologous recombination containing a LacZ reporter gene cassette fused inframe with exon 3 of the Anx A5 gene [26].
Cell lines, cells, induction and detection of necrosis and apoptosis
The murine tumour cell lines Sp2/0 and CT26 were obtained from the American Type Culture Collection (Rockville, MD, USA). The murine B-cell line WEHI 231 was a kind gift of Dr. Dirk Mielenz (Division of Molecular Immunology, Erlangen, Germany) [28]. For the induction of secondary necrosis, the hybridoma Sp2/O cells were irradiated with ultraviolet B light (UV-B; 120 mJ/cm2) and cultured in medium for 24 hrs. Regarding the dying cell population, 75% of the cells were then secondary necrotic and about 25% of the cells were still apoptotic (suppl. Fig. 1). The increase of the PS exposure on the outer membrane leaflet of apoptotic cells can be monitored employing Fluorescein isothiocyanate (FITC)-labelled Anx A5 [29]. The latter binds with high affinity to PS in a Ca2+ dependent manner. Besides the massive disturbance of plasma membrane morphology, the plasma membrane of apoptotic cells is known to remain ion selective for a long time and therefore impermeable for the DNA intercalating propidium iodide (PI). Therefore, apoptotic cells are positive for Anx A5 binding and negative for PI staining. Secondary necrotic cells have undergone apoptosis for a while and then loose their membrane integrity. Consequently, they are positive for Anx A5 binding as wells as for PI, which can now stain the nucleus. Necrotic cells are generally positive for PI staining since they have lost their membrane integrity. Primary necrotic cells are also positive for Anx A5 binding since this protein can bind to PS on the inner leaflet of the disturbed cellular membrane. Primary necrotic cells show a higher staining for PI in comparison to secondary necrotic cells since the latter already have discharged DNA during the apoptotic process via‘blebs’. Primary necrosis in Sp2/O cells was induced by mechanical sheer stress. The cells were forced 2–3 times with high pressure through a very narrow hollow needle. We used a 20G needle with 11/2 length and the aperture was further narrowed using a pincer. Importantly, the necessary bore is dependent on the cell type. We adjusted the final aperture to the cell type, which has to be necrotized. The necrotizing procedure has to be performed very fast to preserve short-lived danger signals. The necrotic status was individually checked by Anx A5-FITC/PI staining. If more than 75% of the cells were necrotic (positive for PI) (suppl. Fig. 1) they were immediately injected into the mice. The WEHI 231 cells for the in vivo phagocytosis assays were necrotized by heat treatment as described previously [14]. Necrosis was verified in each individual experiment before injection of the cells into the peritoneum. More than 90% of the cells stained positive for trypan-blue, PI, and Anx A5-FITC.
Figure 1.
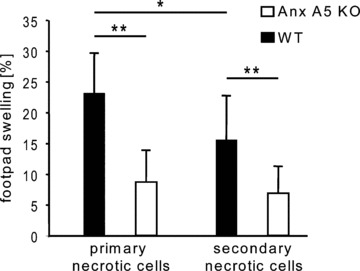
Allogeneic immune response against necrotic cells in WT and Anx A5 KO mice. WT and KO mice were immunized three times i.p. with allo-geneic primary or secondary necrotic Sp2O cells, respectively. The specific cellular immune response was monitored with the delayed type hypersensivity reaction (DTH test) and is indicated as percentage footpad swelling of the mice. Note: WT mice showed a strongly enhanced immune response in comparison to Anx A5 KO animals. This response was also significantly higher against fresh primary necrotic cells in comparison to secondary necrotic cells. Three independent experiments with always four mice per group were performed. KO: knock out; WT: wild type; *P < 0.05; **P < 0.01
Murine macrophages for the cytokine secretion assays were obtained by peritoneal lavage 4 days after i.p. injection of 1.0 ml amylum (starch from corn; Bestfoods, Hamburg, Germany; 2% in PBS) into the mice. 1.5 × 106 macrophages were seeded in 24-well plates in RPMI medium containing 10% foetal calf serum (FCS), 100 U/ml penicillin, 100 mg/ml streptomycin and 200 mM glutamine (R10 medium). The non-adherent cells were removed after 2 hrs and the adherent macrophages were cultured for further 24 hrs in R10.
