Abstract
Objective
The consequences of perioperative venous thromboembolism (VTE) are devastating; identifying patients at risk is an essential step in reducing morbidity and mortality. The utility of perioperative VTE risk assessment in otolaryngology is unknown. This study was designed to risk-stratify a diverse population of otolaryngology patients for VTE events.
Study Design
Retrospective cohort study.
Setting
Single-institution academic tertiary care medical center.
Subjects and Methods
Adult patients presenting for otolaryngologic surgery requiring hospital admission from 2003 to 2010 who did not receive VTE chemoprophylaxis were included. The Caprini risk assessment was retrospectively scored via a validated method of electronic chart abstraction. Primary study variables were Caprini risk scores and the incidence of perioperative venous thromboembolic outcomes.
Results
A total of 2016 patients were identified. The overall 30-day rate of VTE was 1.3%. The incidence of VTE in patients with a Caprini risk score of 6 or less was 0.5%. For patients with scores of 7 or 8, the incidence was 2.4%. Patients with a Caprini risk score greater than 8 had an 18.3% incidence of VTE and were significantly more likely to develop a VTE when compared to patients with a Caprini risk score less than 8 (P <.001). The mean risk score for patients with VTE (7.4) was significantly higher than the risk score for patients without VTE (4.8) (P <.001).
Conclusion
The Caprini risk assessment model effectively risk-stratifies otolaryngology patients for 30-day VTE events and allows otolaryngologists to identify patient subgroups who have a higher risk of VTE in the absence of chemoprophylaxis.
Keywords: venous thromboembolism, prophylaxis, deep venous thrombosis, pulmonary embolism, risk stratification, patient safety, risk factor, Caprini
Venous thromboembolism (VTE), which includes both deep venous thromboses (DVT) and pulmonary emboli (PE), is an important cause of morbidity and mortality in hospitalized patients. Approximately 250,000 VTE events are diagnosed annually in the United States.1 Venous thromboembolism is the most common cause of preventable death in US hospitals and the third most common cause of all hospital-related deaths.2,3 Patients who survive a VTE event are at risk for developing pulmonary hypertension, recurrent PE, and postthrombotic syndrome.4,5 The impressive morbidity and mortality associated with VTE events have prompted groups such as the National Quality Forum, the Center for Medicare & Medicaid Services, the Joint Commission on Accreditation of Health Care Organizations, and the Office of the Surgeon General of the United States to develop initiatives to address this major threat to patient safety.6,7
Venous thromboembolism risk increases with hospitalization for surgery or an acute medical illness, with hospitalized patients having an estimated 150-fold higher VTE risk than community residents.8 This increased risk reflects the higher prevalence of VTE risk factors in hospitalized populations.2 Moreover, surgical patients admitted to the hospital have nearly a 70-fold increase in VTE risk compared with the general population and a 10-fold increased risk compared with surgical outpatients.9 As a result, surgical specialties have studied the incidence of VTE extensively. Earlier studies of hospitalized surgical patients who did not receive thromboprophylaxis provide an estimate of VTE risk.2 In general surgery and gynecologic surgery, the incidences of DVT and fatal PE are 15% to 40% and 0.2% to 0.9%, respectively.10-12 Orthopedic procedures carry a DVT risk of 40% to 60%, with a fatal PE rate of up to 5%.2
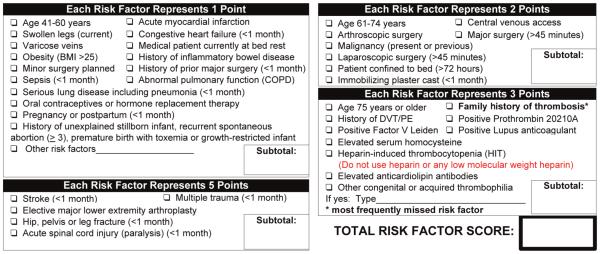
The Caprini Risk Assessment Model (RAM) was developed to provide an individualized VTE risk assessment based on patient-specific risk factors.13-15 The Caprini RAM assigns a weighted risk score to each of approximately 40 VTE risk factors (eg, prior VTE, oncologic status, age). The risk factors are weighted and summed, and the cumulative score is used to risk-stratify patients into 1 of 4 risk levels. Subsequently, clinicians can approximate the patient’s VTE risk and make informed decisions about thromboprophylaxis. A retrospective risk-scoring method based on the Caprini RAM was validated for 30-day VTE events in a series of 8216 general, urology, and vascular surgery patients and provided evidence in support of individual risk assessment.16 The Caprini RAM was validated for 60-day VTE events in 1126 plastic and reconstructive surgery patients.17 Versions of the Caprini RAM have also been tested among medical inpatients and postbariatric body contouring patients.18-20 At our institution, the Caprini RAM has been used to risk-stratify patients and guide perioperative thromboprophylaxis decisions for surgical patients (Figure 1).