Immunization experiments
WT and Anx A5 KO animals were immunized with 5 × 106 secondary necrotic or primary necrotic Sp2/0 cells, respectively. For the injection into the peritoneum, the cells were suspended in 500 μl of Ringer’s solution. The injection was performed three times at day 0, day 21 and day 63, respectively. The delayed type hypersensitivity (DTH) test was carried out 6 days after the final immunization. One million viable Sp2/0 cells in 50 μl PBS were injected into the footpads. The diameter of the footpads was measured before and 18 hrs after the injections. The footpad swelling was calculated by: (diameter of the footpad 18 hrs after the injection – diameter of the footpad before the injection)/(diameter of the footpad before the injection) × 100.
The immune response towards allogeneic CT26 colon carcinoma cells (strain: BALB/c) was monitored by analysis of the tumour mass disappearance. 1 × 106 CT26 cells were injected s.c. in the flank of the mice. From day 5 after injection until day 21, the tumour volume was measured with an electronic calliper.
In vivo phagocytosis by macrophages of necrotic cells
Labelling of the cells
Mouse WEHI 231 cells were labelled with 5-(and 6-)carboxyfluorescein-diacetate succinimidyl ester (CFDASE; Molecular Probes, Leiden, The Netherlands). The stock CFDASE (5 mM) was prepared in DMSO and stored frozen under an atmosphere of the inert gas N2. Cells were re-suspended in PBS at approximately 2 × 106/ml and pre-warmed to 37°C. The stock CFDASE was then added to the suspension (final concentration: 10 μM). The suspension was inverted twice and incubated for 20 min. at 37°C. The cells were next washed twice with four volumes of cold R10 medium. Afterwards, primary necrosis was induced by heat treatment (56°C for 30 min.). The cells were then washed once with PBS/EDTA (5 mM) to remove intrinsic bound Anx A5 and afterwards with PBS only to remove residual EDTA. At the end, the necrotic cells were re-suspended in Ringer’s solution (2 × 106 cells/ml).
In vivo phagocytosis
Macrophages were recruited in the peritoneum of the mice by i.p. injection of 1.0 ml amylum (2% in PBS) 4 days before the injection of the green-labelled necrotic cells. The mice were killed 0.75 hr or 3.5 hrs after the injection of 500 μl of the necrotic cell suspension into the peritoneum and a lavage of the peritoneum was performed with 8 ml R10. The obtained cell suspension was filtered (70 μm filter), the cells washed in R10 and ery-throcytes were removed by hypotonic lysis. Next the cells were stained with mouse F4/80-PE (Caltag, Invitrogen, Karlsruhe, Germany), mouse CD11b-PE (BD Pharmingen, Heidelberg), or the control antibodies, respectively, for analysis by flow cytometry. Phagocytosis of the green-labelled necrotic cells by F4/80 or CD11b positive macrophages was determined and quantified by 2-color flow cytometry as described previously for human cells [14].
Quantification of cytokine secretion by mouse peritoneal macrophages
Mouse peritoneal macrophages (see above) were cultured for 24 hrs in R10. Mechanical stress-induced necrotic Sp2/O cells (1.0 × 106/well) or UV-B induced secondary necrotic Sp2/O cells (1.0 × 106/well) were added to the macrophages in 500 μl of R10 1–2 hrs prior to activation with LPS (100 ng/ml). Sixteen hours after activation, the supernatants were collected. Cytokine concentrations were determined by ELISA using appropriate pairs of monoclonal antibodies specific for mouse IL-10 and TNFα, respectively (BD Pharmingen, Germany).
Staining with X-Gal and FDG
The Anx A5 KO mouse strain was generated by homologous recombination containing a LacZ reporter gene cassette fused inframe with exon 3 (pos. 178) of the Anx 5 gene [25]. Using the specifically expressed Anx A5-LacZ fusion gene, it is possible to isolate cells from the KO mice [30] and to easily monitor the Anx A5 promoter activity. Peritoneal macrophages were stained with 5-bromo-4-chloro-3-indoxyl beta-D-galactoside (X-Gal) for b-galactosidase activity [31], which represents the promoter activity of the Anx A5 gene.
Fluorescein di-β-D-galactopyranoside (FDG; Molecular Probes, Invitrogen) can be used to detect the transient expression of b-galactosidase constructs. Peritoneal macrophages were stained with F4/80-PE or CD11b-PE and additional with FDG as follows: 5 × 105 cells of the F4/80 or CD11b stained cells were re-suspended in 10 μl PBS/FCS. The FDG stock solution was diluted to 2 mM in H2O. 10 μl of this FDG dilution was added to 10 μl of the cell suspension. After vortexing, the cells were incubated for 75 seconds at 37°C. Immediately afterwards, 125 μl of ice cold PBS/FCS was added and the suspension incubated for 2.0 hrs on ice in the dark. FDG positive macrophages could then easily be quantified by flow cytometry (see Fig. 4).