Figure 1.
Caprini venous thromboembolism (VTE) risk assessment.
To date, there are no conclusive or high-quality data exploring the accuracy of perioperative VTE risk assessment in otolaryngology–head and neck surgery. This study was designed to examine if the Caprini RAM could risk-stratify a diverse population of otolaryngology patients for 30-day VTE events.
Methods
Study Design and Participants
The University of Michigan Medical School Institutional Review Board reviewed and approved the study protocol. This study was performed as a retrospective cohort study of all adult patients (≥18 years old) who presented to the Department of Otolaryngology–Head and Neck Surgery at the University of Michigan for surgery requiring hospital admission from September 2003 through June 2010. The cohort included patients admitted to either otolaryngology or neurosurgery services, whose primary surgeon was from the otolaryngology service. Patients admitted to other inpatient services or undergoing outpatient surgeries were excluded. All patients with orders for thromboprophylaxis with subcutaneous heparin or low molecular weight heparin were also excluded from the analysis.
Caprini Risk Assessment
A retrospective VTE risk-scoring method has been developed, tested for reliability, and validated in a large population of surgical patients at our institution.16 We used a similar method in this study and briefly describe its implementation herein. Data for each VTE risk factor included in the Caprini RAM (Figure 1) were abstracted from electronic medical databases. These databases included hospital billing records, which included International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, operating room data, and an institutionally maintained clinical data repository. Risk factors were weighted per the Caprini RAM and summed to determine the total Caprini risk factor score and risk level (low, moderate, high, and highest).
VTE Determination
Diagnostic test results were reviewed to identify DVT or PE events within 30 days of surgery. Deep venous thrombosis required confirmation by venous duplex ultrasound, and a PE event required confirmation by ventilation-perfusion scan or PE-protocol computed tomography (CT) scan. Patients who experienced sudden death were included if a VTE event was confirmed either pre- or postmortem. The Department of Otolaryngology–Head and Neck Surgery retains prospectively maintained morbidity and mortality data that were cross-referenced to ensure the capture of all documented VTE events.
Statistical Analysis
Venous thromboembolism risk factors were identified and Caprini RAM scores were calculated for each patient in the study population. A descriptive analysis of the percentage of patients with each VTE risk factor was conducted. The difference in the percentage of patients with each risk factor was compared between patients who did and did not develop VTE using χ2 or Fisher exact tests. A comparison of the Caprini RAM score between the 2 groups was reported, and because the Shapiro-Wilk test indicated that the distribution of Caprini RAM scores departed slightly from normality (P = .01), the difference in scores was evaluated using the Wilcoxon rank-sum test.
A distribution of the incidence rate of VTE by total risk factor score and the significance of differences were reported. The significance of differences was tested using a χ2 test or Fisher exact test.
Results
A total of 2016 patients were included in the study population. Only 1.4% of the population were classified as low risk (total risk factor score of 0 or 1), 10% were moderate risk (total score of 2), 31% were high risk (total score of 3 or 4), and the remaining 57% were highest risk (total score of 5 or more) (Table 1).
Table 1.
Incidence of VTE in Otolaryngology by Caprini Risk Score
| Caprini RAM | 0-1 | 2 | 3-4 | 5-6 | 7-8 | 9+ | Total |
|---|---|---|---|---|---|---|---|
| VTE, No. (%) | 0 | 1 (0.5) | 1 (0.2) | 7 (0.9) | 7 (2.4) | 11 (18.3) | 27 (1.3) |
| No VTE, No. (%) | 29 (100) | 202 (99.5) | 623 (99.8) | 804 (99.1) | 282 (97.6) | 49 (81.7) | 1989 (98.7) |
| % VTE (95% CI) | NA | 0-2.7 | 0-0.9 | 0.4-1.8 | 1.0-4.9 | 9.5-30.4 | 0.1-0.7 |
Abbreviations: CI, confidence interval; NA, not applicable; RAM, Risk Assessment Model; VTE, venous thromboembolism.
The overall 30-day rate of VTE was 1.3%. The incidence of VTE in patients with a Caprini risk score of 6 or less was 0.5%. For patients with scores of 7 or 8, the incidence was 2.4%. Patients with a Caprini risk score greater than 8 had an 18.3% incidence of VTE (Figure 2).
Figure 2.
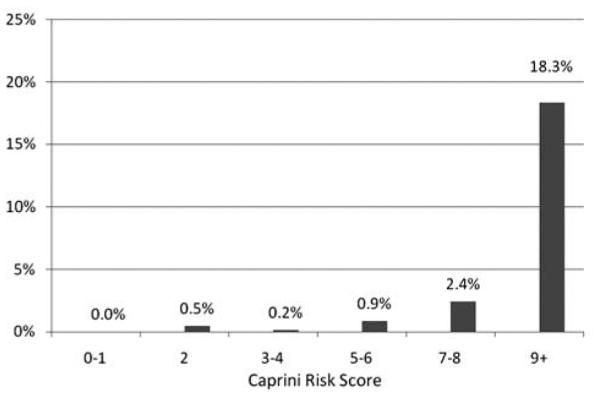
Incidence of 30 day venous thromboembolism (VTE) by estimated Caprini risk score in otolaryngology.
The mean total risk factor score for patients with VTE (n = 27) was 7.4 (median 8). The mean total risk factor score for patients without VTE (n = 1989) was 4.8 (median 5). This difference was significantly different (P < .001). A comparison of VTE risk factors between these 2 groups showed that a significantly higher proportion of patients who developed a VTE had risk factors such as cancer, chronic obstructive pulmonary disease, recent stroke, central venous catheter, and infections, including sepsis and pneumonia (Table 2).
Table 2.
Incidence of VTE Based on Caprini VTE Risk Factors (n = 2016)
| No VTE (n = 1989), No. (%) | + VTE (n = 27), No. (%) | P Valuea | |
|---|---|---|---|
| Age 41-60 y (1 point) | 778 (39) | 10 (37) | .826 |
| Age 61-74 y (2 points) | 535 (27) | 10 (37) | .239 |
| Age >75 y (3 points) | 273 (14) | 5 (19) | .473 |
| Acute myocardial infarction (1 point) | 6 (0.3) | 1 (3.7) | .090 |
| Congestive heart failure (1 point) | 33 (1.7) | 1 (3.7) | .370 |
| Varicose veins (1 point) | 0 | 0 | NA |
| BMI >25 (1 point) | 420 (21) | 7 (26) | .544 |
| Inflammatory bowel disease (1 point) | 11 (0.6) | 0 | 1.000 |
| Sepsis (1 point) | 9 (0.5) | 2 (7.4) | .009 |
| COPD (1 point) | 103 (5.2) | 6 (22) | .003 |
| Pneumonia (1 point) | 19 (1.0) | 2 (7.4) | .031 |
| Oral contraceptives or HRT (1 point) | 1 (0.1) | 0 | 1.000 |
| Pregnancy or postpartum (1 point) | 1 (0.1) | 0 | 1.000 |
| Prior pregnancy complication (1 point) | 0 | 0 | NA |
| Minor surgery (<45 min) (1 point) | 181 (9.1) | 3 (11) | .732 |
| Major surgery (>45 min) (2 points) | 1807 (91) | 24 (89) | .732 |
| Central venous access (2 points) | 57 (2.9) | 10 (37) | <.001 |
| Cancer (2 points) | 1071 (54) | 21 (78) | .013 |
| History of VTE (3 points) | 36 (1.8) | 1 (3.7) | .396 |
| Positive factor V Leiden (3 points) | 9 (0.5) | 0 | 1.000 |
| Other coagulopathy (3 points) | 1 (0.1) | 0 | 1.000 |
| HIT (3 points) | 2 (0.1) | 0 | 1.000 |
| Stroke (5 points) | 15 (0.8) | 4 (15) | <.001 |
| Acute spinal cord injury (5 points) | 0 | 0 | NA |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIT, heparin-induced thrombocytopenia; HRT, hormone replacement therapy; NA, not available; VTE, venous thromboembolism. Bold values are statistically significant.