Figure 4.
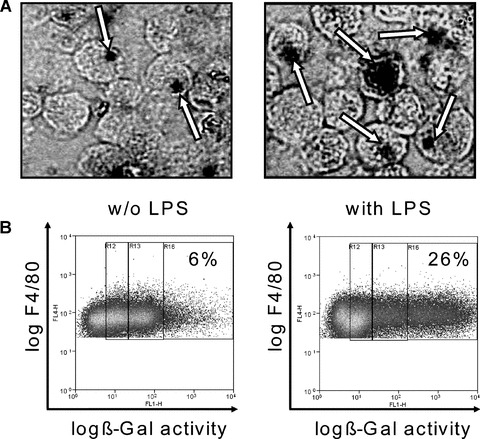
Anx A5 promoter activity of activated macrophages. Macrophages were obtained from the peritoneal lavage of Anx A5 KO mice. Following, the macrophages were cultured for 24 hrs in medium without (w/o) or with LPS. The peritoneal macrophages were stained with 5-bromo-4-chloro-3-indoxyl beta-D-galactoside (X-Gal) for b-galactosidase activity (A), which represents the promoter activity of the Anx A5 gene. The X-Gal staining of macrophages (deep black areas) clearly demonstrates the expression of Anx A5 within the macrophage population (arrows). The b-galactosidase activity was also quantified by staining the Anx A5 knock out macrophages with fluorescein di-β -D-galactopyranoside (FDG) reagent. F4/80 positive macrophages displayed a 4.3-fold higher ß-Gal activity after stimulation with LPS. One out of three representative set of experiments is shown. KO: knock out; WT: wild type; LPS: lipopolysac-charide
Statistical analysis
Statistical analyses were performed using the heteroskedastic two-tailed Student’s t-test for unpaired data. Results were considered statistically significant for P < 0.05 (*) and highly significant for P < 0.01 (**).
Results
The allogeneic immune response against necrotic cells is reduced in Anx A5 KO mice
We injected three times i.p. primary or secondary necrotic Sp2/0 cells in WT or Anx A5 KO mice and determined the allogeneic cellular immune reaction by DTH test. As shown in Figure 1, WT mice showed a median footpad swelling of about 23% or 15% after injection of primary or secondary necrotic cells, respectively. Primary necrotic cells induced a significant higher swelling in comparison to secondary necrotic cells in the WT situation. In contrast, only a very weak swelling was to be observed in Anx A5 KO mice. A highly significant reduction of the allogeneic immune response in comparison to WT animals was observed for primary as well as secondary necrotic cells in Anx A5 KO mice.
The uptake of necrotic cells is enhanced in macrophages of Anx A5 KO mice
Animals were injected i.p. with CFSE-labelled heat-induced primary necrotic cells allowing resident macrophages to interact with the dead cells for up to 3.5 hrs. The mice were killed after 0.75 hr or 3.5 hrs and the uptake of the fluorescent necrotic cells in F4/80 positive macrophages, present in the peritoneal lavage, was monitored by flow cytometry. F4/80 positive peritoneal macrophages of Anx A5 KO mice phagozytosed significantly more primary necrotic cells in comparison to macrophages of WT mice (Fig. 2). This was to be observed at 0.75 hr (Fig. 2A and B) as well as at 3.5 hrs (Fig. 2C and D) after injection of the necrotic cells. The uptake of multiple necrotic cells per phagocyte (high positive macrophages) was highly significantly enhanced in Anx A5 KO mice when compared with Anx A5 WT animals (Fig. 2D, right graph). Similar effects were observed by using mechanical stress-induced primary necrotic cells for the phagocytosis assays (suppl. Fig. 4).
Figure 2.