A χ2 test was used to test the differences in proportion of patients with a risk factor between patients with and without VTE. For risk factors with less than 5 patients, the P value was based on the Fisher exact test.
Patients with a Caprini risk score greater than 8 were significantly more likely to develop a VTE when compared with patients with scores of 7 to 8 (2.4% vs 18.3%, P < .001), 5 to 6 (0.9% vs 18.3%, P < .001), and 3 to 4 (0.2% vs 18.3%, P < .001). In addition, patients with Caprini scores of 7 to 8 were significantly more likely to have 30-day VTE events when compared with patients with scores of 3 to 4 (0.2% VS 2.4%, p = .018).
Of the 27 patients who developed VTE, 11 patients had a confirmed upper and/or lower extremity DVT without PE, and 16 patients had a confirmed PE. There were 0 perioperative mortalities in this group. Seventeen of the 27 incidents of VTE occurred in patients undergoing microvascular free tissue transfer.
Discussion
Perioperative VTE in Otolaryngology
Few studies have examined the incidence of VTE events in the field of otolaryngology–head and neck surgery. Lee and colleagues21 reviewed 9835 otolaryngology operations performed between 1989 and 2004 at a Canadian tertiary care hospital for a postoperative DVT or PE. The authors identified 10 patients (0.10%) with a DVT and 5 patients (0.05%) with a PE. Moreano and colleagues22 reviewed 12,805 otolaryngology operations performed on adults between 1987 and 1994 at the University of Iowa. They identified 34 patients (0.3%) with a DVT, 24 (0.2%) of whom also had a PE. Both the Lee and Moreano studies reported the highest DVT and PE incidence among patients undergoing a head and neck procedure. An increased risk of VTE among patients requiring oncologic head and neck reconstruction was also documented by Chen et al,23despite VTE rates less than 1% in this population. Similarly, in oral surgery patients, VTE events were quite rare but were associated with increasing body mass index (BMI) and longer hospital stays.24In general, these large retrospective analyses, although useful, invariably underestimate VTE incidence due to the dearth of risk stratification, which identifies clinically relevant subgroups with disproportionate levels of risk, thereby increasing the power to detect differences between them.25
The Caprini RAM was recently tested in a large cohort of plastic surgery patients.17 In this study, the incidence of VTE was markedly similar to the findings in our study, with a significantly higher risk among patients with Caprini scores greater than 8. Data are also consistent with the findings from our institution among the general surgery population.16 As the studies by Bahl et al16 and Pannucci et al17 demonstrated, and we corroborate, a diverse group of surgical patients with Caprini scores greater than 8 are at an approximately 20-fold increased risk of VTE, and those with scores of 7 to 8 are at an approximately 5- to 10-fold risk when compared with low-risk patients across surgical specialties.
Evaluation of individual risk factors in our patient population revealed that cancer, chronic obstructive pulmonary disease, recent stroke, central venous catheter, and infections are all associated with a significantly higher rate of VTE, which is consistent with data in other populations. It is somewhat surprising that the population with VTE did not have a higher percentage of patients with a prior history of VTE, but this may be due to either incomplete documentation or a type II error.
Next Steps
The effectiveness of thromboprophylaxis in lowering the rates of DVT, PE, and fatal PE has been well established through many randomized clinical trials.2 A survey indicates that individual practice patterns among otolaryngologists with regard to thromboprophylaxis vary significantly, with the majority of providers not routinely administering prophylaxis.26 Critical to optimizing the risk/benefit ratio of thromboprophylaxis is obtaining an accurate assessment of a patient’s VTE risk. Although several approaches to assess VTE risk and guide thromboprophylaxis have been advocated, recent studies support the use of individualized patient risk assessment to more accurately identify a given patient’s VTE risk.27
Our data provide otolaryngologists with a useful tool to risk-stratify their patients for perioperative VTE events. The Caprini RAM allows otolaryngologists to identify patient subgroups who have a low risk (1 in 200; Caprini score less than 7), medium risk (1 in 42; Caprini score 7-8), and high risk (1 in 5.5; Caprini score greater than 8) of 30-day VTE if no chemoprophylaxis is provided. The next crucial question involves how best to apply this information to decrease the incidence of VTE in this population. Clearly, high-risk patients with Caprini scores greater than 8 merit aggressive thromboprophylaxis; the risk/benefit ratio of such treatments in low-risk and medium-risk otolaryngology patients remains unknown. A disproportionate number of VTE events occurred in patients undergoing microvascular free flaps. We plan further studies designed to describe the impact of thromboprophylaxis upon the incidence of perioperative VTE stratified by risk score, as well as specifically within the free flap population.
Strengths and Weaknesses
We used a large group of patients and employed a validated method of VTE risk stratification, adding weight to the data. The remarkable association between increasing risk score and VTE incidence underscores the biologic plausibility and veracity of our outcomes. The data should be quite generalizable to otolaryngologists in practice, as they span a 7-year period of all patients requiring admission for surgery in a large academic department and serve to risk-stratify patients regardless of specific demographic or clinical factors.
The retrospective nature of the study engenders the element of bias and is a weakness. Caprini risk scores may be underestimated because of incomplete documentation of risk factors, which were not always documented in a manner amenable to retrospective chart abstraction; prior studies suggest that this scoring system underestimates risk in approximately 5% of patients.16 The incidence of VTE may also be underestimated because of patients who were lost to follow-up, including those who may have presented to other institutions with perioperative VTE or those who may have developed a VTE more than 30 days after surgery. Previous work has shown that the risk for perioperative VTE may remain elevated for at least 90 days.9 In addition, patients with asymptomatic VTE events would not be identified, and patients who died without confirmation of a VTE were excluded, yielding an underrepresentation of the true 30-day rate of VTE.
We did not account for the role of sequential compression devices (SCDs) in this cohort, despite the fact that the overwhelming majority of patients had SCDs ordered, because we did not measure compliance with their usage or length of time that they remained on. Although SCDs may have a role in decreasing VTE incidence, studies have demonstrated that noncompliance is nearly ubiquitous and could severely confound the data unless it is rigorously measured.28 Moreover, the role of VTE chemoprophylaxis in otolaryngology has yet to be studied systematically, and this question is not addressed in this study.
Conclusion
The Caprini RAM is an effective and facile tool to predict 30-day VTE events in otolaryngology patients. The Caprini RAM allows otolaryngologists to identify patient subgroups who have a low risk (1 in 200; Caprini score <7), medium risk (1 in 42; Caprini score 7-8), and high risk (1 in 5.5; Caprini score >8) of 30-day VTE in the absence of chemoprophylaxis. Additional research is necessary to establish effectiveness and set specialty-specific guidelines for perioperative thromboprophylaxis among otolaryngology patients.
Footnotes
No sponsorships or competing interests have been disclosed for this article.
Author Contributions
Andrew G. Shuman, initial conception and design of the study, patient care, chart review, analysis and interpretation of data, drafting the article and revising it, and final approval of the version to be published; Hsou Mei Hu, contribution to study design; acquisition of data, statistical analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published; Christopher J. Pannucci, initial conception and design, acquisition of data, analysis and interpretation of data, critically revising the article, and final approval of the version to be published; Christopher R. Jackson, substantial contributions to initial conception and design, chart review, analysis and interpretation of data, drafting the article, and final approval of the version to be published; Carol R. Bradford, initial conception and design, patient care, acquisition of data, analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version to be published; Vinita Bahl, initial conception and design, statistical analysis and interpretation of data, drafting the article and revising it, and final approval of the version to be published.
Disclosures
Competing interests: None.
Sponsorships: None.