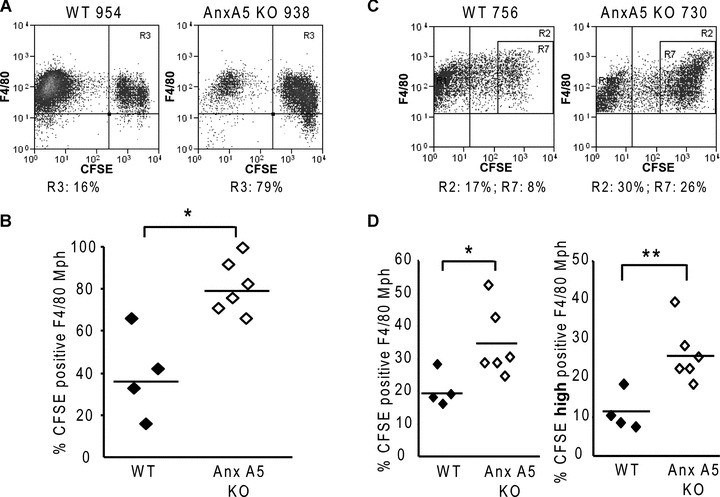
Uptake by macrophages of necrotic cells in presence or absence of endogenous Anx A5. Macrophages in the peritoneum of the mice had contact with CFSE-labelled heat-induced (30 min., 56°C) primary necrotic WEHI 231 cells for 0.75 (A, B) or 3.5 hrs (C, D). Following, a peritoneal lavage was performed and the phagocytosis by macrophages, which were identified with F4/80 staining, of the necrotic prey was determined. In (A) and (C), representative dot plots of the analyses of the phagocytosis by flow cytometry are shown. Macrophages of Anx A5 KO mice showed a significantly enhanced uptake of primary necrotic cells after 0.75 hr (B) as well as after 3.5 hrs (D). Note: The phagocytic ability of peritoneal macrophages of Anx A5 KO mice to take up multiple necrotic cells per phagocyte (high positive macrophages) was highly significantly enhanced when compared with macrophages of WT animals (D, right graph). KO: knock out; WT: wild type; Mph: macrophages; Gates R3, R2:%CFSE positive Mph; Gate R7:% CFSE high positive Mph; One out of two representative set of experiments is shown. *P < 0.05; **P < 0.01
Stimulated macrophages of Anx A5 KO mice show an increased secretion of IL-10 after contact with primary or secondary necrotic cells
We analysed in vitro the secretion of the anti-inflammatory cytokine IL-10 and that of the inflammatory cytokine TNF-α of LPS activated peritoneal macrophages after contact with primary or secondary necrotic cells. The secretion of TNF-α did not significantly differ between macrophages of Anx A5 KO and WT animals (Fig. 3). However, activated macrophages of Anx A5 KO mice showed a higher secretion of IL-10 in response to mechanical stress-induced necrotic cells as wells as to UV-B induced secondary necrotic cells in comparison to WT macrophages (Fig. 3).
Stimulated macrophages express Anx A5
The Anx A5 deficient mouse strain was generated by homologous recombination containing a LacZ reporter gene cassette fused inframe with exon 3 (pos. 178) of the Anx A5 gene. X-Gal staining of macrophages suggested the transcriptional up-regulation of Anx A5 in macrophages after activation with LPS (Fig. 4). We quantified the activity of the endogenous Anx A5 promotor in the KO cells by measuring the b-galactosidase activity of the reporter-gene by staining the Anx A5 knock out macrophages with fluorescein di-β-D-galactopyranoside (FDG) reagent. F4/80 positive macrophages displayed a 4.3-fold higher ß-Gal activity after stimulation with LPS than without stimulation (Fig. 4B).
An allogeneic tumour regresses slower in Anx A5 KO mice
We injected 1 × 106 allogeneic CT26 colorectal tumour cells in the flank of Anx A5 KO or WT mice. At day 5 after injection, a tumour size was measured with an electronic calliper. The tumour size was monitored twice weekly. Importantly, in WT mice the tumour regressed significantly faster in comparison to the Anx A5 KO mice. Figure 5 displays the time course of the regression and final rejection of the allogeneic tumour in Anx A5 KO or WT mice. Three weeks after injection, no tumour was detectable in WT mice while 50% of the Anx A5 KO mice still had a detectable tumour (median tumour diameter = 3.1). Two weeks after the final rejection of the allogeneic tumour we injected viable CT26 cells in the footpads of WT and Anx A5 KO animals to induce a DTH reaction. The latter was detectable in both cases, however, it was slightly, though not significantly reduced in Anx A5 KO animals (mean footpad swelling: 26.5%) in comparison to WT animals (mean footpad swelling: 30.0%) (suppl. Fig. 5).
Figure 5.
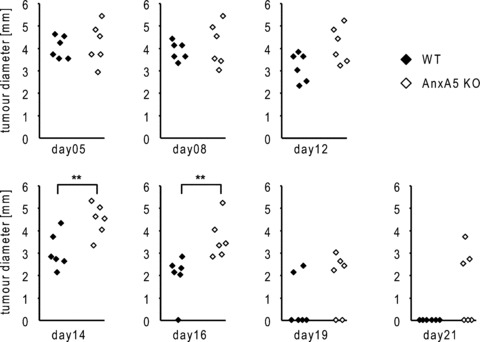
Regression and final rejection of the allogeneic CT26 tumour in WT and Anx A5 KO mice. Allogeneic CT26 colorectal tumour cells were injected in the flank of Anx A5 KO or WT mice. At day 5, a measurable tumour was detectable and the consecutive allogeneic immune response against the tumour was monitored by measuring the tumour diameter with an electronic calliper at the indicated days after the injection. Note: WT mice showed a significant faster regression and final rejection of the tumour in comparison to Anx A5 KO animals. One out of three representative set of experiments is shown. KO: knock out; WT: wild type; **P < 0.01
Discussion
Our immunological experiments in Anx A5 KO and WT mice revealed that Anx A5 reinforces the allogeneic immune response towards primary and secondary necrotic cells as well as allogeneic viable carcinoma cells. It was shown by Win and colleagues that in the syngeneic situation tumours continue to grow and, concurrently, a big necrotic core in the tumour can be observed. However, in the allogeneic situation, the transplanted tumour cells form a detectable tumour only for a short time and then the tumour size regresses very fast, finally leading to the rejection of the tumour [32]. We here showed that the latter appeared faster in WT animals in comparison to Anx A5 KO mice. We do propose that in the Anx A5 KO situation also a decreased induction of cytotoxic T lymphocytes is one possible mechanism for the delayed immune reaction against allogeneic tumour cells.
Over the last years another annexin, namely Anx A1, has emerged as an important anti-inflammatory mediator. It is highly expressed in macrophages and inhibits superoxide production, cell trafficking and phagocytosis (reviewed in [33]). Examinations with bone marrow derived macrophages of Anx A1 KO mice demonstrated that lack of Anx A1 leads to a decreased phagocytosis of various particles and to a higher secretion of inflammatory cytokines by macrophages [34]. Under normal physiological conditions, Anx A1 is, therefore, a mediator of the non- or even anti-inflammatory swift clearance of apoptotic cells by professional phagocytes.
When interfering with the clearance of dying cells, dendritic cells (DC) may acquire modified autoantigens like apoptotic nuclei and nuclear proteins and consequently autoreactive T cells can be activated. This may also be the scenario in chronic autoimmune diseases like systemic lupus erythematosus (SLE) (reviewed in [6] and [35]). An impaired clearance of UV irradiated, apoptotic tumour cells by macrophages can also lead to specific tumour rejection. We previously showed that exogenous Anx A5 decreased apoptotic cell uptake by peritoneal macrophages and concomitantly increased their uptake by DC [11]. When performing experiments with exogenous Anx A5 the effects of endogenous Anx A5 are negligible since it is only present at low concentrations.
Here we show that endogenous levels of Anx A5 act as inflammatory mediator. We demonstrated that Anx A5 is highly expressed by macrophages and that lack of endogenous Anx A5 leads to an increased secretion of anti-inflammatory cytokines by stimulated macrophages. We propose that Anx A5 is expressed and released from macrophages. Afterwards it binds dying and dead cells and, consecutively, the phagocytosis of the latter is partially blocked. Another proof for this demonstrated our experiments with Anx A5 KO macrophages showing a significantly enhanced phagocytosis of necrotic cells. We monitored the in vivo phagocytosis after 0.75 hr and 3.5 hrs. Less macrophages were positive for the labelled necrotic cells after 3.5 hrs in comparison to 0.75 hrs. This is due to digestion of the engulfed material and gave a further hint that uptake and not only binding takes place. We previously have shown that the pathway of necrosis induction does not much influence the rate of phagocytosis by macrophages [14]. Also mechanical stress-induced necrotic cells were favourably taken up by macrophages of Anx A5 KO mice when compared with WT animals (suppl. Fig. 4). However, the consecutive production of cytokines was strongly modulated. We further observed that after contact of the macrophages with the dying and dead cells, the Anx A5 promoter activity was down-regulated (suppl. Fig. 2). We would tend to speculate that after contact with the dying cells the macrophages express less Anx A5 and consequently gain better phagocytic capabilities.