Funding source: None.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006;21:23–29. doi: 10.1007/s11239-006-5572-y. [DOI] [PubMed] [Google Scholar]
- 2.Geerts WH, Bergqvist D, Pineo GF, et al. American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th) 2008;133(6):381S–453S. doi: 10.1378/chest.08-0656. suppl. [DOI] [PubMed] [Google Scholar]
- 3.Michota FA. Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improve ment process. J Gen Intern Med. 2007;22:1762–1770. doi: 10.1007/s11606-007-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, Rooke TW, Silverstein M, et al. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. J Vasc Surg. 2001;33:1022–1027. doi: 10.1067/mva.2001.113308. [DOI] [PubMed] [Google Scholar]
- 6.Rathbun S. Cardiology patient pages: the Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation. 2009;119:e480–e482. doi: 10.1161/CIRCULATIONAHA.108.841403. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services The Surgeon General’s Call to Action to Prevent Deep Venous Thrombosis and Pulmonary Embolism. http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-on-dvt-2008.pdf. Accessed May 31, 2011. [Google Scholar]
- 8.Heit JA, Melton LJ, III, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76:1102–1110. doi: 10.4065/76.11.1102. [DOI] [PubMed] [Google Scholar]
- 9.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339:b4583. doi: 10.1136/bmj.b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolaides A, Irving D, Pretzell M, et al. The risk of deepvein thrombosis in surgical patients. Br J Surg. 1973;60:312. [PubMed] [Google Scholar]
- 11.Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients: results of meta-analysis. Ann Surg. 1988;208:227–240. doi: 10.1097/00000658-198808000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colditz GA, Tuden RL, Oster G. Rates of venous thrombosis after general surgery: combined results of randomized clinical trials. Lancet. 1986;2:143–146. doi: 10.1016/s0140-6736(86)91955-0. [DOI] [PubMed] [Google Scholar]
- 13.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboem bolic disease. Semin Hematol. 2001;38(2):12–19. doi: 10.1016/s0037-1963(01)90094-0. suppl 5. [DOI] [PubMed] [Google Scholar]
- 14.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. doi: 10.1016/j.disamonth.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9:253–262. doi: 10.1023/a:1018770712660. [DOI] [PubMed] [Google Scholar]
- 16.Bahl V, Hu HM, Henke PK, et al. A validation study of a ret rospective venous thromboembolism risk scoring method. Ann Surg. 2010;251:344–350. doi: 10.1097/SLA.0b013e3181b7fca6. [DOI] [PubMed] [Google Scholar]
- 17.Pannucci CJ, Bailey SH, Dreszer G, et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J Am Coll Surg. 2011;212:105–112. doi: 10.1016/j.jamcollsurg.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–2161. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 19.Arcelus JI, Candocia S, Traverso CI, et al. Venous thromboembolism prophylaxis and risk assessment in medical patients. Semin Thromb Hemost. 1991;17:313–318. [PubMed] [Google Scholar]
- 20.Hatef DA, Kenkel JM, Nguyen MQ, et al. Thromboembolic risk assessment and the efficacy of enoxaparin prophylaxis in excisional body contouring surgery. Plast Reconstr Surg. 2008;122:269–279. doi: 10.1097/PRS.0b013e3181773d4a. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Alexander A, Higgins K, Geerts W. The Sunnybrook experience: review of deep vein thrombosis and pulmonary embolism in otolaryngology. J Otolaryngol Head Neck Surg. 2008;37:547–551. [PubMed] [Google Scholar]
- 22.Moreano EH, Hutchison JL, McCulloch TM, et al. Incidence of deep venous thrombosis and pulmonary embolism in otolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 1998;118:777–784. doi: 10.1016/S0194-5998(98)70268-2. [DOI] [PubMed] [Google Scholar]
- 23.Chen CM, Disa JJ, Cordeiro PG, et al. The incidence of venous thromboembolism after oncologic head and neck reconstruction. Ann Plast Surg. 2008;60:476–479. doi: 10.1097/SAP.0b013e31816fd7e7. [DOI] [PubMed] [Google Scholar]
- 24.Forouzanfar T, Heymans MW, van Schuilenburg A, Zweegman S, Schulten EA. Incidence of venous thromboembolism in oral and maxillofacial surgery: a retrospective analysis. Int J Oral Maxillofac Surg. 2010;39:256–259. doi: 10.1016/j.ijom.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 26.Ah-See KW, Kerr J, Sim DW. Prophylaxis for venous thromboembolism in head and neck surgery: the practice of otolar yngologists. J Laryngol Otol. 1997;111:845–849. doi: 10.1017/s0022215100138770. [DOI] [PubMed] [Google Scholar]
- 27.Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199(1):S3–S10. doi: 10.1016/j.amjsurg.2009.10.006. suppl. [DOI] [PubMed] [Google Scholar]
- 28.Cornwell EE, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68:470–473. [PubMed] [Google Scholar]