The use of Anx A5 for therapeutic applications and not only as a marker for apoptotic cell death has become obvious [36–38]. We here showed that macrophages of Anx A5 KO animals displayed a more anti-inflammatory and immunosuppressive potential in response to mechanical stress-induced necrotic cells as well as secondary necrotic cells. We used allogeneic primary or secondary necrotic cells for the immunization experiments in order to get a strong immune response in the WT situation. The mode of cell death strongly influences the immunogenicity of the cells [39, 40] and pro-inflammatory properties of necrotic cells strongly depend on the inductor and the course of necrosis [14, 23, 41].
Taken together, annexins influence the balance of the cytokine milieu leading to inflammatory or anti-inflammatory immune responses. Anx A1 was shown to be an endogenous inhibitory regulator of MAPK activation and IL-6 expression [42]. We here have shown that endogenous Anx A5 leads to a decreased secretion of the anti-inflammatory cytokine IL-10 by activated macrophages. Anx A5 is an important inflammatory modulator of the immune system (reviewed in [9, 43]). Further investigations should focus on combinatory and compensatory effects of various annexins in modulating immune responses; Anx A1 and Anx A5 seem to be antagonists in this scenario.
Acknowledgments
This work was supported by ‘Deutsche Forschungsgemeinschaft’ SFB 643 (project B5), by the ELAN program of the Friedrich-Alexander University of Erlangen-Nürnberg, by the responsif GmbH Erlangen, by the European Commissions [NOTE (TPA4 FP6) and APOCLEAR (QLK3-CT-2002-02017)], by the Programme Alban, the European Union Program of High Level Scholarships for Latin America, scholarship no. ‘E04D047956VE’ to L. E. M., and by the DFG research training grant GK592.
Supporting Information
Fig. S1. UV-B irradiation and mechanical stress induce necrosis in Sp2/O cells. (A) For the induction of secondary necrosis, Sp2/O cells were irradiated with UV-B (120 mJ/cm2) and cultured in R10 medium for 24 hrs. Regarding the dying cell population, 80.4% of the cells were necrotic (AnxA5-FITC positive and PI positive) and 18% of the cells were apoptotic (AnxA5-FITC positive and PI negative). (B) Primary necrosis in Sp2/O cells was induced by mechanical stress. Regarding the dying cell population, 93.0% of the cells were primary necrotic (AnxA5-FITC positive and PIhigh positive). One out of five representative set of experiments is shown.
Fig. S2. Activated macrophages show less AxA5 promoter activity after contact with dying cells. AnxA5 KO macrophages were obtained by a peritoneal lavage. The β-galactosidase activity was quantified by staining the LPS-activated Anx A5 knock-out macrophages with fluorescein di-β-d-galactopyranoside (FDG) reagent. 1 × 106 primary or secondary necrotic WEHI 231 cells were co-incubated for 16 hrs with the LPS-activated macrophages. Activated F4/80 positive macrophages displayed a significant lower Δ-Gal activity after contact with secondary (A) or primary (B) necrotic cells, respectively. * P < 0.05; * * P < 0.01; n = 5.
Fig. S3. Exogenous AnnexinA5 enhances the inflammatory cytokine secretion of activated macrophages after contact with necrotic cells. Activated macrophages of C57BL/6 mice were incubated with secondary necrotic (sec. nec.) Sp2O cells, and the secretion of TNF-α was quantified by ELISA. Note: The macrophages showed a significantly enhanced secretion of TNF-α in the presence of exogenous AnxA5 (1 g/ml); * P < 0.05.
Fig. S4. Uptake by macrophages of mechanical stress-induced necrotic cells in presence or absence of endogenous AnxA5. Peritoneal macrophages had in vivo contact for 0.75 hrs with CFSE-labelled WEHI 231 cells, which were necrotized by mechanical stress. Afterwards, a peritoneal lavage was performed and the phagocytosis by macrophages of the necrotic prey was determined by flow cytometry. Macrophages of AnxA5 KO mice showed a significantly enhanced uptake of primary necrotic cells; Mph: macrophages; * P < 0.05; n =7.
Fig. S5. Delayed type hypersensitivity (DTH) reaction of AnxA5 KO and WT mice 2 weeks after the rejection of the allogeneic CT26 colorectal tumour (at day 42 of the experiment shown in the new Fig. 5).The DTH reaction was induced by injection of 6 × 106 viable CT26 cells in the right footpad. As control, we injected PBS in the left footpad. Twenty-four hours later, the footpad swelling was measured with an electronic calliper. P = 0.37; n = 6.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
References
- 1.Hirt UA, Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10:1156–64. doi: 10.1038/sj.cdd.4401286. [DOI] [PubMed] [Google Scholar]
- 2.Hanayama R, Tanaka M, Miwa K, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 3.Miyanishi M, Tada K, Koike M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–9. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 4.Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–30. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 5.Scannell M, Flanagan MB, DeStefani A, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 6.Michlewska S, McColl A, Rossi AG, et al. Clearance of dying cells and autoimmunity. Autoimmunity. 2007;40:267–73. doi: 10.1080/08916930701357208. [DOI] [PubMed] [Google Scholar]
- 7.Perretti M, D’Acquisto F. Novel aspects of annexin 1 and glucocorticoid biology: intersection with nitric oxide and the lipoxin receptor. Inflamm Allergy Drug Targets. 2006;5:107–14. doi: 10.2174/187152806776383170. [DOI] [PubMed] [Google Scholar]
- 8.McColl A, Michlewska S, Dransfield I, Rossi AG. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. Sci World J. 2007;7:1165–81. doi: 10.1100/tsw.2007.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaipl US, Munoz LE, Rodel F, et al. Modulation of the immune system by dying cells and the phosphatidylserine-ligand annexin A5. Autoimmunity. 2007;40:254–9. doi: 10.1080/08916930701357331. [DOI] [PubMed] [Google Scholar]
- 10.Stach CM, Turnay X, Voll RE, et al. Treatment with annexin V increases immunogenicity of apoptotic human T-cells in Balb/c mice. Cell Death Differ. 2000;7:911–5. doi: 10.1038/sj.cdd.4400715. [DOI] [PubMed] [Google Scholar]
- 11.Bondanza A, Zimmermann VS, Rovere-Querini P, et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med. 2004;200:1157–65. doi: 10.1084/jem.20040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Zandbergen G, Bollinger A, Wenzel A, et al. Leishmania disease development depends on the presence of apoptotic pro-mastigotes in the virulent inoculum. Proc Natl Acad Sci USA. 2006;103:13837–42. doi: 10.1073/pnas.0600843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krahling S, Callahan MK, Williamson P, Schlegel RA. Exposure of phosphatidylserine is a general feature in the phagocytosis of apoptotic lymphocytes by macrophages. Cell Death Differ. 1999;6:183–9. doi: 10.1038/sj.cdd.4400473. [DOI] [PubMed] [Google Scholar]
- 14.Bottcher A, Gaipl US, Furnrohr BG, et al. Involvement of phosphatidylserine, alphav-beta3, CD14, CD36, and complement C1q in the phagocytosis of primary necrotic lymphocytes by macrophages. Arthritis Rheum. 2006;54:927–38. doi: 10.1002/art.21660. [DOI] [PubMed] [Google Scholar]
- 15.Kenis H, Van Genderen H, Deckers NM, et al. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp Cell Res. 2006;312:719–26. doi: 10.1016/j.yexcr.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Voll RE, Herrmann M, Roth EA, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 17.Proskuryakov SY, Gabai VL, Konoplyannikov AG, et al. Immunology of apoptosis and necrosis. Biochemistry. 2005;70:1310–20. doi: 10.1007/s10541-005-0263-4. [DOI] [PubMed] [Google Scholar]
- 18.Bilyy RO, Antonyuk VO, Stoika RS. Cytochemical study of role of alpha-d-mannose- and beta-d-galactose-containing glycoproteins in apoptosis. J Mol Histol. 2004;35:829–38. doi: 10.1007/s10735-004-1674-z. [DOI] [PubMed] [Google Scholar]
- 19.Rovere P, Sabbadini MG, Fazzini F, et al. Remnants of suicidal cells fostering systemic autoaggression. Apoptosis in the origin and maintenance of autoimmunity. Arthritis Rheum. 2000;43:1663–72. doi: 10.1002/1529-0131(200008)43:8<1663::AID-ANR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 22.Hanley PJ, Musset B, Renigunta V, et al. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci USA. 2004;101:9479–84. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz LE, Franz S, Pausch F, et al. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J Leukoc Biol. 2006 doi: 10.1189/jlb.0306166. [DOI] [PubMed] [Google Scholar]
- 24.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 25.Brachvogel B, Welzel H, Moch H, et al. Sequential expression of annexin A5 in the vasculature and skeletal elements during mouse development. Mech Dev. 2001;109:389–93. doi: 10.1016/s0925-4773(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 26.Brachvogel B, Dikschas J, Moch H, et al. Annexin A5 is not essential for skeletal development. Mol Cell Biol. 2003;23:2907–13. doi: 10.1128/MCB.23.8.2907-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flaherty MJ, West S, Heimark RL, et al. Placental anticoagulant protein-I: measurement in extracellular fluids and cells of the hemostatic system. J Lab Clin Med. 1990;115:174–81. [PubMed] [Google Scholar]
- 28.Mielenz D, Vettermann C, Hampel M, et al. Lipid rafts associate with intracellular B cell receptors and exhibit a B cell stage-specific protein composition. J Immunol. 2005;174:3508–17. doi: 10.4049/jimmunol.174.6.3508. [DOI] [PubMed] [Google Scholar]
- 29.Koopman G, Reutelingsperger CP, Kuijten GA, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 30.Brachvogel B, Pausch F, Farlie P, et al. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp Cell Res. 2007;313:2730–43. doi: 10.1016/j.yexcr.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 31.Singh MB, Knox RB. Quantitative cyto-chemistry of beta-galactosidase in normal and enzyme deficient (gal) pollen of Brassica campestris: application of the indigogenic method. Histochem J. 1984;16:1273–96. doi: 10.1007/BF01003726. [DOI] [PubMed] [Google Scholar]
- 32.Win S, Uenaka A, Nakayama E. Immune responses against allogeneic and syngeneic tumors in aged C57BL/6 mice. Microbiol Immunol. 2002;46:513–9. doi: 10.1111/j.1348-0421.2002.tb02728.x. [DOI] [PubMed] [Google Scholar]
- 33.Kamal AM, Flower RJ, Perretti M. An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem InstOswaldo Cruz. 2005;100:39–47. doi: 10.1590/s0074-02762005000900008. [DOI] [PubMed] [Google Scholar]
- 34.Yona S, Heinsbroek SE, Peiser L, et al. Impaired phagocytic mechanism in annexin 1 null macrophages. Br J Pharmacol. 2006;148:469–77. doi: 10.1038/sj.bjp.0706730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaipl US, Sheriff A, Franz S, et al. Inefficient clearance of dying cells and autoreactivity. Curr Top Microbiol Immunol. 2006;305:161–76. doi: 10.1007/3-540-29714-6_8. [DOI] [PubMed] [Google Scholar]
- 36.Boersma HH, Kietselaer BL, Stolk LM, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46:2035–50. [PubMed] [Google Scholar]
- 37.Kenis H, Hofstra L, Reutelingsperger CP. Annexin A5: shifting from a diagnostic towards a therapeutic realm. Cell Mol Life Sci. 2007;64:2859–62. doi: 10.1007/s00018-007-7297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Genderen HO, Kenis H, Hofstra L, et al. Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbamcr.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Larmonier N, Billerey C, Rebe C, et al. An atypical caspase-independent death pathway for an immunogenic cancer cell line. Oncogene. 2002;21:6091–100. doi: 10.1038/sj.onc.1205738. [DOI] [PubMed] [Google Scholar]
- 40.Zitvogel L, Kroemer G. Death, danger, and immunity: an infernal trio. Immunol Rev. 2007;220:5–7. doi: 10.1111/j.1600-065X.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 41.Popescu AT, Vidulescu C, Stanciu CL, et al. Selective protection by phosphatidic acid against staurosporine-induced neuronal apoptosis. J Cell Mol Med. 2002;6:433–8. doi: 10.1111/j.1582-4934.2002.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YH, Toh ML, Clyne CD, et al. Annexin 1 negatively regulates IL-6 expression via effects on p38 MAPK and MAPK phosphatase-1. J Immunol. 2006;177:8148–53. doi: 10.4049/jimmunol.177.11.8148. [DOI] [PubMed] [Google Scholar]
- 43.Munoz LE, Frey B, Pausch F, et al. The role of annexin A5 in the modulation of the immune response against dying and dead cells. Curr Med Chem. 2007;14:271–7. doi: 10.2174/092986707779941131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item
Supporting info item


